INTRODUCTION
By the end of 2009, 33·3 million HIV-infected people were living worldwide. Of these, 30·8 million were adults including 15·9 million women and 14·9 million men. There were 2·6 million individuals newly infected with AIDS in 2009 including 2·2 million adults and 370 000 children aged <15 years [1]. In 2009, an estimated 1·2 million people were living with AIDS in the USA [2] while an estimated 54 000 adults and children were newly diagnosed with HIV [1]. The Michigan Department of Community Health (MDCH) estimates that there are 18 200 people currently living with HIV/AIDS in Michigan, of these, 14 871 were reported by October 2010 [3].
Viral hepatitis is caused by an infection with any of the five distinct hepatitis viruses. However, in terms of morbidity and mortality, hepatitis B and C viruses are the most important. Around 2 billion people globally have been infected with hepatitis B virus (HBV) while about 350 million live with chronic infection. Less than 1% of the population in Western Europe and North America suffers from chronic infection with HBV [4].
According to the World Health Organization (WHO), there are about 180 million people infected with hepatitis C virus (HCV) which is 3% of the world's population. Approximately, 3–4 million individuals are newly infected each year, 70% of whom will develop chronic hepatitis. Currently, there are 3·9 million individuals in the USA with chronic HCV, with prevalence as high as 8–10% in Blacks [5].
The Centres for Disease Control and Prevention (CDC) reported 4519 cases of acute hepatitis B in the USA in 2007. As many HBV infections are either asymptomatic or never reported, the actual number of new infections is estimated to be nearly tenfold higher or about 43 000 persons in the USA in 2007 [6]. In Michigan, there were 142 cases of acute HBV in 2008 while the number of confirmed cases of chronic hepatitis B was 1340 in the same year [7, 8].
In 2007, 849 cases of confirmed acute hepatitis C were reported in the USA. However, the CDC estimates that about 17 000 new HCV infections occurred that year, taking into account asymptomatic infection and underreporting. There are about 3·2 million persons in the USA with chronic HCV infection [9]. In 2008, there were 125 cases of acute hepatitis C and 7167 confirmed cases of chronic hepatitis C in the state of Michigan [10, 11].
With the recent decrease in many common opportunistic infections in HIV/AIDS patients, HBV and HCV co-infection have emerged as major contributors to morbidity and mortality. In the USA, the estimated prevalence of HCV ranges from 15% to 30% in people living with HIV/AIDS to as high as 50–90% in persons who acquired HIV infection through injecting drug use. HCV is one of the most important causes of chronic liver disease and cirrhosis in the USA and HCV infection progresses more rapidly to cause liver damage in HIV-infected persons. HCV associated end-stage liver disease is currently a major cause of death in people living with HIV/AIDS [Reference Sherman12]. Individuals with HIV are found to have higher rates of HBV chronicity, higher HBV replication, lower ALT levels and lower rates of seroconversion to anti-HBe and anti-HBs [Reference Puoti13]. HIV-infected adults progress to chronic hepatitis B at a rate about five times higher than HIV-uninfected adults [Reference Bodsworth, Cooper and Donovan14].
In Michigan, the Adult and Adolescent Spectrum of Disease (ASD) surveillance project collected data from the medical records of HIV patients at two major medical centres in Detroit, between 1990 and 2004. Based on this project, hepatitis C was the most common hepatitis co-infection in HIV-infected individuals. Out of the 1790 individuals in care and in ASD in 2001–2003, 353 (20%) were diagnosed with HCV infection at some time during ASD follow-up, while 207 (12%) were diagnosed with HBV infection [15].
As co-infection with HBV and HCV is associated with an increased morbidity and mortality in HIV/AIDS patients, it is imperative that the magnitude of the co-infection is estimated in the HIV/AIDS population in Michigan along with the concomitant risk factors for co-infection. This would allow for better prevention and control of these infections in the HIV/AIDS population. Therefore, the purpose of this study was to estimate the prevalence of hepatitis co-infection and identify the factors associated with co-infection in HIV-infected individuals residing in the state of Michigan in the years 2006–2009.
