INTRODUCTION
Vitamin D deficiency has reached pandemic proportions with significant impact on the induction and outcome of many chronic diseases [Reference Holick1]. The benefits of a vitamin D replete state remain poorly recognized [Reference Bikle2]. In addition to the potential benefits of vitamin D on innate immunity there is increasing evidence for existence of an antimicrobial effect. Over 20 years ago, Feindt & Ströder [Reference Feindt and Ströder3] demonstrated by in vitro experiments that vitamin D3 proved lethal or markedly inhibited the growth of several bacterial strains including Staphylococcus aureus. Several studies have indicated a potential role for vitamin D in respiratory tract infections in both children and adults [Reference Roth4, Reference Karatekin5]. Vitamin D receptor haplotypes may determine the susceptibility to HIV infection in intravenous drug abusers [Reference De la Torre6]. Moreover, antiviral and anti-tuberculous effects of vitamin D have also been described [Reference Li7, Reference Liu8].
Clostridium difficile is a growing problem in hospitals, especially among elderly patients [Reference O'Connor9], C. difficile infection is the most frequent cause of healthcare-associated infectious diarrhoea in industrialized countries [Reference Gravel10, Reference Bujanda and Cosme11]. C. difficile has demonstrated progressive increase in virulence and is often refractory to treatment [Reference Dubberke and Wertheimer12]. The increasing prevalence of the spread of C. difficile in the community, virulence and frequent relapse has created an urgent need to identify new effective treatments for this infection.
About one-third of the US population is colonized with S. aureus [Reference Noskin13], and the rates of S. aureus bacteraemia are increasing [Reference Engemann14]. S. aureus was the most common cause of nosocomial infections reported in the National Nosocomial Surveillance System between 1990 and 1996 [Reference Engemann14, Reference Rubin15]. Staphylococcal infections were associated with high costs and large numbers of deaths in the New York City metropolitan area [Reference Rubin15]. S. aureus has a profound impact on length of hospitalization and patient outcomes [Reference Noskin13].
Given the economic burden of these two infections, we initiated a study to determine if vitamin D status was related to the healthcare outcomes and costs associated with infections with these two organisms.
METHODS
This study was conducted at James H. Quillen Veterans Medical Facility in the Southeastern United States. The Research and Development committee at VAMC and the institutional review board at the affiliated university approved the study. Data were obtained electronically through retrospective review after personal information was redacted. The sample included all patients diagnosed with either methicillin-sensitive Staphylococcus aureus (MSSA) or C. difficile infections from 2000 to 2008 that also had serum 25-hydroxyvitamin D [25(OH)D] analysis run within 3 months of the initial diagnosis. The 25(OH)D assay was done by immunochemiluminometric assay (Labcorp, USA). The costs were estimated by the technical guidelines via the Decision Support System and clinical National Data Extracts standardized by the VA as previously reported [Reference Peiris, Bailey and Manning16]. Costs in the year following diagnosis were broken down into separate in-patient and outpatient categories (i.e. laboratory, pharmacy, radiology, surgery, primary care, emergency room, etc.). For each of the cost categories, a total amount (US$) was analysed. Dichotomous service utilization variables were constructed to represent whether a patient had incurred that type of cost. No cost in a particular category meant that service was not used. Fees refer to costs incurred by the VA as a result of consultation and/or procedures performed in the private sector.
Utilizing diagnosis and procedure codes specified in the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9) [Reference Napier17], the database was queried for patients with a discharge diagnosis code of 008·45 reflecting C. difficile/pseudomembranous colitis, patients with staphylococcal infections were identified using the ICD-9 code 041·11.
The final sample contained 52 patients, 29 with staphylococcal and 23 with C. difficile infections (see Table 1). There were no significant differences between patients with S. aureus and patients with C. difficile regarding their 25(OH)D level [24·6 vs. 22·6; t(50)=0·47, P=0·63] or status [58·6% deficient vs. 60·9% deficient; χ2(1)=0·03, P=0·87]. Thus, remaining analyses were performed using the combined sample of 52 patients.
Table 1. Sample characteristics: vitamin D status (deficient/non-deficient)
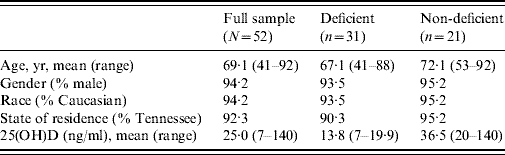
Apart from 25(OH)D levels, none of the differences between the groups were statistically significant.
