INTRODUCTION
Streptococcus pneumoniae is a leading cause of morbidity and mortality from community-acquired pneumonia (CAP) worldwide, and can cause invasive disease, for example, bacteraemia, meningitis, and septic arthritis [1, Reference Melegaro2]. There are currently two pneumococcal vaccines used in the UK, where all vaccine programmes are publically funded without cost to the user at the point of care. The pneumococcal polysaccharide vaccine (PPV23), which contains antigens from 23 serotypes of S. pneumoniae, was introduced in 1992 for high-risk groups [those with asplenia or dysfunction of the spleen, chronic respiratory, heart, renal or liver disease, diabetes (requiring insulin or oral hypoglycaemic drugs), immunosuppression, individuals with cochlear implants, and individuals with cerebrospinal fluid (CSF) leaks]. The PPV23 programme was expanded to cover all persons aged ⩾65 years in April 2005 (phased introduction with vaccination initially recommended to all those aged >80 years in August 2003 and then to those aged >75 years in April 2004). The pneumococcal conjugate vaccine (PCV), which previously contained antigens from seven serotypes (PCV7) and since 2010 has covered 13 serotypes (PCV13), was introduced for those aged <2 years at a higher risk of pneumococcal infection in 2002, and was subsequently included in the routine childhood immunization programme in September 2006.
There is controversy about the effectiveness of PPV23 in older adults [Reference Fine3–Reference Huss6]. Only one small randomized controlled trial has demonstrated protective efficacy of PPV23 against pneumonia [Reference Maruyama7]. Evidence from observational studies has suggested that PPV23 is effective in reducing the incidence of invasive pneumococcal disease (IPD) (protective efficacy of about 45%) [Reference Jackson8–Reference Foster10], but not pneumonia or mortality, although the validity of this evidence has been questioned [Reference Huss6]. There is no controversy regarding PCV7, which has been demonstrated to be effective in reducing vaccine serotype pneumococcal disease in children in the USA [11]. Furthermore, a reduction in IPD in all age groups in the USA has been attributed to herd immunity following the introduction of PCV7 into routine childhood immunization programmes [Reference Lexau12, Reference Whitney13]. PCV7 has subsequently been introduced across Europe, where early studies have demonstrated a reduction in IPD rates in children and adults [Reference Rodenburg14–Reference Foster17]. However, vaccine serotype coverage by PCV7 in the USA (>80%) was greater than that in Europe (60–70%) at the time of its introduction and a greater increase in non-PCV serotype disease has subsequently been recorded in European countries [Reference Rodenburg14–Reference Hausdorff18]. Because it is not guaranteed that the effect of vaccination will be the same in all populations, even within the same country, high-quality surveillance data is essential at both local and national levels to monitor the epidemiology of pneumococcal disease.
The population of Hull (~264 500) has a much higher prevalence of comorbid illness and unhealthy behaviour, such as smoking, and a shorter life expectancy than the average for England, and is known to be more deprived with over 50% of the population living within the most deprived national quintile [19, 20]. The population of the East Riding of Yorkshire (ERoY) (~346 000), in comparison, is relatively affluent with a longer life expectancy and better health indicators than averages for England [19, 21]. In both Hull and ERoY, PPV23 has been recommended for all those aged ⩾65 years since 2002, anticipating the national recommendation by 3 years. The local Health Protection Unit (HPU), in contrast to many other areas of the UK, has been actively collecting patient-level surveillance data from primary-care General Practitioners (GPs) on PPV23 vaccination uptake since its introduction.
The purpose of this study was to describe the epidemiology of IPD and pneumonia in Hull and ERoY over an 8-year period during which PPV23 and PCV7 vaccination programmes were introduced. Our primary hypothesis was that a reduction in the incidence of IPD and pneumonia in the target groups for vaccination would have occurred during the study period. Secondary hypotheses were that: a reduction in the incidence of IPD and pneumonia would have occurred in other age groups as a result of herd immunity, particularly in relation to PCV7 introduction; vaccine serotype IPD would have decreased; and that there would be an association between the incidence of IPD and the degree of deprivation within a locality.