METHODS
A retrospective cohort study was conducted from 1 January 2006 to 31 December 2009 in Michigan, USA to meet the objectives of the study. Information on the participants was obtained from the enhanced HIV/AIDS Reporting System (eHARS) maintained by the HIV/STD/VH/TB Epidemiology Section and the Michigan Disease Surveillance System (MDSS) which is the state-wide communicable disease reporting system maintained by the Surveillance and Infectious Disease Epidemiology Section of the MDCH [16]. The information on HIV-infected individuals was obtained from eHARS while information on HBV and HCV infections was gathered from the MDSS. To enable analysis of HBV and HCV infections in HIV/AIDS individuals, a record linkage was established between the HIV/AIDS surveillance database and the MDSS. For our study, individuals of all ages infected with HIV/AIDS, from all the counties of Michigan, were included in the dataset and matched with all HBV and HCV cases during the study period to obtain a prevalence estimate, and the factors associated with HBV/HCV/HIV/AIDS co-infections were identified. As information on all hepatitis cases in this HIV/AIDS population was obtained, no sampling was performed.
Ethical approval was obtained from the Institutional Review Board at Michigan State University and MDCH. To ensure confidentiality and privacy of the participants, an employee of the HIV/STD/VH/TB Epidemiology Section performed the record linkage. The resulting database was de-identified according to Health Insurance Portability and Accountability Act (HIPAA) guidelines on public health information (there were no interviews or contact with the participants). Information on a number of characteristics of the individuals was obtained from eHARS and MDSS. For this study, variables included were sex, race, age at HIV diagnosis, current HIV status (status to December 2009) and HIV transmission risk. The outcome variable was a binary indicator for ‘co-infection’. An HIV/AIDS individual was categorized as co-infected if he/she had been concurrently infected with confirmed hepatitis B or hepatitis C virus (acute and chronic) based on the CDC case definition [17–20] and residing in the state of Michigan during the period 2006–2009. The rationale for creating the co-infection variable was based on the fact that the three diseases share similar routes of transmission [Reference Portelinha21]. Any HIV/AIDS case which had a diagnosis of acute or chronic hepatitis B or C before 2006 was excluded from the study. The HIV/AIDS case definition was based on the CDC's 1993 revised classification system of HIV [22]. Statistical analysis was performed in SAS software version 9.2 (SAS Institute Inc., USA). Descriptive statistics were generated to examine the distribution of characteristics. Logistic regression was used to assess factors associated with the outcome of co-infection. Univariable analyses provided the unadjusted association of factors with co-infection and the corresponding odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Multivariable analysis was conducted to evaluate the association of factors with co-infection while adjusting for confounding effects of other variables. All possible interactions were checked and entered into the final model based on statistical significance and/or biological plausibility. For interpretation, adjusted ORs (aORs) and their respective 95% CIs were utilized.
RESULTS
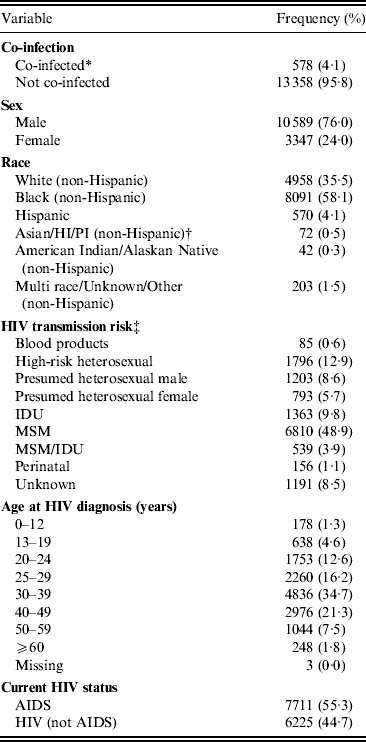
A total of 13 936 individuals were living with HIV/AIDS during the study period. About 76% were male. The race distribution was Black (58·1%), White (35·5%), Hispanic (4·1%), multiracial (1·5%), Asian/Hawaiian/Pacific Islander (0·5%), and American Indian/Alaskan Native (0·3%). The prevalence of HIV–hepatitis co-infection in these individuals was 4·1% (n = 578, 95% CI 3·82–4·48) (Table 1). The majority of co-infected cases were either chronic hepatitis B (n = 250, 1·8%) or chronic hepatitis C (n = 307, 2·2%). For HIV transmission risk categories, the majority of HIV/AIDS-infected individuals were men who have sex with men (MSM) (48·9%) followed by high-risk heterosexual (HRH) (12·9%), injecting drug user (IDU) (9·8%), heterosexual male (8·6%), unknown risk (8·5%), heterosexual female (5·7%), MSM/IDU (3·9%), perinatal (1·1%) and blood products (0·6%). With respect to age at HIV diagnosis, most HIV/AIDS-infected individuals were in the following age groups 30–39 years (34·7%) followed by 40–49 years (21·3%), 25–29 years (16·2%), and 20–24 years (12·6%). With respect to current HIV status, there were more individuals with AIDS (55·3%) compared to HIV only (44·7%).
