INTRODUCTION
Invasive group A streptococcal (GAS) disease is a condition of major public health importance. Worldwide it is estimated that severe GAS disease (including acute rheumatic fever and invasive infections) is responsible for over 500 000 deaths annually [Reference Carapetis1]. Globally there are 1·78 million new cases of severe GAS disease each year [Reference Carapetis1]. Case-fatality rates for GAS toxic shock syndrome and GAS necrotizing fasciitis, two severe manifestations of invasive GAS disease, are 36% and 24%, respectively, in the USA [Reference O'Loughlin2].
The identification of predictors of severe outcomes in patients presenting with invasive GAS disease may aid clinicians in their management of this serious infection. Ekelund and colleagues studied 397 cases of invasive GAS disease identified through a national surveillance programme in Denmark [Reference Ekelund, Lemcke and Konradsen3]. The prevalence of gastrointestinal (GI) complaints in the 72 patients who died was 17%. In comparison, this prevalence was only 9% in the patients who survived their invasive GAS infection [Reference Ekelund, Lemcke and Konradsen3]. The objective of this study was to determine if the presence of diarrhoea and/or vomiting early in the course of invasive GAS disease is associated with either of two severe outcomes: GAS necrotizing fasciitis, or hospital mortality.
METHODS
Source of patients and inclusion criteria
Demographic and clinical data from an epidemiological study of invasive GAS disease that was conducted in Florida, the methods of which are described in detail elsewhere, were accessed [Reference Mulla, Leaverton and Wiersma4]. The study population was comprised of 257 patients who were hospitalized throughout the state of Florida between August 1996 and August 2000 for invasive GAS disease and reported to the Florida Department of Health (Tallahassee, Florida).
Invasive GAS disease was defined as isolation of group A Streptococcus from a normally sterile site (e.g. blood, cerebrospinal fluid, joint fluid, pleural fluid, or pericardial fluid), and a clinically compatible presentation. The definition of a clinically compatible presentation was one of several entities, including pneumonia, bacteraemia in association with cutaneous infection (e.g. cellulitis, erysipelas, or infection of a surgical or nonsurgical wound), deep soft-tissue infection (e.g. myositis), meningitis, peritonitis, osteomyelitis, septic arthritis, postpartum sepsis (i.e. puerperal fever), neonatal sepsis, and non-focal bacteraemia. The original study also included cases of necrotizing fasciitis if GAS was isolated from a non-sterile site.
For each case of invasive GAS disease that was reported by a county health department to the Florida Department of Health, a three-page surveillance case report form was completed after review of the patient's medical record. This chart review was usually performed by an epidemiology staff member at the reporting county health department. Cultures that were positive for GAS (e.g. blood, body fluids, and wounds) were recorded on the case report forms.
The case report form contained a section which prompted the chart reviewer to check off the diseases caused by GAS including necrotizing fasciitis. Another section required the chart reviewer to check off various signs and symptoms that were present within 48 h of admission including diarrhoea and vomiting [Reference Mulla5]. Risk factors for invasive GAS infection including diabetes were also captured by this case report form. Antimicrobial therapy that was received during the hospitalization was also noted on the form.
Statistical analysis
Cross-sectional analyses were conducted. The risk factor of interest was GI complaints. A binary exposure variable was created using the vomiting and diarrhoea check boxes. If either of these boxes were checked then the patient was considered to have GI complaints. Two binary outcomes were studied: GAS necrotizing fasciitis (present/absent), and hospital mortality. There were too few documented cases of GAS toxic shock syndrome in our dataset to use this variable as an outcome in multivariable analyses.
The data were analysed using the SAS System for Windows Release 9.1.3 (SAS Institute, USA). Initially, the associations between GI complaints and various factors were evaluated using the χ2 test for categorical variables and the Wilcoxon rank sum test for the age variable. Unadjusted and adjusted odds ratios (aORs) for the two outcomes were calculated from logistic regression models. The GI ORs were considered to be statistically significant if the 95% confidence intervals for the population ORs did not include the null value of 1.