METHODS
Surveillance and data collection
The computer databases of the microbiology laboratories of Hull and East Yorkshire Hospitals (HEY) NHS Trust and of hospitals from three NHS Trusts bordering Hull and ERoY (comprising eight hospitals in total that routinely receive admissions of individuals resident in Hull and ERoY) (Supplementary Table S1, available online), were retrospectively interrogated in order to identify pneumococcal isolates cultured from samples retrieved from normally sterile body sites (blood, CSF, and joint, pleural or ascitic fluids), from 1 January 2002 to 31 December 2009. Information from all four Trust microbiology laboratories was obtained for the complete time period 2002–2009 with the exception of one, North Lincolnshire and Goole (NLG) Hospitals NHS Foundation Trust, where data for the years 2002 and 2003 were unavailable. The main NLG hospital concerned lies outside the boundaries of Hull and ERoY and, in relation to this study, serves a small catchment area of just one postcode district (Goole, DN14). In order to account for the missing data, the population numbers for that postcode for 2002 and 2003 were subtracted from all the population denominators used in the calculation of incidence rates. In addition, positive pneumococcal PCR results for residents of Hull and ERoY were retrospectively identified from the reports received from supra-regional testing laboratories held by the HPU. Individuals were classified as a case of IPD if S. pneumoniae was cultured from a normally sterile body site and/or pneumococcal DNA was detected by PCR of blood and/or CSF. Demographic details of cases (including postcode district) were obtained from the Trust microbiology and/or pathology databases. Only individuals resident in Hull and ERoY, defined by the postcode of residence lying within the recognized geographical boundaries of the Hull and ERoY Primary Care Trusts (PCTs), were included. Information concerning the origin of pneumococcal isolates cultured from samples received within the first 24 h of admission, and the serotype of isolates (tested by the Respiratory and Systemic Infection Laboratory, Colindale, London, UK) were recorded. In order to avoid the same patient episode being included more than once, further subsequent isolates from specimens taken within 30 days of the index isolate(s) were excluded.
In order to make an assessment of the contribution of changing laboratory and/or clinical (sampling) practice in relation to the diagnosis of IPD, information on the number of blood cultures submitted to microbiology laboratories and changes in laboratory practices were obtained. Numbers of hospital admissions were also obtained from hospital trust information services in order to calculate the incidence of blood culture sampling.
For the years 2005/2006 to 2009/2010, information on population vaccination uptake was derived from the results of PPV uptake surveys, performed and published by the Health Protection Agency (HPA), concerning the populations covered by Hull and ERoY PCTs, using the same geographical boundaries as defined above [22]. For the years 2001/2002 to 2004/2005, in which HPA surveys were not performed, vaccination uptake was calculated using data from the HPU PPV vaccination surveillance database. PPV vaccination status of IPD cases aged ⩾65 years was ascertained by matching the identity of IPD cases with individuals in the HPU vaccination surveillance database. Both the national HPA and the local HPU datasets provided population numbers vaccinated (and not vaccinated) by year, which were used as denominator populations (person-years) in the calculation of PPV23 effectiveness as detailed below. Date of death (if applicable) of IPD cases was recorded from the National Health Service Spine Summary Care database.
Hull and ERoY PCTs provided data on the number of hospital admissions of residents of Hull and ERoY with a diagnosis of pneumonia (2002–2009), which was established by searching the Healthcare Purchaser System database (containing information from the Secondary Uses Service, the national central repository for healthcare data) for the following ICD-10 codes in the primary diagnostic field: J18.0 bronchopneumonia, J18.9 pneumonia (unspecified), J13X pneumococcal pneumonia, J18.1 lobar pneumonia and J15X bacterial pneumonia not elsewhere classified; residence in Hull and ERoY was defined by residential postcode and/or GP registration lying within the PCT geographical boundaries outlined above. As an unrelated ‘observational control’ (for data validation purposes), the numbers of admissions of residents with a primary diagnosis of fractured neck of femur over the same time period were established by the same method for the following ICD-10 codes: S720 fracture of neck of femur, S721 pertrochanteric fracture, S722 subtrochanteric fracture. To replicate the method used to identify IPD cases and compare the incidence of IPD with another community-acquired infectious disease for which a vaccine is not available, the number of confirmed invasive group B meningococcal infections in residents of Hull and ERoY from 2002 to 2009, whose samples were obtained from the same hospitals, was determined from records held at the HPU. [Invasive group B meningococcal infections (diagnosed when group B meningococcus is cultured or diagnosed by PCR from a sample retrieved from a sterile body site, and there are clinical signs and symptoms indicative of infection), are subject to statutory notification to HPUs in the UK.]
Statistical analyses and hypotheses testing
Descriptive statistics are presented as numbers and percentages. Crude incidence rates were calculated using mid-year population estimates for Hull and ERoY published by the Office for National Statistics (ONS) [19]. Age- and sex-specific incidence rates were calculated. The 95% confidence interval (CI) formula used is based on the Poisson distribution.
Poisson regression was undertaken to compare the incidence rates annually; and was also undertaken to compare incidence rates within age groups and by gender in order to detect changes in incidence (as used in a previous UK IPD study) [Reference Foster10]. χ2 tests were used to compare the proportions of IPD cases caused by vaccine and non-vaccine serotypes. PPV23 effectiveness was estimated using the screening method:
where IR=incidence rate [Reference Orenstein23].