Statistical analysis
25(OH)D level was analysed both as a continuous and a dichotomous variable. Vitamin D deficiency was defined as a 25(OH)D level of <20 ng/ml [Reference Holick1]. Statistical analyses were performed using SPSS software, version 14.0 (SPSS Inc., USA). All variables were checked for outliers and normality of distribution before analyses were performed. Correlations and χ2 analysis were used to examine the association between type of infection and 25(OH)D level and deficiency status. t tests were used to examine the associations between vitamin D status and the cost and service utilization variables.
RESULTS
In the outpatient setting, vitamin D deficiency in patients with C. difficile and staphylococcal infections were associated with significantly increased total outpatient costs and fee-based consultation (Table 2). Laboratory expenses had a trend towards higher costs in the vitamin D-deficient group but did not reach statistical significance.
Table 2. Costs incurred in staphylococcal and clostridial infections in relation to vitamin D status (N=52)
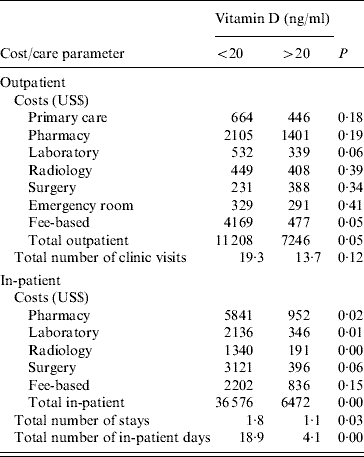
25(OH)D <20 ng/ml deemed vitamin D deficient.
As shown in Table 2, the differences between the two groups were most clearly seen in the in-patient group with enhanced laboratory, pharmacy and radiology costs. These differences resulted in vitamin D-deficient patients with C. difficile/staphylococcal infections having costs more than five times higher than the non-deficient patients. The total length of hospital stay was four times greater in the vitamin D-deficient group. In addition the total number of hospitalizations was also significantly greater in the vitamin D-deficient group. Surgery costs demonstrated a tendency to be higher in the vitamin D-deficient group but failed to reach statistical significance.
DISCUSSION
Our findings in a Veterans' population with C. difficile and staphylococcal infections indicate a link between vitamin D deficiency and adverse outcomes including increased healthcare utilization and expenses. This report is, to the best of our knowledge, the first to indicate that vitamin D deficiency may play an important role in limiting the success of traditional therapies in these two bacterial infections.
There is an increasing incidence of both S. aureus and C. difficile infections resulting in increased healthcare costs, morbidity and mortality. The traditional use of antibiotics has been implicated in the induction of superinfections with S. aureus and in some cases may play a more direct role in the induction of infections such as C. difficile. Given the prevalence of vitamin D deficiency in Veterans [Reference Peiris, Bailey and Manning16], we believe that vitamin D has an important antimicrobial role with the potential to offer substantial cost savings linked to hospitalization for C. difficile and staphylococcal infections.
The link between suboptimal vitamin D status and infections is not new. An association between bacterial vaginosis and maternal vitamin D deficiency has been reported in pregnant women [Reference Bodnar, Krohn and Simhan18]. In patients on regular haemodialysis, serum 25(OH)D levels were significantly correlated with serum H. pylori-specific IgG antibody titres even when adjusted for age and duration of dialysis [Reference Nasri and Baradaran19]. In HIV-infected adults an inverse relationship between 1,25(OH)2D (the active form of vitamin D) level and mortality and positive correlations between 1,25(OH)2D levels and CD4+ cell counts have been observed [Reference Haug20].
Serum 25(OH)D levels were inversely associated with recent upper respiratory infections in the Third National Health and Nutrition Examination Survey [Reference Ginde, Mansbach and Camargo21]. In Indian children aged <5 years, subclinical vitamin D deficiency was a significant risk factor for severe acute lower respiratory tract infection [Reference Wayse22]. There may be a dose-related effect since supplementation with 2000 IU of vitamin D in a study by Li-Ng and colleagues was not associated with decreased upper respiratory tract infections [Reference Li-Ng23]. The levels reached following supplementation in that study were ∼35 ng/ml (88 nmol/l). These values are significantly below the values seen in humans exposed to liberal sunlight, in whom the values are ∼60 ng/ml. It may also be possible that factors other than serum vitamin D level play a role in susceptibility to infection. The vitamin D receptor is ubiquitous; however, genetic polymorphisms in the vitamin D receptor may also determine the risk of acquiring infections such as tuberculosis [Reference Leandro24, Reference Wilbur25].