A stratified analysis revealed that the association between GI symptoms and the presence of necrotizing fasciitis was modified by age, i.e. age was an effect-measure modifier [Reference Rothman, Greenland and Lash6]. Therefore all of the aORs for necrotizing fasciitis were stratified by age: 0–54 years vs. ⩾55 years). The GI OR for the outcome of GAS necrotizing fasciitis was also adjusted for sex, race (white vs. other), diabetic status, treatment in the hospital with one or more of the penicillins and/or cephalosporins, and treatment with clindamycin. The GI OR for hospital mortality was adjusted for age (a continuous variable), sex, race, diabetic status, penicillins and cephalosporins, clindamycin, and the presence of necrotizing fasciitis. Intravenous immunoglobulin G therapy was not included as a covariate in any of the models due to the fact that very few of the 257 patients received it (n=4) and a large number (n=101) had missing values for this variable.
Two approaches to the logistic regression analyses were taken. In the first approach, only the records that were free of missing values for all of the variables under study were included. This technique is referred to as a complete-subject analysis [Reference Rothman, Greenland and Lash6], and it reduced the sample size from 257 patients to 138 patients.
In the second approach, multiple imputation was used to replace any missing values for the independent variables and the two dependent variables [Reference Rothman, Greenland and Lash6–Reference Yuan8]. This method allowed for the analysis of the entire sample (n=257). Imputation methods predict and complete the missing values using the observed data [Reference Rothman, Greenland and Lash6]. Multiple imputation replaces each missing value with a group of plausible values and then these datasets are analysed using standard procedures and then combined in an appropriate manner. Data were assumed to be missing at random (MAR) [Reference Little and Rubin7, Reference Yuan8]. If the probability that a value is missing does not depend on the missing value but does depend on observed quantities (values of variables that were measured) then the missing-data mechanism is called MAR. The majority of the literature on multivariate incomplete data assumes that the data are MAR [Reference Little and Rubin7]. While it is impossible to test the MAR assumption without additional information, including a larger set of predictors in the imputation model may make the MAR assumption more plausible [Reference Horton and Kleinman9]. Both of our imputation models contained the mortality variable, the necrotizing fasciitis variable, diarrhoea, vomiting, age (continuous), race, sex, diabetes, clindamycin, and the β-lactam antibiotic variable. The Markov chain Monte Carlo method (MCMC) was implemented. MCMC creates multiple imputations by using simulations from a Bayesian prediction distribution [Reference Yuan8]. This technique assumes multivariate normality. In contrast, the majority of our predictors were categorical variables. However, there is evidence that this imputation method performs well even when the distributions are not normal/Gaussian [Reference Allison10].
The mi procedure in SAS was used to perform 20 imputations and the mianalyze procedure was used to combine the results of the logistic regression models [Reference Yuan8]. A prior distribution is required to obtain the posterior distribution of parameters [Reference Yuan8]. We specified a non-informative prior (Jeffreys' prior). Time-series and autocorrelation plots were inspected for undesirable trends [Reference Allison10]. The minimum, maximum, and round options were used initially. Since the rounding of imputed binary variables may lead to biased results [Reference Horton, Lipsitz and Parzen11, Reference Bernaards, Belin and Schafer12], we repeated the process described above without applying minima and maxima to any of the imputed independent variables and without rounding these variables. For each dichotomous outcome (GAS necrotizing fasciitis, and death) we continued to apply a minimum of 0 and a maximum of 1.
Analyses of this database were deemed exempt from formal review by the Institutional Review Board of the Texas Tech University Health Sciences Center School of Medicine at El Paso.
RESULTS
The demographic and clinical characteristics of the study sample are displayed in Table 1. The group of patients who had GI symptoms within 48 h of admission had a median age of 48 years while those who were free of GI symptoms within 48 h of admission had a median age of 53 years. Males were underrepresented in the group presenting with GI symptoms. The prevalence of diabetes in the GI group was about half that found in the comparison group (10·4% vs. 20·0%).