In order to make comparisons, incidence density rates were calculated separately for the populations of Hull and ERoY, for the entire study period using ONS population estimates as the denominator. Incidence density rates were also calculated by postcode district separately for Hull and ERoY for the entire study period using resident population estimates (residence defined by postcode, as above) supplied by Hull and ERoY PCTs from the primary care, general practice (GP), patient registration file as the denominator. Incidence density rates were directly age-standardized to the European Standard Population and 95% CIs were calculated according to the preferred method of the Association of Public Health Observatories (APHO) (utilizing pre-formulated APHO spreadsheet tools), using Byar's approximation (for numerators above 389) or computed from the Poisson distribution (for numerators ⩽389) [24]. To compare incidence rates between Hull and ERoY and between postcode districts, Poisson regression was undertaken. A Mann–Whitney test was undertaken to compare age at death between Hull and ERoY and χ2 tests were used to compare case-fatality. A population-weighted mean deprivation score [index of multiple deprivation (IMD) 2007] was calculated for each postcode district and was supplied by the PCTs [25]. Spearman's rank correlations were calculated to explore the relationship between deprivation and incidence of IPD which was assumed to be nonlinear in nature.
To explore the relationship between numbers of blood cultures submitted and IPD incidence, Spearman's rank correlations were calculated. Poisson regression was undertaken to compare blood culture-sampling incidence rates between annual data in order to assess any potential changes in sampling practices. In the same manner as for IPD, Poisson regression was undertaken to compare incidence rates for fractured neck of femur and for invasive group B meningococcal disease.
A P value of <0·05 was considered to indicate statistical significance. No adjustments were made for multiple testing in keeping with the recommendations of Perneger, who stated that ‘simply describing what tests of significance have been performed, and why, is generally the best way of dealing with multiple comparison’ [Reference Perneger26]. All data were recorded and analysed using PASW Statistics v. 18 (IBM, USA). Data and information sources are summarized in Supplementary Table S2 (online).
Advice was sought from the National Research Ethics Service (NRES) prior to study commencement; this work was classified as surveillance and did not require formal review by an ethics committee.
RESULTS
Study sample
Of the 653 patients with IPD (730 positive microbiological samples), 350 (53·6%) were male; their median age was 68 years (55·7% aged ⩾65 years, age range 0–102 years). The age and gender distribution of IPD cases is shown in Figure 1. The ratio of male to female IPD cases was 1·16. There were 668 (91·5%) pneumococcal isolates cultured from blood, 17 (2·3%) from CSF, 17 (2·3%) from pleural fluid, eight (1·1%) from synovial fluid and three (0·4%) from ascitic fluid; PCR results indicative of pneumococcal infection were retrieved for 16 samples: 10 (1·4%) from CSF and six (0·8%) from blood.

Fig. 1. Age and gender of invasive pneumococcal disease (IPD) cases.
In total, 561 (85·9%) of the 653 individuals had presented to the three hospitals of HEY Hospitals NHS Trust, 49 (7·5%) to the two hospitals of Scarborough and North East Yorkshire (NEY) NHS Trust, 27 (4·1%) to York Teaching Hospital (YTH), and 16 (2·5%) to the two hospitals of North Lincolnshire and Goole (NLG) Hospitals NHS Foundation Trust.
Incidence of IPD
The number of confirmed cases of IPD increased from 65 in 2002 to 99 in 2009, corresponding to a rise in overall incidence of IPD from 11·8 (95% CI 9·14–15·1) to 16·4 (95% CI 12·4–18·9) per 100 000 (Table 1) (Poisson regression, P=0·13). The incidence of IPD by age range is shown in Figure 2. The incidence of IPD in those aged ⩾65 years (the target group for PPV23) increased from 40·0 (95% CI 28·3–54·9) to 45·5 (95% CI 33·5–60·3) per 100 000 from 2002 to 2009 (Poisson regression, P=0·69). In contrast, the incidence of IPD in those aged 15–64 years significantly increased from 5·0 (95% CI 3·0–8·0) to 11·2 (95% CI 8·2–15·0) per 100 000 (Poisson regression, P=0·02) over the study period. There was no significant change in the incidence of IPD in those aged 5–14 years (Poisson regression, P=0·91); nor in those aged <5 years (includes the target group for PCV7) over the study period (Poisson regression, P=0·54) or post-PCV7 introduction (Poisson regression, P=0·34).

Fig. 2. Incidence of invasive pneumococcal disease (IPD) by age range.
