The use of antibiotics is a major determinant for the development of resistance, and antibiotic resistance has been seen as a serious threat to the health of patients admitted to healthcare settings [Reference Gyssens1]. Optimizing the use of antibiotics is a key strategy in multi-faceted solutions to prevent the development of healthcare-acquired infections (HCAIs) due to multidrug-resistant microorganisms. It has been shown that antibiotic use varies widely between different hospitals, with excessive use [Reference Vander2, Reference Werner3] suggesting that antibiotic stewardship interventions may result in improved antibiotic use. Such interventions could be achieved through identifying factors that influence antibiotic use, and as such facilitate inter-hospital antibiotic use comparison and the undertaking of robust benchmarking [Reference Kuster4–Reference Polk6]. High antibiotic use in a particular setting is often justified, by clinicians, as relating to differences in patients’ morbidities [Reference Kuster4–Reference Polk6]. Measuring patients’ comorbidity is essential in determining the disease burden. Comorbidity can be adjusted by integrating patient's age with the Charlson comorbidity index, to create an age-adjusted comorbidity index [Reference Charlson7, Reference Aldeyab8]. The age-adjusted comorbidity index is used when comorbidity and age are considered essential factors in outcome assessment. In addition, the use of alcohol-based hand rub has been shown to influence the incidence of HCAIs and demonstrated as an effective method to reduce the HCAI rates in hospitals [Reference Jarlier9–Reference Vernaz13]. As such, we hypothesized that increasing (or decreasing) alcohol-based hand rub usage might be associated with an increase (or decrease) of antibiotic use in healthcare settings. In addition, patients with a greater age-adjusted comorbidity index are more ill and therefore more likely to become colonized and infected, thus, increasing antibiotic use.
The objective of this study was to evaluate the effect of age-adjusted comorbidity and alcohol-based hand rub on monthly hospital antibiotic usage figures using time-series analysis. Time-series analysis is defined as a group of techniques aimed at fitting a mathematical model to a data time-series in order to predict its future behaviour, and explain its characteristics as well as other factors influencing the series. Time-series analysis has previously been shown to be a successful method for investigation of antibiotic use and resistance, with the key advantage of being able to address the problem of autocorrelation existing between consecutive observations and to transform observed data into a series of independent values [Reference Aldeyab10–Reference Aldeyab14].
The study was conducted in the Antrim Area Hospital in Northern Ireland, UK, a 426-bed teaching hospital serving a population of about 420 000. The hospital provides all acute, general medical and surgical services. The study involved collecting monthly data retrospectively (April 2005–March 2010), obtained from the hospital information systems, on possible predictive factors for antibiotic use in the study site hospital. This included proxy measures for infection control practices (i.e. alcohol-based hand rub/litres), and for case mix (i.e. age-adjusted comorbidity scores). Age-adjusted comorbidity data was obtained from Hospital Episode Statistics (HES) and was calculated by combining the age factor with the Charlson comorbidity index score; paediatric data were excluded [Reference Charlson7, Reference Aldeyab8]. Using this approach, the age-adjusted comorbidity score for a patient was the summation of the Charlson comorbidity score with 1 risk point added for each decade of age over 40 years (e.g. ⩽40 years, 0; 41–50 years, +1; 51–60 years, +2; 61–70 years, +3, etc. [Reference Charlson7, Reference Aldeyab8]. Data were modelled as mean monthly scores. The modelled outcome was the monthly antibiotic usage figures (systemic antibiotics). Monthly quantities delivered to each ward were determined from the pharmacy computer systems and were converted into defined daily doses (DDDs) and expressed per 100 bed-days; paediatric antibiotic use data were excluded. The modelled antibiotic use included all antibiotic classes.
The study was ecological in design. Following a major Clostridium difficile infection (CDI) outbreak in the study site hospital in January 2008, the Trust restricted the use of fluoroquinolones and second- and third-generation cephalosporins (which together formed 10% of total antibiotic use); the use of cephalosporins was low before the outbreak in the Antrim Hospital [Reference Aldeyab8, Reference Aldeyab15]. The Trust's infection control policies were consistent with national and international guidelines. The CDI outbreak ended following the implementation of an action plan that involved improving communication, antibiotic stewardship, infection control practices, environmental hygiene and surveillance; this has been detailed elsewhere [Reference Aldeyab15].