Table 1. Demographic and clinical characteristics of 138 patients hospitalized for invasive group A streptococcal disease
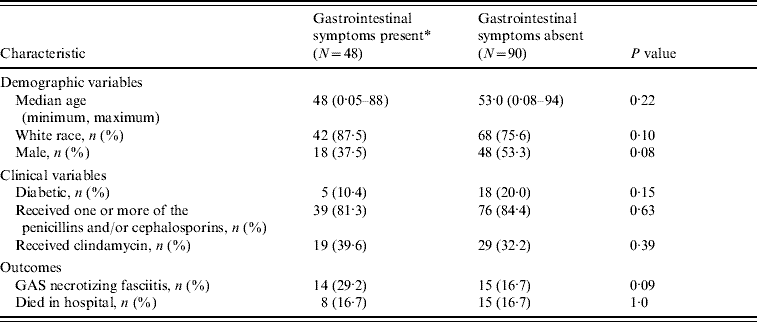
* Defined as vomiting and/or diarrhoea within 48 h of admission.
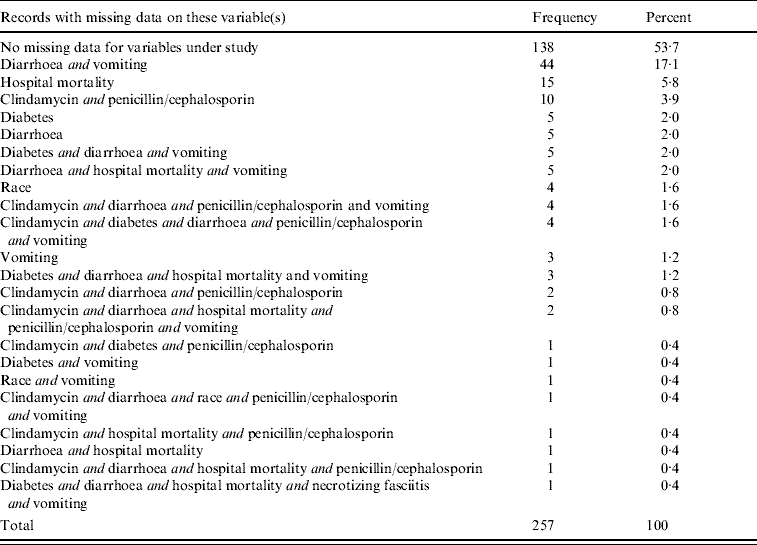
Table 2 summarizes the missing data patterns found in the entire dataset of 257 patients. A total of 138 patient records (53·7%) had complete data on the variables of interest. Forty-four of the 257 patients (17·1%) had missing values for both the diarrhoea variable and the vomiting variable.
Table 2. Missing data patterns found in the entire dataset (N=257)
Adjusted ORs for the two outcomes, GAS necrotizing fasciitis and hospital mortality, are given in Tables 3 and 4. One of the two ORs from the complete-subject analysis (Table 3) indicated that in patients who were aged <55 years, those who had GI complaints early in their hospitalization were more than four times as likely to have a diagnosis of necrotizing fasciitis than patients who did not have GI complaints early in their hospitalization (aOR 4·64, P=0·03). None of the ORs in the older (⩾55 years) patients were statistically significant (Table 3). The GI OR for GAS necrotizing fasciitis from the imputed dataset where rounding was used was attenuated but still indicated a substantial increase in the odds of having necrotizing fasciitis (aOR 3·86, P=0·02) in the group of patients who were aged <55 years. In the younger age group, multiple imputation without rounding resulted in a GI OR for GAS necrotizing fasciitis that was similar to the OR from the complete case analysis. None of the three GI ORs for hospital mortality were statistically significant (Table 4).
Table 3. Adjusted odds ratios (aOR)Footnote * for the outcome of necrotizing fasciitis comparing IGASD patients who had gastrointestinal symptoms within 48 h of admission with IGASD patients who did not have gastrointestinal symptoms within 48 h of admission (the results are stratified by age, an effect modifier)
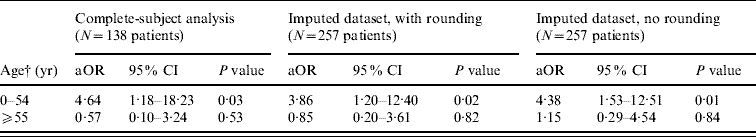
IGASD, Invasive group A streptococcal disease; CI, confidence interval.
* Adjusted for race, sex, diabetes, and receipt of penicillins or cephalosporins, and clindamycin.