Table 1. Number of cases, incidence and mortality of invasive pneumococcal disease per 100 000 by year
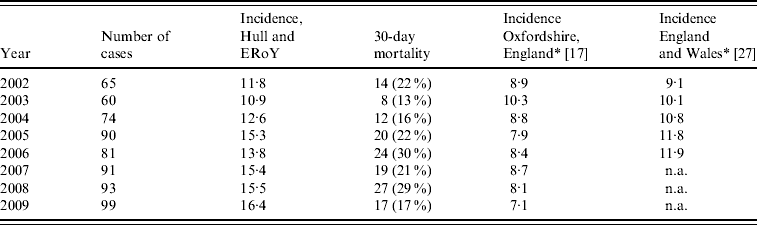
ERoY, East Riding of Yorkshire; n.a., not available.
* Rates were measured July–June, therefore 2002 refers to rates of 2001/2002, 2003 to 2002/2003 and so on; rates for 2007–2009 for England and Wales were not available.
The incidence rates for IPD in Hull and ERoY are compared to published national rates and those recently published for the population of Oxfordshire, England in Table 1.
Vaccination uptake
Over the same period, vaccination uptake for PPV in the ⩾65 years age group increased from 49% (95% CI 48·6–49·3) to 70% (95% CI 69·5–70·0) and uptake of PCV7 in those aged <2 years had reached 98% (95% CI 95·3–100·1) by 2009 (Fig. 3).

Fig. 3. Population vaccine uptake. (Vaccination uptake is calculated from 1 April to 31 March.) Data for 2001 represents uptake from 1 April 2001 to 31 March 2002, and so on.
Blood culture sampling, laboratory methods and antimicrobial susceptibilities
The number of blood cultures received by HEY Hospitals NHS Trust (where 85·9% of the IPD cases were diagnosed) each year and the incidence of IPD is shown in Figure 4. There was no significant correlation between the numbers of blood cultures submitted and the incidence of IPD (Spearman's rank correlations, r s=0·48, P=0·23). The incidence of blood culture sampling in those admitted to HEY hospitals actually decreased over the study period from 103 (95% CI 101–104) to 91 (95% CI 89–92) per 1000 admissions (Poisson regression, P<0·001). In addition there were no reported major changes in laboratory methodology in relation to the diagnosis of IPD or pneumonia over the study period. Antimicrobial susceptibilities were available for all 714 culture isolates. Resistance to penicillin occurred in eight (1·1%) isolates and resistance to macrolides in 63 (8·8%); resistance was stable over the study period.

Fig. 4. Incidence of invasive pneumococcal disease (IPD) and number of blood culture samples received by Hull and East Yorkshire Hospitals NHS Trust.
Mortality following IPD
A total of 141 (21·6%) IPD cases had died within 30 days of the invasive pneumococcal isolate. This included five deaths in those aged <5 years (10·2% of IPD cases aged <5 years), zero in those aged 5–14 years, 30 in those aged 15–64 years (13·3% of cases aged 15–64 years), and 106 in those aged ⩾65 years (29·1% of cases aged ⩾65 years). Within 1 year 240 (36·8%) cases had died, of which 50 (20·8%) were aged 15–64 years and 185 (77·1%) were aged ⩾65 years (there were no additional deaths between 30 days and 1 year in those aged <5 years). The case-fatality rate within 30 days was non-significantly higher in men (24·3% vs. 18·5%) (χ2, P=0·07).
Pneumococcal serotypes
A serotype result was available for 478 (75%) of the 639 culture-positive IPD cases. The number and proportion of IPD cases for which a serotype result was available increased over time (Table 2). Overall, 34 distinct pneumococcal serotypes were detected. Of the isolates 426 (89%) were PPV23 serotypes and 157 (33%) were PCV7 serotypes. The proportion of IPD cases caused by serotypes covered by both PPV23 and PCV7 (the PCV7 serotypes) did not change significantly prior to PCV7 introduction (range 39–61%, χ2, P=0·34); however, post-PCV7 introduction fell sharply (from a baseline of 52% to 12%, χ2, P<0·001), and the proportion of IPD cases caused by PPV23 serotypes increased post-PCV7 (from 39% to 69%, χ2, P<0·05). The proportion of IPD cases caused by non-vaccine serotypes appeared to increase post-PCV7 introduction (from 9% to 19%), although this was non-significant (χ2, P=0·26) (Table 2).
Table 2. Cases of invasive pneumococcal disease by pneumococcal serotype

PPV, Pneumococcal polysaccharide vaccine; PCV, pneumococcal conjugate vaccine.
* Serotypes: 4, 6b, 9V, 14, 18c, 19f, 23f.