Table 1. Distribution of basic characteristics of the HIV/AIDS-infected individuals in Michigan (n = 13 936)

* Co-infection = any HIV-infected individual that is infected by hepatitis B and C virus (acute and chronic).
† Asian/Hawaiian, Pacific Islander.
‡ Heterosexual female = female who denies being injecting drug user and has had sex with a man; MSM = men who have sex with men; IDU = injecting drug user.
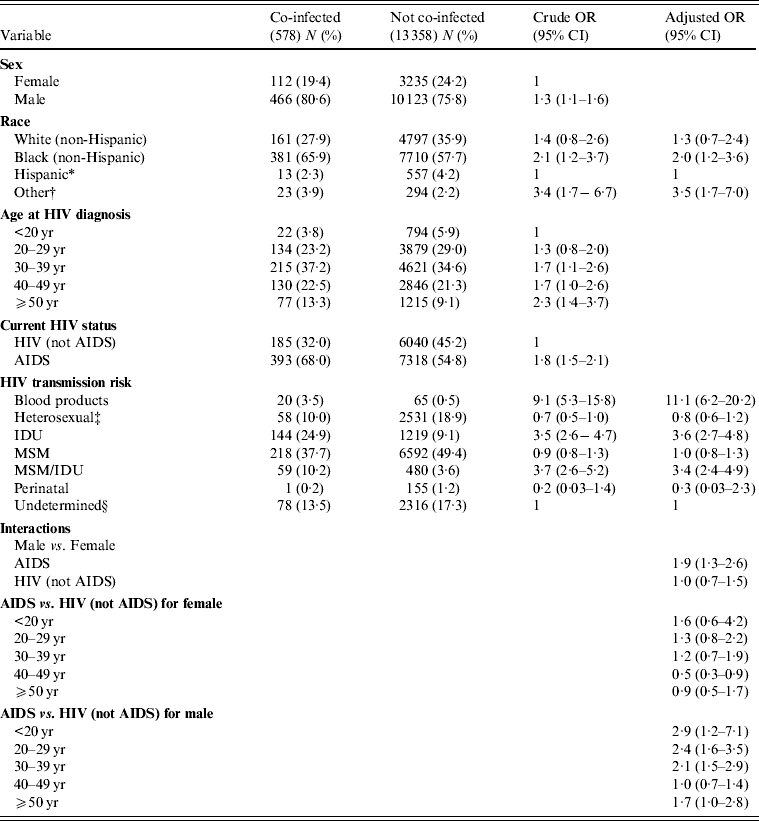
For univariable analysis, the variable HIV transmission risk was reclassified into blood products, heterosexual, IDU, MSM/IDU, MSM, perinatal, and undetermined [3] (Table 2). The variable race was categorized into Black, White, Hispanic and Other whereas age at HIV diagnosis was reclassified into <20, 20–29, 30–39, 40–49, and ⩾50 years age groups [15, Reference Kellerman23]. Those individuals who were co-infected were more likely to be male, of other race followed by black and white race compared to Hispanics and predominantly having AIDS compared to HIV (not AIDS) (Table 2). They were also more likely to have had a transmission risk through blood products, MSM/IDU and IDU compared to an undetermined transmission. Furthermore, HIV diagnosis at an older age showed a significant association with hepatitis co-infection compared to diagnosis at age <20 years (Table 2). The multivariable analysis was based upon statistical significance and biological plausibility of the variables. After adjusting for all other variables in the model, there was a significant association between co-infection and being male and of Black race (OR 2·0, 95% CI 1·2–3·6) and being of Other race (OR 3·5, 95% CI 1·7–7·0) compared to Hispanic race. In addition, a significant association was found between co-infection and transmission risk categories of blood products (OR 11·1, 95% CI 6·2–20·2), IDU (OR 3·6, 95% CI 2·7–4·8) and MSM/IDU (OR 3·4, 95% CI 2·4–4·9) after adjustment. The final model also included two interactions; one between sex and current HIV status and the other between current HIV status and age at HIV diagnosis (Table 2). The first interaction shows that the effect of gender differs if the individual's current HIV status is AIDS (OR 1·9) or HIV positive (OR 1·0) in relation to co-infection. The second interaction indicates that the effect of current HIV status in males compared to females differs across different age groups at HIV diagnosis in relation to co-infection (Table 2).
