INTRODUCTION
Between 2000 and 2009, the annual number of reported cases of legionellosis in the USA more than tripled from 1110 to 3522 [1]. Of the legionellosis cases reported with detailed diagnoses, 99·5% were Legionnaires' disease (LD), a type of pneumonia with a high case-fatality rate, while the remaining 0·5% of legionellosis cases took the form of Pontiac fever (PF), an influenza-like, self-limiting illness. Although most legionellosis cases are not associated with known outbreaks, legionellosis outbreaks can be highly disruptive and costly to the facilities and communities affected [Reference Fraser2–Reference Den4]. Reported legionellosis outbreaks with both LD and PF cases are relatively rare [Reference Jones5], and most known outbreaks in which both legionellosis syndromes were documented had no more than a few LD cases [Reference Fraser2, Reference O'Mahony3, Reference Girod6–Reference Foster, Gorton and Waller16].
On 19 July 2010, reports of illness of unknown aetiology in eight employees were received by the occupational health staff at military base A. Over the next several days additional illnesses with similar symptoms were reported in employees who worked in the same area as the original eight cases. On 22 July, buildings A and B, the two buildings most affected by the outbreak (Fig. 1), were evacuated. Laboratory testing of specimens from two of the ill persons confirmed a diagnosis of LD on 26 July. A team consisting of personnel from U.S. Army Public Health Command, the Michigan Air National Guard, the Michigan Department of Community Health, and the Centers for Disease Control and Prevention (CDC) investigated the outbreak in order to characterize cases, identify the outbreak's source and scope, and recommend interventions to prevent future cases.

Fig. 1 [colour online]. Legionnaires' disease and Pontiac fever cases and possible sources of the outbreak, military base A, July–August 2010.
METHODS
Outbreak threshold
In the USA, a location that has no history of previous legionellosis outbreaks and does not provide healthcare is generally considered to have a legionellosis outbreak if two or more individuals develop legionellosis after being exposed to that location at around the same time [17, Reference Tablan18].
Case definitions and case finding
A confirmed case of LD was defined as radiographically confirmed pneumonia with laboratory evidence of Legionella infection in a person with onset of illness between 1 July 2010 and 12 August 2010 and within 10 days of exposure to the base's eastern office complex (Fig. 1). Laboratory evidence of Legionella infection included at least one of the following: isolation through culture of any Legionella organism from respiratory secretions, lung tissue, pleural fluid, or other normally sterile fluid; detection of Legionella pneumophila serogroup 1 antigen in urine; or seroconversion, specifically a ⩾fourfold rise in specific serum antibody titre to L. pneumophila serogroup 1 between acute and convalescent titres. An individual was considered to have been exposed to the eastern office complex if they reported spending any time in or immediately adjacent to that area. The focus on the base's eastern office complex was due initially to the distribution of the first identified cases and ultimately to the lack of any laboratory-confirmed legionellosis in base workers who had not been exposed to that area. A probable case of LD was defined as radiographically confirmed pneumonia without laboratory confirmation of Legionella infection in a person with onset of illness on or after 1 July 2010 and within 10 days of exposure to the eastern office complex. PF cases were defined as fever, either subjective or measured, in a person with onset of illness on or after 1 July 2010 and within 3 days of exposure to the eastern office complex, and with at least one of the following symptoms: headache, cough, shortness of breath, myalgia, vomiting, and/or diarrhoea.
LD and PF cases were identified in part through mandatory reporting of legionellosis cases from healthcare providers and laboratories to area public health authorities [19]. Medical records were abstracted for all hospitalized cases using a standardized data abstraction form. In addition, military units on the 5·6 square-mile base conducted active surveillance for employees with recent illness. Employees with recent illnesses were interviewed with screening questionnaires to determine if they had symptoms consistent with legionellosis. Base employees who were reported to local health departments as having legionellosis or who reported recent symptoms consistent with either form of legionellosis on the screening questionnaire were interviewed using a detailed standardized questionnaire that captured information on demographics, medical history, recent illnesses, symptoms, medical diagnosis and treatment, and exposures on and off the base.
Retrospective cohort study
The cohort study included all persons who were working in buildings A and B, the buildings in the base's eastern office complex (Fig. 1) that were most affected by the outbreak immediately prior to their evacuation on 22 July. Standardized questionnaires similar to those used to interview ill base employees were administered.