A multivariate autoregressive integrated moving average model (ARIMA) was built, using Box–Jenkins methodology [Reference Aldeyab10, Reference Aldeyab14], to relate age-adjusted comorbidity index and alcohol-based hand rub to the use of antibiotics in the study site hospital. All variables were logarithmically transformed. Models were identified by determining ARIMA model orders (p, d, q) using autocorrelation and partial autocorrelation. The model parameters were then estimated by the unconditional least squares method. After identification of the multivariate transfer function models, the cross-correlation function was determined by estimating the correlations between the series describing age-adjusted comorbidity index and alcohol-based hand-rub use at different time lags, and the antibiotic use series. Significance tests for parameter estimates were used to eliminate unnecessary terms in the model. Finally, the adequacy of the model was checked as detailed elsewhere [Reference Aldeyab10, Reference Aldeyab14]. All time-series analyses were performed using EViews 6 software (QMS, USA).
The findings of the study showed that the average observed monthly antibiotic use was 98·32 DDDs/100 bed-days (range 73·78–133·18), and a significant increased trend was observed (P = 0·0004). There was also a significant increase over time in the use of alcohol-based hand rub (average monthly use 1·01 litres/100 bed-days, range 0·10–2·09, P < 0·0001), while no significant trend was observed for the age-adjusted comorbidity (average monthly scores 2·62, range 2·34–2·97, P = 0·342). The results of this study showed that monthly antibiotic use had significant positive relationships with age-adjusted comorbidity index (concomitant effect, coefficient 1·103, P = 0·0002), and had a negative relationship with the use of alcohol-based hand rub (2-month delay, coefficient −0·069, P = 0·0533; Table 1). A stochastic term was introduced into the model, i.e. an autoregressive term (AR) with a lag time of 3 months (Table 1), which reflected autocorrelation existing in the antibiotic use series in the study site hospital. The determination coefficient (R 2) of the final model was 0·37, i.e. 37% of the variation in the monthly antibiotic use, in the study site hospital, was explained by the predictive factors used in the study. Plots for monthly antibiotic use vs. mean age-adjusted comorbidity index, and alcohol-based hand rub are presented in Figure 1.
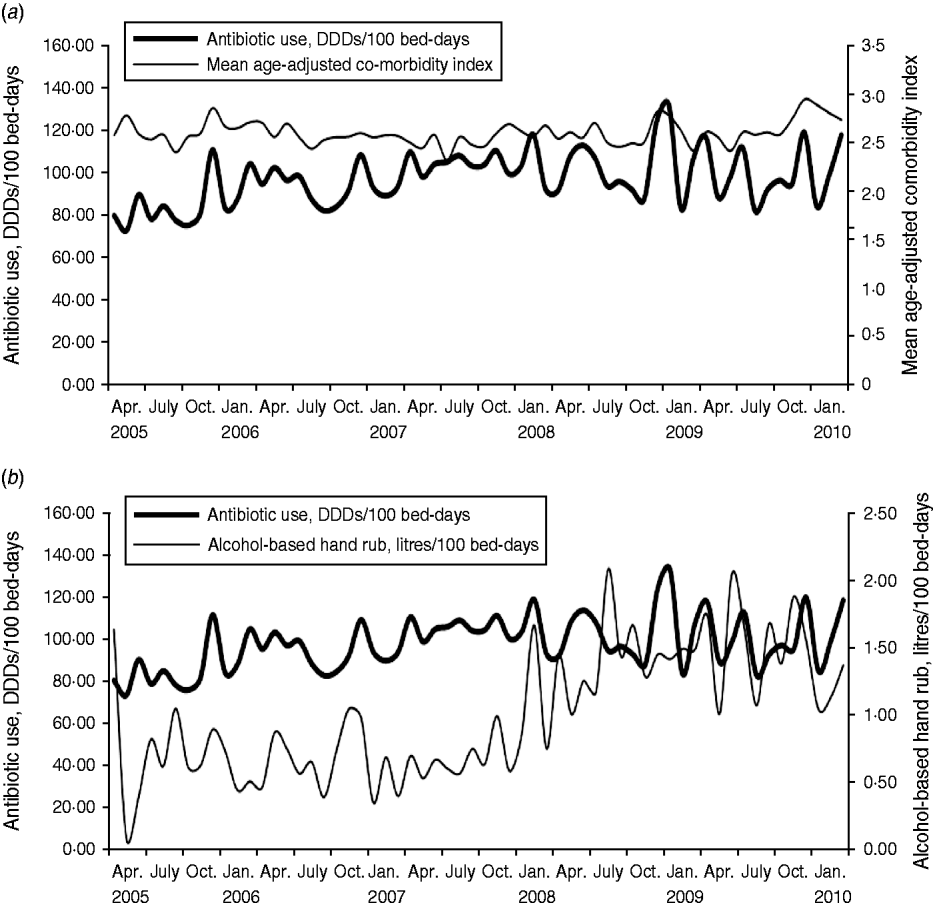
Fig. 1. Monthly antibiotic use vs. (a) mean age-adjusted comorbidity index (P = 0·0002), and (b) alcohol-based hand rub (P = 0·0533), April 2005–March 2010. DDD, Defined daily dose.