Table 2. Multivariable analysis of different factors associated with co-infection in HIV-infected individuals in the state of Michigan

OR, Odds ratio; CI, confidence interval.
* Based on lowest risk [Reference Kellerman23].
† Other = Multiracial, Asian, Hawaiian & Pacific Islander, Alaskan Native, American Indian.
‡ Heterosexual = presumed heterosexual female and high-risk heterosexual.
§ Undetermined = unknown (males and females with no identified risk) and presumed heterosexual male.
DISCUSSION
In this study, about 4% of HIV/AIDS-infected individuals were co-infected with hepatitis viruses. Although this prevalence estimate consists of both hepatitis B and C cases, it is lower than the estimate reported in other studies on chronic hepatitis B in the USA [Reference Kellerman23, Reference Chun24]. However, these studies were focused upon hospital (prevalence 7%) [Reference Kellerman23, Reference Anderson, Guest and Rimland25] or military (prevalence 11%) populations [Reference Chun24]. A cross-sectional analysis of the US Adult AIDS clinical trials group data estimated a prevalence of 16·1% for HIV–HCV co-infection [Reference Sherman12] whereas another study reported a prevalence of 31·6% for this co-infection [Reference Anderson, Guest and Rimland25]. Although these studies reported a higher prevalence than our study, it should be noted that one study was clinic based [Reference Anderson, Guest and Rimland25] while the other was conducted on a relatively small sample from a cohort [Reference Sherman12].
A systematic review of HIV–hepatitis co-infection in sub-Saharan Africa reported a prevalence of 15% for HBV and 7% for HCV [Reference Barth26]. In Brazil, a study on HIV, HCV and HBV co-infections reported a prevalence of 4·4% which is comparable to our study [Reference Portelinha21]. The majority of HIV/AIDS–hepatitis co-infected individuals in our study were male [Reference Kellerman23] and of Black [Reference Kellerman23] or Other race groups. Notably, Other race which included multiracial groups, Asian, Hawaiian and Pacific Islanders along with Alaskan Natives and American Indians had a stronger association with co-infection than the Black and White populations. This association needs to be explored further to identify racial subgroups which might be at a higher risk for hepatitis co-infections.
Co-infected individuals were more likely to be associated with HIV transmission risk of blood products followed by IDU and MSM/IDU. These findings are similar to a study in Georgia which identified blood transfusion as a significant contributor to HIV–hepatitis co-infection [Reference Kuniholm27]. It is important to note that in spite of improved testing of viral pathogens in blood [Reference Dwyre, Fernando and Holland28], transmission through blood products is identified as a source of infection in our study. A study conducted in Brazil identified heterosexual transmission as a major risk factor [Reference Victoria29] whereas other studies have identified IDU as a significant factor [Reference Reuter30]. Our study also identified IDU and MSM/IDU as significant factors contributing to co-infection [Reference Lincoln, Petoumenos and Dore31]. Older age at HIV diagnosis showed a significant association with co-infection which is consistent with other studies conducted in the USA [Reference Chun24, Reference Tedaldi32]. It is quite likely that these individuals had HIV infection earlier on but were diagnosed later and additionally, had more chances of acquiring hepatitis infection through similar transmission routes because the duration of exposure to viral hepatitis is more likely to be higher in older individuals [Reference Portelinha21]. Another explanation could be that they are more immunologically compromised when they are infected with HIV at an older age compared to a younger age making them more likely to develop chronic hepatitis infection. Individuals that had progressed to AIDS were more likely to be associated with co-infection which could mean that infection with hepatitis could have led to rapid progression from HIV to AIDS status [Reference Dorrucci33]. Additionally, there may be other factors specific to AIDS which could lead these individuals to acquire hepatitis B or C infections. It is also possible that AIDS status contributed to development of chronic hepatitis infections [Reference Romeo34].