Clinical laboratory methods
Clinical sputum specimens from two hospitalized base employees were retrieved from clinical laboratories and tested at the CDC Legionella laboratory. Once Legionella were cultured from a specimen, the species, serogroup, and monoclonal antibody (MAb) patterns [Reference Sanden, Cassiday and Barbaree20, Reference Joly21] of the isolate were determined. Sequence-based typing was also performed to further characterize isolates according to seven-gene profiles of select isolates (flaA, pilE, asd, mip, mompS, proA, neuA) [Reference Gaia22, Reference Ratzow23]. Consenting base employees with recent illnesses consistent with legionellosis provided urine samples for L. pneumophila serogroup 1 urine antigen testing, which was performed at the Walter Reed Army Institute of Research (WRAIR) using the BinaxNOW Legionella urinary antigen kit (Alere Inc., USA). Some urine samples were collected before the employees were seen by medical providers for their illnesses.
Environmental investigation
An environmental assessment was conducted to identify the locations of all possible sources of warm, aerosolized water in the base's eastern office complex. The maintenance, environmental testing, and treatment histories of likely sources of the outbreak, including all potable water systems, were reviewed with local engineers and base contractors. In addition, the temperature, free chlorine concentration, and pH of water found in these sources were measured. The base environmental science officers provided detailed meteorological data for June and July 2010, including hourly temperature, wind speed, and wind direction. Daily base precipitation, mean temperature, and mean dew-point data for June, July, and August 2010 were also obtained from the National Climatic Data Center (NCDC) [24].
Bulk water and biofilm swab samples were collected from two cooling towers in the base's eastern office complex on 6 August 2010. The samples were tested at the CDC Legionella laboratory using previously published standard procedures [25]. The swab samples and filtered bulk-water samples were plated on buffered charcoal-yeast extract (BCYE) media with and without antibiotics and with and without acid treatment. Plates were incubated at 35°C in 2·5% CO2. Suspected Legionella colonies were verified on biplates, and L. pneumophila species underwent serogrouping [Reference Thacker, Wilkinson and Benson26]. MAb pattern determination and sequence-based typing were also performed on Legionella isolates if they were the same species and serogroup as clinical isolates [Reference Joly21, Reference Fry27].
Statistical analysis
Team members on site entered patient data and survey responses into a database developed with Snap survey software, version 10 (Snap Surveys, UK). Analyses were performed with SAS software, version 9.2 (SAS Institute Inc., USA). Relative risks (RRs) and 95% confidence intervals (CIs) were calculated for exposure variables. Confirmed and probable LD cases were analysed separately and together. Multivariate logistic regression was performed and included all variables identified as being statistically significant (P < 0·05) during univariate analysis. The multivariate models assessed whether the likelihood of developing LD vs. not developing legionellosis and the likelihood of developing PF vs. not developing legionellosis in members of buildings A and B cohorts were affected by building exposure, age, or reported history of any chronic diseases. Times between patients' first medical visit for their legionellosis and the date of urine antigen sample collection were compared using the exact Wilcoxon rank-sum test. The difference between the date of the first medical visit and the urine antigen sample collection was set to zero in the multivariate models if patients provided urine antigen samples prior to seeking medical care. The base's daily mean relative humidity was calculated using the daily mean temperature and dew point provided by the NCDC and formulae detailed by Ricketts et al. [Reference Ricketts28, Reference Ricketts29].
Human subjects determination
This study was determined to be an emergency public health investigation exempt from institutional review board (IRB) review.
RESULTS
Case finding
We identified seven confirmed LD cases, 22 probable LD cases, and 38 PF cases (Table 1), all with exposure to the eastern office complex (Fig. 1). All 29 confirmed and probable LD cases had onset of symptoms within an 18-day period, with a peak of five confirmed and probable LD cases having symptom onset on 17 July (Fig. 2). Although seven (100%) confirmed and six (27%) probable LD cases were hospitalized, no deaths resulted from the outbreak (Table 1). LD patients were significantly more likely than PF patients to report shortness of breath (RR 1·6, 95% CI 1·2–2·2) and abdominal pain (RR 1·9, 95% CI 1·1–3·3) (Table 2).

Fig. 2. Epidemic curve of legionellosis outbreak at military base A.
Table 1. Characteristics of cases with confirmed Legionnaires' disease (LD), probable LD, and Pontiac fever

Table 2. Symptoms of Legionnaires' disease and Pontiac fever cases
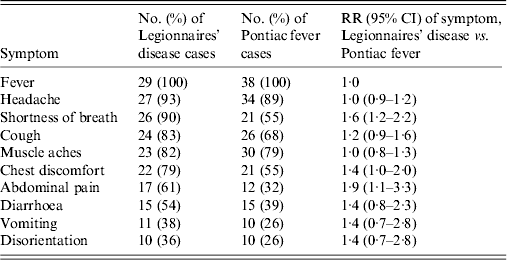
RR, Relative risk; CI, confidence interval.