Animal studies also lend support the antimicrobial effects of vitamin D. In an experimental model of turkey osteomyelitis, vitamin D treatment resulted in reduced bacterial presence in tissue and improved mortality [Reference Huff26]. In studies in mice, an overload of vitamin D had a protective effect against some strains of Trypanosoma cruzi infections [Reference Silva27].
The role of vitamin D as an antimicrobial agent acting through multiple mechanisms is becoming increasingly recognized. These mechanisms have been reviewed by Bikle [Reference Bikle2] and indicate a potential boost in innate immunity by vitamin D. Gombart et al. [Reference Gombart, Borregaard and Koeffler28] proposed that 1,25-dihydroxyvitamin D3 [1,25D(3)] induced expression of the human cathelicidin antimicrobial peptide (CAMP) gene. Their findings have since been confirmed by others [Reference Li7] and indicate that vitamin D increases the body's production of naturally occurring antimicrobial peptides such as cathelicidin [Reference Li7]. Cathelicidin is required for the 1,25D(3)-triggered antimicrobial activity against intracellular Mycobacterium tuberculosis [Reference Liu8]. Furthermore, Liu et al. [Reference Liu8] observed that sera from African-American individuals, known to have increased susceptibility to tuberculosis had low vitamin D levels and were inefficient in cathelicidin messenger RNA induction. Thus the link between vitamin D-mediated and toll-like receptor activation may also play a role in susceptibility to infections. In patients initiating chronic haemodialysis, low baseline levels of cathelicidin are independently associated with an increased risk of death attributable to infection [Reference Gombart, Borregaard and Koeffler28].
The current recommendations for vitamin D intake while adequate for preventing rickets are clearly inadequate for optimal health. It is clear that much larger doses of vitamin D than previously surmised, e.g. a 300 000 IU bolus, can be used safely to treat vitamin D deficiency [Reference Leventis and Kiely29]. The potential to boost cathelicidin by short-term intensive high-dose vitamin D replacement and thereby influence infectious processes remains to be seen.
Our study does have certain inherent limitations. The current study involves Veterans and given its retrospective nature not all factors which could have influenced outcomes could be controlled. The strength of our study, similarly to previously published studies, comes from documentation of an important and unique association [Reference Doll and Hill30]. The present conclusions could also be explained by increased severity of illness in the vitamin D-deficient group. However, the mean serum levels of 25(OH)D found in the deficient group in our study are comparable to levels in elderly adults in winter [Reference Scragg, Khaw and Murphy31] and 25(OH)D levels of <10 ng/ml have been reported in 35–50% of male university students living in Riyadh, Saudi Arabia [Reference Sedrani32]. As such, it is likely that vitamin D deficiency contributes to healthcare costs for these two bacterial infections rather than merely reflecting the degree of illness. Furthermore, our findings are consistent with the benefits of a vitamin D replete state to improve innate immunity in critically ill patients with sepsis [Reference Jeng33]. Testing for vitamin D remains suboptimal and as such an adequate sample size in order to extend these observations to methicillin-resistant S. aureus was not possible. However, given the significance of our findings, we believe our conclusions should provide impetus to initiate additional studies to evaluate this important issue.
In conclusion, we believe that achieving a vitamin D replete state should be given high priority when treating C. difficile and staphylococcal infections, and possibly other infections. Studies by Ginde et al. [Reference Ginde, Liu and Camargo34] indicate that the prevalence of vitamin D deficiency has become more marked in recent years and as such appropriate replacement may offer immune and antimicrobial benefits at minimal cost. This benefit may be added to the long list of health benefits associated with adequate vitamin D reserves including improved well-being and enhanced longevity [Reference Autier and Gandini35] with a projected significant savings in healthcare costs [Reference Grant36]. Moreover, the possible synergy with antibiotics by the therapeutic effects of a vitamin D-replete status remains an exciting avenue for further exploration.
ACKNOWLEDGEMENTS
We thank Nancy Milligan and the Mountain Home VAMC library staff and Johnson City Medical Center Library staff for their assistance. We appreciate the helpful comments by Felix Sarubbi, M.D. and Jonathan Moorman, M.D., Division of Infectious Diseases, ETSU and Dr Guha Krishnaswamy, Division of Allergy Immunology, ETSU.
DECLARATION OF INTEREST
None.