Table 1. Multivariate time-series analysis model for monthly antibiotic drug use (R2 = 0·37)

* Represents the delay necessary to observe the effect (in months).
† Indicates the size and the direction of the effect.
‡ AR, Autoregressive term representing the past value of antibiotic use.
The findings of this study showed that the use of alcohol-based hand rub was negatively correlated with antibiotic usage figures, i.e. an increase in the use of alcohol-based hand rub was associated with a decrease in antibiotic use. Alcohol-based hand rub is considered a modifiable factor and as such can be identified as a target for quality improvement programmes. Such findings highlight the importance of encouraging the use of alcohol-based hand rub as a potential method to reduce antibiotic use. In addition, the model included an adjustment for patient case-mix characteristics (i.e. Charlson comorbidity index). Measuring comorbidity is considered an essential criterion in determining disease burden, thus providing risk-adjustment criteria for case-mix purposes [Reference Charlson7, Reference Aldeyab8]. The effect of the comorbidity index on the incidence of HCAIs might be linked to the intensity of the nursing required with a concomitant increase in workload, thus influencing compliance with hand hygiene and other infection control precautions [Reference Aldeyab8].
It is interesting to note that identifying proper predictive factors for antibiotic use may help in establishing risk-adjusted models for explaining antibiotic use in hospitals and for benchmarking purposes. Benchmarking is a technique that aims to compare healthcare process measures or outcomes to internal or external standards, for the purpose of quantifying variability in practices and to identify performance deviations from normative standards [Reference Ibrahim and Polk16]. Making valid comparisons would require antibiotic usage figures to be risk-adjusted in order to control for inter-hospital differences that may confound the benchmarking. The framework for such risk-adjusted models is to adjust for different hospital characteristics and differences in patient case mix by including possible predictor variables in a regression model with overall proper goodness of fit [Reference Ibrahim and Polk16]. Several predictors were identified in previous studies, e.g. case mix index, proportion of patient days in intensive care, surgery and medical wards, input from infectious disease consultant, and number of infections. However, the inclusion of infection control efforts is still lacking in most benchmarking studies of antimicrobial use [Reference Ibrahim and Polk16].
This study has the advantage of using a robust analytical method. Time-series analysis is an advanced regression technique that takes into account the problem of dependency existing between consecutive observations and identifying lag effects between an input and output series, while allowing for the addition and estimation of other explanatory factors [Reference Aldeyab10–Reference Aldeyab14]. However, the study has some limitations. First, quasi-experimental studies at the population level may provide associations that do not correlate with associations at the individual patient level. Second, the study, would have benefited if other possible predictors (e.g. other proxy measures for infection control and clinical activity) had been used. Such variables are likely to be involved in the 63% of the variance which was not explained by the model presented. Third, there is a possibility that the new hospital policy to restrict specific antibiotics following a CDI outbreak in 2008, and to increase usage of alcohol-based hand rub was the reason for the association between alcohol use and antibiotic use, rather than a reduction in infections subsequent to increased use of alcohol-based hand rub. However, the nature of the analysis employed, which compared monthly variation over a 5-year study period, may have reduced the latter confounding effect.
In conclusion, the present research identified significant association between age-adjusted comorbidity index, alcohol-based hand rub use and antibiotic use. While age-adjusted comorbidity is considered a non-modifiable factor, it is possible to influence the use of alcohol-based hand rub in hospitals. Time-series analysis provides a suitable methodology for identifying and measuring possible predictive variables that explain antibiotic use in healthcare settings. Future research should examine the relationship between infection control practices and antibiotic use, identify other infection control predictive factors for hospital antibiotic use, and evaluate the impact of enhancing different infection control practices on antibiotic use in a healthcare setting.
ACKNOWLEDGEMENTS
We thank Dominique L. Monnet (ECDC, Stockholm, Sweden) for expert advice and assistance on an earlier version of the manuscript.
DECLARATION OF INTEREST
None.