† For the complete-subject analysis 79 patients were aged <55 years while 59 patients were aged ⩾55 years. For the imputed datasets, these figures were 140 patients and 117 patients, respectively.
Table 4. Adjusted odds ratios (aOR)Footnote * for the outcome of hospital mortality comparing IGASD patients who had gastrointestinal symptoms within 48 h of admission with IGASD patients who did not have gastrointestinal symptoms within 48 h of admission

IGASD, Invasive group A streptococcal disease; CI, confidence interval.
* Adjusted for age (continuous), race, sex, diabetes, and receipt of penicillins or cephalosporins, and clindamycin, and necrotizing fasciitis.
In an attempt to confirm that GI symptoms preceded rather than followed the development of necrotizing fasciitis, the admitting diagnoses of the 29 necrotizing fasciitis patients were examined. A total of 27 of our 29 necrotizing fasciitis patients had an admitting diagnosis listed on their health department case report form (Table 5). Of these 27 patients, only two had an admitting diagnosis of necrotizing fasciitis (Table 5).
Table 5. Admitting diagnosisFootnote * of 27 patients with group A streptococcal necrotizing fasciitis
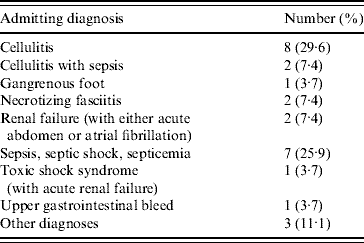
* Admitting diagnoses were not available for an additional two patients with group A streptococcal necrotizing fasciitis.
DISCUSSION
Invasive GAS infection has a fulminant nature and necrotizing fasciitis is one of its most severe manifestations. Rapid detection and rapid, appropriate treatment are critical in reducing the risk of mortality in these patients. Our population-based epidemiological analysis of 138 patients hospitalized for invasive GAS disease found that the presence of diarrhoea and/or vomiting early in the course of the infection was associated with necrotizing fasciitis in younger (0–54 years) but not older individuals. We could only find one other similar controlled, epidemiological study [Reference Ekelund, Lemcke and Konradsen3]. This comparable study was conducted by Ekelund et al. [Reference Ekelund, Lemcke and Konradsen3]. Ekelund and colleagues did not examine the association between GI symptoms and the development of necrotizing fasciitis in a cohort of patients with invasive GAS disease, but they did examine the relationship between GI symptoms and mortality in patients carrying the diagnosis of invasive GAS infection [Reference Ekelund, Lemcke and Konradsen3]. They found that fatal cases were almost twice as likely as survivors (17% vs. 9%, P<0·05) to have only GI complaints as the primary symptom [Reference Ekelund, Lemcke and Konradsen3]. In contrast, we did not detect a link between GI complaints and hospital mortality in our study.
The common cause of GI symptoms and necrotizing fasciitis may be streptococcal exotoxins. These exotoxins mediate their damage by increasing cell membrane permeability. This permeability increase is by one of three possible mechanisms depending on the specific toxin present. The permeability change is due to (1) altered lipid composition of the membrane, (2) detergent-like activity of the toxin, or (3) trans-membrane pore formation [Reference Lewis, Berg and Kleine13]. This altered permeability leads to cell death by disrupting cell function, cell lysis, or influx of ions such as calcium [Reference Lewis, Berg and Kleine13]. Cell death in the presence of living group A streptococci has been observed since the 1960s when Quinn & Lowry demonstrated that cell types of various human tissues were all destroyed by exposure to living Streptococcus pyogenes [Reference Quinn and Lowry14]. Given these results it is likely that regardless of the site of localization of the initial GAS infection, the bacteria could gain access to the systemic circulation via permeabilization of the epithelial barrier by destruction of individual cells comprising the barrier. This effect would be similar to the mechanism of damage observed in the small intestine by ischaemia reperfusion injury [Reference Osborne15]. The localized damage following superior mesenteric blockade resulted from the sloughing of cells from the villi. This loss of cells resulted in a breach of the epithelial barrier which in the case of GAS-mediated damage would expose the host to invasion by bacteria. This is the possible mechanism by which GI symptoms are correlated with the subsequent observation of necrotizing fasciitis. This would explain why GI exposure, a simple columnar epithelia, would be more likely to lead to systemic translocation than pharyngeal exposure, a stratified squamous epithelia.