All individuals with laboratory-confirmed legionellosis associated with the base had exposure to the eastern office complex. Among the occupants of buildings A, B, and C, there were 34 (five confirmed LD, 11 probable LD, 18 PF), 10 (one confirmed LD, three probable LD, six PF), and five (all PF) legionellosis cases, respectively (Fig. 1). The remaining 18 legionellosis cases (one confirmed LD, eight probable LD, nine PF), occurred in individuals who primarily worked outdoors. These included two probable LD and two PF cases in military police based at building D, two probable LD cases in contractors working outdoors on a building E construction project, and one confirmed and one probable LD case in base maintenance workers laying cable to the building E site along the western edge of the eastern office complex. None of the maintenance staff working in other areas of the base during the outbreak reported illnesses. At least one patient with confirmed LD and ten patients with probable LD stated that they did not enter building A during their LD incubation periods. The last two cases of LD and the last case of PF had onset of symptoms 9 days and 2 days, respectively, after the 22 July evacuation of buildings A and B. No cases occurred in occupants of buildings A and B, which were reoccupied in September 2010, after the remediation of the outbreak source.
Cohort study
Of the 369 people working in buildings A and B at the beginning of the outbreak, 267 (72%) participated in the cohort study. Occupants of building A had a RR of 6·3 (95% CI 2·2–18·4) for developing LD and a RR of 4·7 (95% CI 1·9–11·5) for developing PF compared to occupants of building B (Table 3). Compared to younger persons, individuals aged ⩾50 years had RRs of 3·6 (95% CI 1·5–8·8) for developing LD and 2·0 (95% CI 1·0–4·4) for developing PF. Reporting a history of any chronic disease carried RRs of 2·5 (95% CI 1·1–5·7) for LD and 1·8 (95% CI 0·8–3·9) for PF. Multivariate analysis indicated that only being an occupant of building A was significantly associated with an increased risk of LD [adjusted odds ratio (aOR) 6·9, 95% CI 2·2–22·0] and PF (aOR 5·5, 95% CI 2·1–14·5). Patients' characteristics were not associated with a greater risk of developing LD relative to PF (data not shown).
Table 3. Risk factors for development of Legionnaires' disease (LD) or Pontiac fever (PF) instead of remaining unaffected, buildings A and B cohorts

RR, Relative risk; CI, confidence interval; aOR, adjusted odds ratio; n.a., not applicable.
PF cases were excluded from the calculations of whether characteristics were associated with an increased risk of developing LD instead of remaining unaffected and LD cases were excluded from the equivalent calculations for PF.
* Characteristic used in multivariate models calculating aORs for whether individuals with specified characteristics developed LD instead of remaining unaffected or developed PF instead of remaining unaffected.
Clinical laboratory results
Of the two sputum samples tested, one specimen of poor quality was negative for growth on culture after 14 days, but the other was successfully cultured for L. pneumophila. This isolate was determined to be L. pneumophila serogroup 1, type Knoxville 1 MAb pattern (1, 2, 3) with sequence type (ST) 222.
Based on chart review and urine specimen testing at WRAIR, all seven confirmed LD cases and one PF case had positive urine antigen tests (Table 1), while 14 probable LD cases and 13 PF cases had negative urine antigen tests. Urine specimens from five confirmed LD cases, ten probable LD cases, and seven PF cases had both a known date of urine sample collection and a known date of the patient's first receiving medical care for legionellosis. The samples from confirmed LD cases were collected a median of 2 days (range 0–23) after the first visit for legionellosis-related medical care, while the negative samples from probable LD cases were collected a median of 14 days (range –4 to 25) after the first medical visit (P = 0·19). The positive sample from a PF case was collected the same day as the patient's first medical visit while the negative samples from PF cases were collected a median of 12 days (range –2 to 27) after the first medical visit (P = 0·43). All of the LD cases with known dates of first medical visit for legionellosis and at least seven of the PF cases with known first legionellosis medical visit dates received antibiotics.
Environmental investigation
Two water-based cooling towers, equipped with drift eliminators to prevent the spread of water droplets, were located in the base's eastern office complex. One was exposed to the elements at ground level outside building A and the other was inside building C (Fig. 1). All other buildings on base used air-based cooling systems. The two water-based cooling towers were typically turned off and drained during the winter. Building A's cooling tower had been restarted on 19 June while building C's tower had been reactivated earlier in spring 2010. The occupants of building A reported that the building was warm even after the cooling tower was restarted, leading many employees to leave their office windows open. Each building on base had a separate potable water system supplied by the base water system, which in turn received water from the local municipality. No whirlpool spas or decorative fountains were located on the base.