† Serotypes: 1, 2, 3, 5, 7f, 8, 9N, 10a, 11a, 12f, 15b, 17f, 19a, 20, 22f, 33f.
Vaccine effectiveness
Of the 364 IPD cases aged ⩾65 years, 212 (58%) were known to have received PPV23 prior to their episode of IPD. The median time from vaccination with PPV23 to IPD episode was 4 years and 2 months (range 2 months to 13 years). Serotype information was available for 164 (77%) of the previously vaccinated cases; 138 (84%) of these had infections caused by PPV23 serotypes and were therefore considered ‘vaccine failures’. Overall incidence in the ⩾65 years age group known to have previously received PPV23 was 41·5 (95% CI 36·1–47·5) per 100 000 person-years compared to 48·8 (95% CI 41·4–57·2) per 100 000 person-years in those without a record of vaccination prior to IPD; PPV23 effectiveness was thus estimated to be 15% (95% CI −4·6 to 31).
Incidence of hospitalization with pneumonia
The numbers and incidence of hospitalizations with pneumonia increased every year of the study period from 833 cases (143/100 000, 95% CI 133–153) in 2002 to 1250 (207/100 000, 95% CI 195·5–218·6) in 2009 (Poisson regression, P<0·001), an increase in incidence of 45%. The incidence of pneumonia by age range is shown in Figure 5. Incidence increased significantly in adults, with an increase of 44% in those aged ⩾65 years [from 543 (95% CI 498–590) to 781 (95% CI 729, 836) per 100 000 (Poisson regression, P<0·001)] and 72% in those aged 15–64 years [from 48·8 (95% CI 42·1–56·4) to 84·1 (95% CI 75·4–93·6) per 100 000 (Poisson regression, P<0·001)] from 2002 to 2009. Incidence in those aged <5 years did not change significantly between 2002 and 2005, but decreased in 2006–2009, from 309 (95% CI 250–378) to 167 (95% CI 126–218) (Poisson regression, P<0·001) post-introduction of PCV7. Incidence in those aged 5–14 years did not change significantly over the study period (Poisson regression, P=0·90).

Fig. 5. Incidence of hospitalization with pneumonia by age range.
Incidence of invasive group B meningococcal infection and of hospitalization with fractured neck of femur (‘observational controls’)
Reports of 98 invasive group B meningococcal infections were identified; the incidence of these infections was highly variable year to year and did not change significantly overall from 2002 to 2009 (Poisson regression, P=0·78) (Fig. 6). There were 5035 hospital admissions with a primary diagnosis of fracture neck of femur over the study period and similarly there was no significant change in incidence (Poisson regression, P=0·22) (Fig. 6).

Fig. 6. Incidence of invasive group B meningococcal infection and of hospitalization with fractured neck of femur.
Geographical distribution of disease, vaccination uptake and deprivation indices
Over the study period, the overall age-standardized incidence of IPD in Hull was 14·3 (95% CI 12·8–16·0) per 100 000 person-years and was considerably higher than that in ERoY, 8·8 (95% CI 7·7–9·9) per 100 000 person-years. The incidence of IPD was significantly higher in Hull in the ⩾65 years (Poisson regression, P<0·001) and the 15–64 years (Poisson regression, P<0·001) age groups, but not in children aged <14 years (Table 3). Similarly, the overall age-standardized incidence of hospitalization with pneumonia was higher in Hull (148·3, 95% CI 143–153 per 100 000 person-years) compared to ERoY (111·2, 95% CI 107–115 per 100 000 person-years) over the study period. The incidence of pneumonia was significantly higher in Hull for all age groups except those aged <5 years (Table 3).
Table 3. Incidence density of invasive pneumococcal disease (IPD) and pneumonia by age in Hull and East Riding of Yorkshire (ERoY), 2002–2009

CI, Confidence interval.
There was no significant difference in mean PPV23 uptake between Hull and ERoY over the study period [62·7% (95% CI 62·6–62·9) and 63·3% (95% CI 63·2%, 63·5%), respectively] (χ2, P=0·07). Mean uptake of PCV7 was marginally higher in ERoY from 2006 to 2009 [74·4% (95% CI 72·9–76·0) compared to 70·8% (95% CI 69·4%, 72·3%)] (χ2, P=0·01), although it exceeded 94% in both areas by 2008.
A higher incidence of IPD was noted in the most deprived Hull postcode district and incidence of IPD was significantly related to the postcode IMD score (Spearman's rank correlation, R 2=0·62, P=0·04) (Fig. 7). The same pattern was not noted in ERoY (data not shown), where there were less cases overall and a larger number of postcode districts.