To our knowledge, interactions between sex and current HIV status and sex, current HIV status and age at HIV diagnosis in relation to co-infection are documented for the first time in this study. Based on these results, males currently having AIDS were more likely to be co-infected with hepatitis B or C than females. It is possible that there could be some risk factors more prevalent in males having AIDS, compared to females, which could result in them acquiring hepatitis infection more commonly than females. A survey on adolescent males reported that bisexually active adolescent males were more likely to engage in AIDS-related risk behaviours such as having multiple sex partners, unprotected intercourse, sexually transmitted disease, and injecting drug use [Reference Goodenow, Netherland and Szalacha35]. Conversely, it is also quite likely that females practice more protective behaviours like needle exchange use and carrying clean syringes compared to males as reported in a study on female IDUs [Reference Montgomery36]. This might lead to them having a lower prevalence of HIV/AIDS and/or co-infection. Additionally, when this relationship was stratified by age at HIV diagnosis, males currently having AIDS were more likely to be co-infected at age ⩽39 years or age ⩾50 years. This association indicates that co-infection is more likely in males having AIDS at a younger age than females of comparable groups. It is conceivable that certain high-risk behaviours or practices associated with the younger age groups could lead to a higher prevalence of co-infection compared to older age groups. However, males currently having AIDS who were aged ⩾50 years at HIV diagnosis were also found to be associated with co-infection. It is possible that some high-risk behaviours or practices at a younger age that are identified with these men and persist with them at an older age, make them more likely to acquire hepatitis co-infections. Additionally, as the duration of exposure to hepatitis is likely to be higher in older individuals and persons with AIDS compared to younger individuals with HIV infection only, these factors could interact with high-risk behaviours or practices specific to male gender leading to a significant association with co-infection as observed in this study.
We were not able to obtain reliable information on HBV vaccination status for study participants nor were we able to obtain complete information on anti-retroviral therapy for HIV-infected individuals. Additionally, tests for CD4 counts were not routinely performed on most HIV cases which limited our analysis. However, a study conducted in the USA did not identify either CD4 counts or HIV RNA level as a significant predictor of HIV–HCV co-infection [Reference Tedaldi32]. As the reporting of HBV and HCV infection through the MDSS is based on a passive surveillance system, there is a high likelihood of underreporting and under-detection of cases. However, the issues with the current hepatitis surveillance system are not limited to Michigan but have been identified nationwide. According to a report from the Institute of Medicine in 2010, the current hepatitis surveillance systems do not provide accurate estimates of current disease burden and are not contributing enough to programme planning and evaluation [Reference Colvin and Mitchell37]. In addition, the HIV surveillance system in Michigan is based on data for those persons who have been confidentially reported by name. Data for infected individuals who have not been tested, have been tested only anonymously, or have been tested by name but not reported, are not included which could lead to underreporting of cases [Reference Kellerman23]. However, the strength of our study lies in the fact that it is a population-based study encompassing most if not all HIV-infected individuals as well as persons having hepatitis B and C co-infection residing in Michigan.
Although the prevalence of HIV and hepatitis co-infections in Michigan is not higher than other studies in the USA, it does represent a public health problem especially considering the vulnerability of the HIV-infected population. Furthermore, this study identified males of different races as well as of Black race to be at a higher risk of hepatitis co-infections. The majority of studies indicated that individuals of Black race are at a higher risk of HIV infection as well as for hepatitis co-infection; however, our results indicate that other races might be at a higher risk for hepatitis co-infections. Currently having AIDS and being of older age at diagnosis were found to be important predictors of hepatitis co-infection. An important finding was the correlation of transmission through blood products with co-infection which indicates that additional measures should be taken to address this issue. Other high-risk populations identified in this study were IDUs and MSM/IDUs.The study also demonstrated interactions between current HIV status, sex and age at HIV diagnosis. The importance of these findings needs to be explored further in order to implement preventive measures and interventions to reduce the prevalence of hepatitis co-infections. The results of this study help to elucidate subpopulations within the HIV-infected population for whom HBV vaccination would be most beneficial, and certainly reinforces the recommendation that all persons with HIV, or at high risk of acquiring HIV or HBV, be vaccinated for HBV. Finally, future studies should be conducted keeping in mind the changing epidemiology of HIV–hepatitis co-infections to prevent comorbidities in an already high-risk immunocompromised population of HIV-infected individuals.
ACKNOWLEDGEMENTS
This work was supported by the Dissertation Completion Fellowship awarded by the Graduate School and the Department of Epidemiology at Michigan State University. The authors thank the staff at MDCH, especially the HIV/STD/VH/TB section, for their continuous support during the study and Dr Kim Kirkey for providing valuable insight regarding surveillance of hepatitis infections.
DECLARATION OF INTEREST
None.