Since the distribution and timing of cases suggested that airborne spread of Legionella from one of the cooling towers was the most likely cause of the outbreak, the environmental testing focused on these towers. At the time of sample collection, building A's cooling tower water temperature was 26·5°C while building C's cooling tower water temperature was 34·0°C. No chlorine was detected in the water of either cooling tower. L. pneumophila serogroup 4 was recovered from building C's cooling tower while L. pneumophila serogroup 1, type Knoxville 1 MAb pattern (1, 2, 3) with ST222 was found in building A's cooling tower, which matched the L. pneumophila isolated from a case sputum sample.
The base experienced a large amount of rainfall on 11 and 12 July, which preceded the beginning of the cluster of LD cases by 1–2 days (Fig. 3 a). The second and third highest daily average relative humidity readings for the 30 days after the restart of building A's cooling tower were on 12 and 13 July, at 85% and 86%, respectively (Fig. 3 b). Other clusters of high relative humidity followed on 18–20 July and 23–24 July. In addition, much of July 2010 was quite warm, with 3–18, 21–24, and 26–28 July all having maximum daily outdoor temperatures above 26·7°C. Between 1 and 25 July 2010 the wind at the base often travelled to the south and west; thus, the wind came from the lake and travelled past building A's cooling tower towards buildings A and B and the eastern office complex (Fig. 1).

Fig. 3 [colour online]. (a) Precipitation and (b) daily mean temperature and relative humidity at military base A, 19 June–14 August 2010.
DISCUSSION
A single source caused 29 confirmed and probable LD cases as well as 38 PF cases and disrupted operations on a military base in the summer of 2010. Multiple findings indicate that the cooling tower of building A was the source of this mixed legionellosis outbreak. First, the outbreak featured an epidemic curve similar to those of previous cooling-tower-related legionellosis outbreaks [Reference Fraser2, Reference Klaucke30–Reference Brown33] and different from the more indolent patterns of multiple previous potable-water-related LD outbreaks [Reference Silk34–36]. Second, the wide dispersion of cases suggested airborne spread of Legionella bacteria. Third, the rates of legionellosis were highest immediately around building A's cooling tower. Fourth, multiple confirmed and probable LD patients denied entering building A. Finally, the clinical Legionella isolate matched the environmental isolates from building A's cooling tower. As in multiple past outbreaks [Reference Klaucke30, Reference Addiss31, 37, Reference Fiore38], the activation of the cooling tower on 19 June was probably key in triggering the outbreak which followed a few weeks later. The large rainstorm that occurred 2 days before the first LD case and the high temperatures and relative humidity present throughout the outbreak may also have contributed since several other legionellosis outbreaks linked to cooling towers have been preceded by similar conditions [Reference Addiss31, Reference Phares32].
The large numbers of both LD and PF cases in this outbreak were unusual. Several environmental factors were present to promote the outbreak. First was the ability of building A's cooling tower to amplify large amounts of Legionella. Second, weather conditions promoted the aerosolization of Legionella and the blowing of that aerosol towards the base. Third, a large number of workers were in close proximity to building A's cooling tower. This combination of factors is similar to those present during three previous outbreaks with large numbers of both LD and PF cases [Reference Fraser2, Reference O'Mahony3, Reference Mitchell13, Reference Pendergrast39]. To our knowledge, all mixed LD and PF outbreaks with identified sources have been directly associated with cooling towers [Reference Fraser2, Reference Girod6, Reference Nicolay8, Reference Mitchell13, Reference Bell14, Reference Pendergrast39]; warm, stagnant pools of cooling tower sump water aerosolized by nearby air-conditioning units [Reference O'Mahony3]; or indoor heated spas and hot tubs [Reference Goldberg7, Reference Benin9–Reference Euser, Pelgrim and Den12, Reference Okada15, Reference Foster, Gorton and Waller16], not with potable water systems. The lack of mixed legionellosis cases involving showers and sinks, in contrast to multiple LD-only outbreaks due to potable water systems [Reference Silk34–36] suggests that it would be very unusual for those systems to generate high enough concentrations of aerosolized PF agent to cause a mixed legionellosis outbreak. If so, the presence of PF cases in a future LD outbreak would suggest that a potable water system was not the outbreak's cause. Future LD outbreak investigations should seek out possible PF cases in the exposed population since such cases could provide valuable clues about the outbreak's source as well as its size and scope.