Fig. 7. Age-standardized incidence density rate of invasive pneumococcal disease (IPD) in Hull 2002–2009 by postcode district* and postcode index of multiple deprivation (IMD) score. [* Each point represents one postcode district in Hull with population >20 000 (HU3-9). HU1 and HU2 were excluded as the population of both is <4000.]
There was no significant difference in case fatality within 30 days for IPD between Hull and ERoY [20% vs. 23%, respectively (χ2, P=0·26)], nor median age at death (which appeared lower in Hull at 78 years compared to 80·5 years in ERoY), (Mann–Whitney, P=0·40).
DISCUSSION
Enhanced surveillance of infection and vaccine uptake from 2002 has shown that despite increasing uptake of both vaccines, and contrary to our primary hypothesis, the overall incidence of IPD and pneumonia has increased. Importantly, the incidence rates for IPD presented are likely to be an underestimate of the true rates: many individuals will not present to hospitals (particularly the very elderly); diagnosis of IPD is dependent on appropriate samples being sent at the appropriate time (ideally blood cultures prior to antimicrobial therapy); and some residents of Hull and ERoY may have presented elsewhere for diagnosis and treatment. Whereas other UK studies have explained an observed increase in IPD incidence as an artefact of increased sampling [Reference Trotter27, Reference Ihekweazu28], our findings do not support this as the number of blood cultures performed remained relatively stable during the study period (and the incidence of blood culture sampling appeared to decrease). The dramatic increase in the incidence of hospitalization for pneumonia, in keeping with a recent large epidemiological study in England and Wales, also suggests that the burden of pneumococcal disease is increasing in this locality [Reference Trotter29].
A reduction in IPD was not demonstrated in the main target group for PPV23 or overall. The protective effect of PPV23 at the population level was found to be low (and may be overestimated due to underreporting of PPV23 vaccination status), and is consistent with the recent assessment by the Joint Committee on Vaccination and Immunization (JCVI) UK, that the effectiveness of PPV23 is poor in those aged ⩾65 years [30]. At the patient level, many ‘vaccine failures’ were identified. However, the increasing incidence of IPD may be unrelated to vaccination; for example, risk factors for IPD may have increased in adults during the study period. In contrast, we found a significant reduction in the incidence of pneumonia in children aged <5 years following the introduction of PCV7. While the reduction in the incidence of IPD in children was not statistically significant, we acknowledge that the number of IPD cases in children was small and the study may therefore have been performed in too small a population area to detect the anticipated target population effects of PCV7 seen in other studies [11–Reference Whitney13] [the population aged <5 years was 32 900 (5·4% of the total population) in 2009] [19]. However, there was no evidence of herd immunity in the larger older cohort during the post-PCV (post-2006) period. Whether this is due to a delayed beneficial effect yet to be detected, the relatively small size of our study compared to some reports, natural fluctuation of disease incidence, or another reason is unclear.
The incidence rates of IPD in Hull and ERoY are higher than those reported nationally and are considerably higher than those in Oxfordshire. National surveillance requires voluntary reporting of clinically significant isolates by individual microbiology laboratories whereas in this study, information on relevant isolates was actively sought. Therefore, the higher incidence rates for Hull and ERoY may reflect the more inclusive methodology. However, this does not explain the large difference in rates between Hull and ERoY and Oxfordshire, where enhanced surveillance was also performed, and suggests a genuinely higher burden of IPD in our locality compared to more affluent and healthier areas of the UK. It is also notable that rates of IPD are falling in Oxfordshire. Together with the observed marked differences in the incidence of both IPD and hospitalization for pneumonia between Hull and ERoY, this study highlights the likely presence of important health inequalities within the population of the UK. Interestingly, HEY Hospitals NHS trust was recently identified by the Dr Foster group as having a higher mortality rate compared to the average acute UK hospital and it is suspected that this, at least in part, is explained by the recognized high level of comorbidity and risk-taking behaviour in the local population [31]. The higher incidence of IPD in the most deprived Hull postcode district, the apparent relationship between the incidence of IPD and postcode IMD score within Hull postcode districts, and the differences in the incidences of IPD and pneumonia between Hull and the more affluent ERoY, also suggests that social deprivation is an important contributor to IPD, in keeping with a previous study of children [Reference Grant32]. Deprivation covers a broad range of issues and refers to unmet needs resulting from a lack of resources of all kinds, not just financial [25]. The IMD score reflects the measurement of seven distinct domains of deprivation (differentially weighted and combined) which include: income deprivation, employment deprivation, health deprivation and disability, education skills and training deprivation, barriers to housing and services, living environment deprivation, and crime [25]. In every domain the population of Hull is considered more deprived than the average for England, and even within Hull there are also marked inequalities [20, 25]. It is conceivable that deprivation affecting each of the seven domains might contribute to increased pneumococcal carriage and disease (e.g. it can be speculated that overcrowded living conditions might lead to increased transmission and carriage of pneumococcus; and poverty, disability and low educational level might contribute to delayed presentation of infection and increased likelihood of invasive disease). However, further research (at the individual level) is indicated to confirm that such a relationship exists and to identify the important underlying social determinants.