Bryant et al. [Reference Bryant16] recently conducted an animal study to determine the mechanism responsible for the rapid destruction of tissue observed in GAS necrotizing fasciitis/myonecrosis. These investigators found that GAS toxins initiated a cascade of events leading to thrombosis of microvasculature and ischaemic necrosis. Specifically, the authors found that GAS toxins stimulated the formation of platelet/polymorphonuclear leukocyte complexes and these aggregates contributed to vascular occlusion and regional tissue necrosis. Their results suggest that the toxin streptolysin O is involved in the formation of these platelet/neutrophil complexes [Reference Bryant16]. This platelet-induced coagulation could also lead to ischaemic injury in the area around the occlusion which could give bacteria access to the interstitial compartment in localized tissue spaces around the occlusion.
Bisno and colleagues [Reference Bisno, Cockerill and Bermudez17] described the clinical manifestations of 15 patients with GAS necrotizing fasciitis. They found that 47% of these cases had gastroenteric symptoms such as nausea, vomiting, and diarrhoea, at the time of their initial outpatient presentation. Staphylococcus aureus is well-known for its ability to cause foodborne intoxication [Reference Chin18]. Both group A streptococci and coagulase-positive staphylococci express a family of pyrogenic toxin superantigens which can produce a variety of signs including emesis and diarrhoea [Reference Schlievert19]. In fact, streptococcal pyrogenic exotoxin (spe) serotype A is more similar to staphylococcal enterotoxins B and C than to other streptococcal or staphylococcal toxin serotypes [Reference Stevens and Kaplan20, Reference Bohach21].
Strengths of our study include the control of several potential confounders and the use of multiple imputation to address missing values. About 46% of the patient records in our analysis were missing values for one or more of the variables under study. There is no agreed upon upper limit for the percentage of records with missing values beyond which a data analyst should not attempt multiple imputation. In theory, a large missing rate is not a critical issue as long as the assumptions of MAR and multivariate normality are met and the sample size is large because the multiple imputation takes into account the uncertainty caused by the high missing rate.
Most of the literature on multivariate missing data assumes that the data are MAR, a term that is usually synonymous with the phrase ‘ignorable non-response’ [Reference Little and Rubin7]. Unfortunately, however, without additional information it is impossible to test whether or not the assumption of MAR is true [Reference Horton and Kleinman9]. If the missing data mechanism is not MAR, i.e. non-ignorable non-response is present, then biased estimates will result if the data analyst assumes the mechanism is MAR [Reference Little and Rubin7]. If the data are not MAR then one will have to model the missing data mechanism. However, estimating the missing data mechanism with some level of confidence is rarely feasible [Reference Little and Rubin7]. Little & Rubin state that, ‘the formulation of non-ignorable models that are superior to ignorable models is very context-specific and not easy’ [Reference Little and Rubin7].
A limitation of this study and most cross-sectional studies is the ambiguity regarding temporality: did the suspected risk factor or correlate truly occur before the outcome? In our analysis, it is possible that patients developed GAS necrotizing fasciitis first and then some days later developed GI symptoms; however, this is unlikely given the results our analysis of the admitting diagnoses (Table 5). Nonetheless, our study case report forms lacked detailed data on the timing of certain clinical events and therefore further investigation is warranted.
Diarrhoea and emesis may be early clinical manifestations of severe invasive GASl infections [Reference Ekelund, Lemcke and Konradsen3]. A large prospective cohort study would be required to definitively determine the utility of screening emergency-room patients with suspected invasive GAS disease or sepsis for GI symptomatology. This type of study would not be feasible for a single institution although countries who conduct regional or national active surveillance of invasive GAS infections would be equipped to determine the positive predictive value of GI signs and symptoms and other clinical features in the rapid identification of GAS necrotizing fasciitis.
If GAS necrotizing fasciitis is suspected then prompt consultations with surgeons and infectious disease physicians are indicated. Clinicians who are treating individuals presumed to be in the early stages of invasive GAS disease should take note of GI symptoms and remain vigilant for the development of a GAS necrotizing soft-tissue infection.
DECLARATION OF INTEREST
None.