This sizable mixed outbreak of LD and PF provided an opportunity to evaluate the factors that lead to each form of legionellosis. Similar to at least one earlier mixed outbreak [Reference Burnsed10], the positive L. pneumophila serogroup 1 urine antigen tests of seven of the LD cases and one of the PF cases suggest that this outbreak may have involved a mixture of live and dead Legionella capable of causing both diseases [Reference Edelstein40]. There also seems to have been a substantial dose-response relationship between the amount of material inhaled from the cooling tower and individuals' risk of developing either LD [Reference Brown33] or PF as well as a relatively rapid drop-off in the concentration of the material from the cooling tower as the distance from the tower increased. For example, occupants of building A had significantly higher risks for developing each disease than occupants of building B, who were only slightly farther away from building A's cooling tower. In addition, it seems reasonable that host characteristics played a role in determining which form of legionellosis the patients from buildings A and B developed since all of them were exposed to the same source capable of causing both forms [Reference Bell14]. However, as in an earlier mixed legionellosis outbreak involving dozens of LD and PF cases [Reference Mitchell13], we did not identify any specific patient risk factors for developing one form of legionellosis rather than another.
Several features of the base's disease surveillance and response efforts may have reduced the harm caused by the outbreak. The base occupational health staff's detection of a cluster of employee illnesses probably brought the outbreak to the attention of base administrators more quickly than would have occurred in the absence of an employee illness monitoring system. In turn, the base leadership's shutting down the buildings most affected by the outbreak on 22 July 2010 may have played a role in ending the outbreak. The periods between the evacuations of buildings A and B and the symptom onset dates of the last LD and PF cases were within previously observed incubation periods for LD [Reference O'Mahony3, Reference Den4] and PF [Reference Jones5] and consistent with the proposition that no legionellosis-causing exposures occurred after buildings A and B were shut down. Additional cases might have occurred if the base leadership had been less aggressive in responding to the outbreak. Earlier identification of the outbreak as involving legionellosis would have aided its management, highlighting the importance of clinical testing for LD [1].
Our study has a number of limitations. The case definition of PF was sensitive but not specific, so some of the illnesses classified as PF may have been due to other aetiologies. In addition, the lack of laboratory confirmation of the probable LD cases leaves open the possibility that some probable LD cases also may have been due to other aetiologies, although the fact that the outbreak did not coincide with the respiratory illness season makes other aetiologies less likely [Reference Marston41]. More LD cases might have had laboratory confirmation if more of the patients with probable LD had undergone urine antigen testing and if a larger proportion of the urine antigen tests had been collected at patients' first legionellosis-related medical visits. The likely initiation of antibiotics soon after those first visits may have reduced the sensitivity of the urine antigen testing performed on samples collected later [Reference Kohler, Winn and Wheat42].
The findings from this mixed legionellosis outbreak caused by a cooling tower have a number of implications for the prevention and control of future US legionellosis outbreaks, which are likely to occur in substantial numbers given rising numbers of reported US legionellosis cases [1]. Preventive maintenance for cooling towers and other potential sources of Legionella-laden aerosols [37] is essential. For example, treating a shutdown cooling tower's water system with adequate levels of biocide or sodium hypochlorite as part of the process of restarting the cooling tower can reduce the risk of outbreaks [37]. In addition, whenever possible, the risks from cooling towers should be reduced by placing the towers far from fresh-air intakes and downstream from public areas [37]. Public health officials investigating LD outbreaks should search for related PF cases to better define the size, scope, and cause of the outbreak. Clinicians should consider both the diagnosis of LD and PF in an ill patient with probable exposure to Legionella in the context of a LD outbreak and should take care to report any identified legionellosis cases to the public health authorities. Legionellosis appears to be a growing problem in the USA, possibly due to an ageing population as well as climatic conditions, particularly increased temperatures, which favour Legionella growth. Proper maintenance of water and cooling systems [37], early recognition of outbreak sources, and prompt identification and treatment of cases are all essential to minimize the harm it causes.
ACKNOWLEDGEMENTS
We gratefully acknowledge the staff of the military base for their participation in this investigation. We also thank the numerous personnel at the U.S. Army, the CDC, the Michigan Department of Community Health, the Michigan Air National Guard, and area hospitals for their contributions to this investigation. The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of U.S. Army Public Health Command, the CDC, the Michigan Department of Community Health, or the Michigan Air National Guard.
Financial support was received from U.S. Army Public Health Command and the U.S. Centers for Disease Control and Prevention.
DECLARATION OF INTEREST
None.