The increased burden of pneumococcal disease observed in this study is not explained easily by changes in population demographics; although the numbers of elderly did increase, the growth in the population aged ⩾65 years (4·6%) was modest over the 8-year period and similar to the increase (3·5%) in the general population. The proportion of the population aged >80 years was unchanged (4·3% in 2002, 4·6% in 2009) [19]. Although ERoY has an older population compared to Hull and England, the population of Hull has a lower proportion of elderly, thus the combined population structure of Hull and ERoY is similar to that of England [19]. The increases in the incidence of IPD and pneumonia overall were largely driven by increases in the adult age groups. The significant increase in both IPD and pneumonia in the 15–64 years age group could be explained by an increasing prevalence of risk factors for pneumococcal disease known to be at a high level in the young adult population of Hull, such as intravenous drug use, high alcohol intake, and smoking [20, 25], although further research is required to confirm this; such factors should be important targets for future local health promotion and research. The increase is not explained by antibiotic resistance. We were not able to assess if the prevalence of pneumococcal carriage in the population also increased. There were no major changes in laboratory equipment or blood culture methodology and, while PCR was used more frequently in later years, the number of cases diagnosed by this method was low (14 cases, 2%) spread over the study period (1/59 cases in 2003 vs. 4/95 cases in 2009). Uptake of both PPV23 and PCV7 was higher in Hull and ERoY compared to the averages for England [22, 33], and although there was marginally higher uptake of PCV7 in ERoY compared to Hull, PCV7 uptake in both areas was high following the first full year of vaccine implementation; PPV23 uptake was similar in both areas. Thus our observations cannot be attributed to low vaccine coverage and the marked differences between Hull and ERoY are unlikely to be explained by differential vaccine uptake.
The reduction in PCV7 serotypes noted after 2006 is consistent with the observations of other studies and supports the effectiveness of this vaccine in reducing vaccine serotype disease. However, although an increase in non-PCV7 serotypes after 2006 was to be expected and in keeping with other studies, the observed overall increase in the incidence of IPD was surprising and in contrast to other settings where IPD rates have been falling post-PCV7 [Reference Rodenburg14–Reference Foster17]. It is therefore conceivable that a change in the sero-epidemiology of IPD in our locality, in the period following PCV7 implementation, has contributed to an increased overall burden of disease; this appears to have mostly been due to an increase in non-PCV7, PPV23 serotypes. A recent large cohort study showed that some pneumococcal serotypes result in higher mortality (and presumably therefore more severe, non-fatal illness) compared to others [Reference Harboe34]. Those serotypes covered by PCV7 are associated with relatively lower 30-day mortality compared to many non-PCV, PPV serotypes and non-vaccine serotypes [Reference Harboe34]. One consequence of PCV7 introduction in our locality, therefore, may have been replacement of PCV7 serotypes with serotypes more likely to cause severe disease, although there was no clear evidence of a significant increase in mortality, which was 19% in the 2002–2005 period compared to 24% in the 2006–2009 period (χ2, P=0·20). Differences in the predisposition of serotypes to colonize and invade the human respiratory tract might have also contributed to the overall increase in IPD incidence [Reference Hausdorff, Feikin and Klugman35]. The notable increase in mortality (from 22% to 37%) between 30 days and 1 year after the diagnosis of IPD entirely occurred in adults (>16 years), especially those aged ⩾65 years, and suggests that IPD is a marker of poor 1-year survival. This is likely to be due to comorbidity, but other explanations, such as the triggering of an inflammatory response resulting in cardiovascular disease should be investigated.
Our results suggest that the current approach to pneumococcal vaccination in our locality should be reviewed. Possible alternative strategies include a switch to the use of conjugate vaccines in those aged ⩾65 years (and high-risk patients of any age) [Reference Dransfield36], and the development and use of conjugate vaccines that cover a higher proportion of circulating serotypes. Studies are currently being performed to assess the effectiveness of conjugate compared to polysaccharide vaccines in older age groups [Reference Goldblatt37], but it remains unclear as to whether this will be a more cost-effective strategy than PPV23. The UK has recently switched to PCV13 for the vaccination of those aged <2 years and it will be interesting to observe the impact of this in our locality on IPD and pneumonia both within this age group and in the overall population. The results also highlight the importance of tackling the likely underlying public health determinants of IPD and pneumonia.
We acknowledge that this study has a number of limitations. The observational design means that the observed trends cannot be definitively attributed to vaccine effectiveness and could be due to natural fluctuation. It is, however, interesting to note that the incidence of invasive meningococcal group B infections did not increase over the study period. The estimate of vaccination uptake was based on data from primary-care practitioners both at a local and national level. In contrast to other areas in England, a financial incentive is offered in Hull and ERoY by the HPU for information on PPV23 uptake. The vaccination surveillance database is updated continuously, with active measures taken annually to identify and contact non-responding GP practices. Nevertheless, PPV23 uptake is likely to be underestimated. There are also limitations to assessing deprivation by postcode districts, which may contain both areas of deprivation and comparative affluence. Use of the IMD score, which is calculated relative to populations in other postcode districts within the same region, presents some challenges for interpretation when the whole region is relatively deprived (as in Hull). In addition, there are recognized limitations in using population estimates for the postcode districts derived from primary care (GP) registrations (PCTs utilize this data in the absence of ONS population estimates at this geographical level) which tend to overestimate population size compared to ONS estimates [38]; incidence density rates of IPD in the postcode districts might be therefore be underestimated, and if there was differential registration between populations of different postcode districts consistently over the 8-year study period then the association between deprivation and IPD by postcode district, might be either over- or under-estimated. We tried to account for differences in age distribution within postcode districts by using directly age-standardized data. The identification of those hospitalized with pneumonia relied on ICD-10 based case-coding performed after hospital discharge. While a change in coding practice and/or clinical practice (e.g. lower threshold for hospital admission) may have occurred over the study period, this is unlikely to have resulted in the magnitude of the observed increases in pneumonia incidence. Indeed the relatively constant incidence of the observation control, i.e. hospitalization with fractured neck of femur, would suggest that there was not a systematic change in coding practice or other gross bias in data collection over the study period and thus provides supportive evidence of a true increase in pneumonia incidence. There are many causes o pneumonia and the increase cannot be solely attributed to an increase in the incidence of S. pneumoniae infection, although S. pneumoniae is well recognized to be the commonest cause of CAP worldwide. We included only patients given a primary (first position) diagnostic code for pneumonia in order to increase the likelihood of identifying true cases of CAP. Finally, the proportion of isolates sent to the reference laboratory for typing was particularly low in the first year (2002), but was higher thereafter (between 64% and 89%); this could have affected our results as it is possible, for example, that isolates from patients with more severe pneumococcal infection are more likely to be sent for serotyping. Despite the identified limitations, this study provides valuable information on the epidemiology of IPD and pneumonia and the early effects of vaccination in a relatively deprived UK population and suggests that the impact of vaccination cannot always be assumed to be consistent across populations.
In conclusion, the burden of IPD and pneumonia increased in Hull and ERoY from 2002 to 2009 despite improved uptake of both PPV23 and PCV7 vaccines. The underlying determinants of this trend are unclear, and may be independent of vaccine introduction, but should be explored. Existing vaccination programmes alone might not be sufficient to reverse the burden of pneumococcal disease, particularly in areas with a high prevalence of comorbid illness and social deprivation, and our findings are consistent with the JCVI assessment that PPV23 effectiveness is poor in those aged ⩾65 years [Reference Ihekweazu28]. Further investigation of the social determinants of pneumococcal disease is required. Surveillance of pneumococcal serotype distribution and monitoring of non-PCV serotype-associated disease is imperative to inform future PCV formulations. Awareness of pneumococcal disease by healthcare professionals remains of paramount importance.
NOTE
Supplementary material accompanies this paper on the Journal's website (http://journals.cambridge.org/hyg).
ACKNOWLEDGEMENTS
The hospitals involved in the surveillance were as follows: Hull Royal Infirmary, Castle Hill, Princess Royal, York, Scunthorpe General, Scarborough District General, Bridlington and Goole and District. East Riding of Yorkshire and Hull Primary Care Trusts provided population data. The authors acknowledge the help of Alexander Anderson, Peter Cowling, Dan Weiand and Naomi Thompson for their help in providing and interpreting the hospital data; Emma Smith, Paul Thomas, Jeremy Newton and Helen Fields for their help in providing and interpreting data from the Healthcare Purchaser System Database; Mandy Porter and Adrian Wensley for help with statistical analyses; and Tracey Docwra and Pawel Wlodarczyk for technical assistance.
DECLARATION OF INTEREST
None.












