INTRODUCTION
Aujeszky's disease (AD, pseudorabies) is a notifiable disease caused by Suid herpesvirus 1 [SuHV1, syn. Aujeszky's disease virus (PrV) or Aujeszky's disease virus (ADV)], which belongs to the family Herpesviridae, subfamily Alphaherpesvirinae, genus Varicellovirus [Reference Mettenleiter1]. Members of the family Suidae (true pigs) are the only natural hosts for ADV, although the virus can infect other mammals including cattle, sheep, dogs, cats, and rats. AD is normally fatal in these other species [Reference Pensaert, Kluge and Pensaert2]. Carnivores, in particular, can easily become infected by the oral route via consumption of ADV-contaminated meat and are often indicators for the presence of ADV on pig farms. The disease has a worldwide distribution particularly in regions with dense populations of domestic pigs. Because of the substantial economic losses AD causes to the pig industry, it represents one of the most dangerous diseases in domestic pigs. Due to increased control efforts and the strict implementation of national eradication programmes which have either been based on initial large-scale vaccination with attenuated or gE-deleted vaccines, AD virtually disappeared from domestic pigs in several parts of the world in recent decades. In Europe, ADV has been eliminated from domestic pig populations in Austria, Cyprus, Czech Republic, Denmark, Finland, France (except single départments), Hungary, Luxembourg, The Netherlands, Sweden, Switzerland, Slovakia, and Great Britain (England, Scotland, Wales), mainly using the so-called DIVA (‘differentiating infected from vaccinated animals’) strategy. In Germany, nationwide elimination of AD was achieved in 2003 [Reference Müller3]. In countries officially recognized as AD-free, vaccination against ADV is prohibited. AD is still endemic in Eastern and Southeastern Europe.
Despite the tremendous progress made to control and eliminate the disease in domestic pigs, ADV infections appear to be widespread in populations of non-domestic swine, including feral pigs, wild boar and hybrids, across the world. First evidence for the occurrence of ADV in wild swine was reported from the USA, Italy, the former Yugoslavia, and The Netherlands in the mid-1980s [Reference Müller, Conraths and Hahn4]. In recent years, ADV infections in wild boar populations have also been reported from further European countries including the Czech Republic, France, Slovenia, Croatia, Poland, Russia, Switzerland, and Spain [Reference Szweda5–Reference Sedlak, Bartova and Machova12]. It was speculated that variants of ADV may have found ecological niches in populations of wild boar on the one hand or that wild boar populations represent the actual historical reservoir for the virus on the other [Reference Müller, Conraths and Hahn4].
As far as ADV infections in wild boar are concerned, East Germany has been intensively studied. Recent serosurveys in wild boar from the northeastern federal states of Brandenburg and Mecklenburg-Western Pomerania produced evidence for local seroprevalences up to 25% [Reference Oslage13–Reference Kaden17]. However, these studies were fragmentary. Annual, area-wide serological monitoring of wild boar populations and central collection of data had already been implemented in the former German Democratic Republic (GDR) in 1985 as laid down in national decrees [18]. With the unification of both German states, the animal health legislation changed in East Germany. As a result, the serological monitoring of wild boar populations was no longer mandatory, but conducted on a voluntary basis from 1991 onwards. The objectives of this study were to conduct a descriptive epidemiological analysis of the results obtained during serological monitoring for the presence of ADV infections in wild boar populations in East Germany since 1985 and to gain new insights into the long-term evolution and spread of ADV infections in its wild host reservoir.
MATERIALS AND METHODS
Study area
The study area (50° 26′ to 54° 67′ N, 9° 81′ to 15° 51′ E) in the eastern part of Germany with an east–west axis of about 360 km and a north–south axis of 520 km encompasses the territory of the former GDR and West Berlin covering an area of 108 589 km2 (Fig. 1). Since 1990, it has comprised of the German federal states of Mecklenburg-Western Pomerania (MW), Brandenburg (BB), Saxony-Anhalt (ST), Saxony (SN), Thuringia (TH) and Berlin (BE) bordering the Baltic Sea in the North, Poland in the East, the Czech Republic and Bavaria in the South, and Lower Saxony and Hesse in the West.

Fig. 1. Map of the study area showing the estimated cumulative Aujeszky's disease virus seroprevalence in wild boar of affected districts (n=66) for the years 2006–2008. The rivers Elbe, Havel, Saale, Spree and Odra are indicated.
Sampling
Between 1985 and 2008, blood samples were obtained from the heart ventricle and/or thoracic cavity of wild boar shot during the year, especially during the main hunting season in autumn and winter (end of October to end of January). The animals were either sampled by members of local hunting associations or by state forest officers and the samples were sent to the regional veterinary laboratories by courier. Delivery of blood samples took 1–4 days. Subsequently, samples were centrifuged at 1000 g for 10 min, the serum recovered was stored in aliquots at −20°C until testing. Data on the location, date and time of sampling was recorded. Information on the sex and age of the animals was available for two federal states (MW, BB) for the years 1991–1994 and 2006–2008. The age of shot wild boar was determined using tooth eruption patterns [Reference Matschke19]. Animals were divided into three categories consisting of ages <12 months, 12 to <24 months (juveniles) and ⩾24 months (adults).
Hunting statistics
To estimate population densities of wild boar and their dynamics, official hunting statistics of the respective federal states for the years 1985–1996 were used. Hunting statistics are based on ‘hunting years’, i.e. covering the period from 1 April of a given year until 31 March of the following year. Wild boar density was determined using the hunting index of population density (HIPD) which is defined as the number of wild boar shot/km2 and year [Reference Bögel20].
Serology
For the detection of ADV-specific antibodies, two different serological assays were used as established at the respective regional veterinary laboratories. From 1985 to 1991, sera were exclusively examined in a seroneutralization test (SNT) following a prescribed standard operating procedure to allow comparison of results obtained. The SNT was conducted essentially as described previously [Reference Dahle21] without guinea-pig complement using a plaque-purified field ADV (strain Stendal/64) as test virus. Because of the occasional poor quality (haemolysis, bacterial contamination), prior to testing, blood samples were pre-diluted in PBS 1:2, heat inactivated at 56°C and centrifuged at 800 g for 5 min. Subsequently, samples were tested in duplicate with twofold serum dilutions starting at 1:2. The virus neutralizing antibody (VNA) titre was defined as the dilution of the test serum showing a 50% reduction of the test virus (50% neutralizing dose, ND50). Sera with VNA titres ⩾1:4 were considered positive [Reference Bitsch, Eskildsen, Wittmann and Hall22]. From 1990 to 2008, the SNT was replaced by commercial full ADV antigen or ADV-glycoprotein B (gB)-based enzyme-linked immunosorbent assays (ELISAs) provided by different manufacturers (HerdCheck Anti-ADVgpI, IDEXX, Germany; Chekit® Aujeszky ELISA, Dr Bommeli AG, Switzerland; SvanovirTM PRV-gB-Ab, Svanova Biotech AB, Sweden) (Table 1). Identification of positive and negative sera was performed according to the manufacturers' instructions [Reference Müller15, Reference Lutz23].
Table 1. Number of wild boar sera tested from the study area on the presence of ADV-specific antibodies per federal state between 1985 and 2008
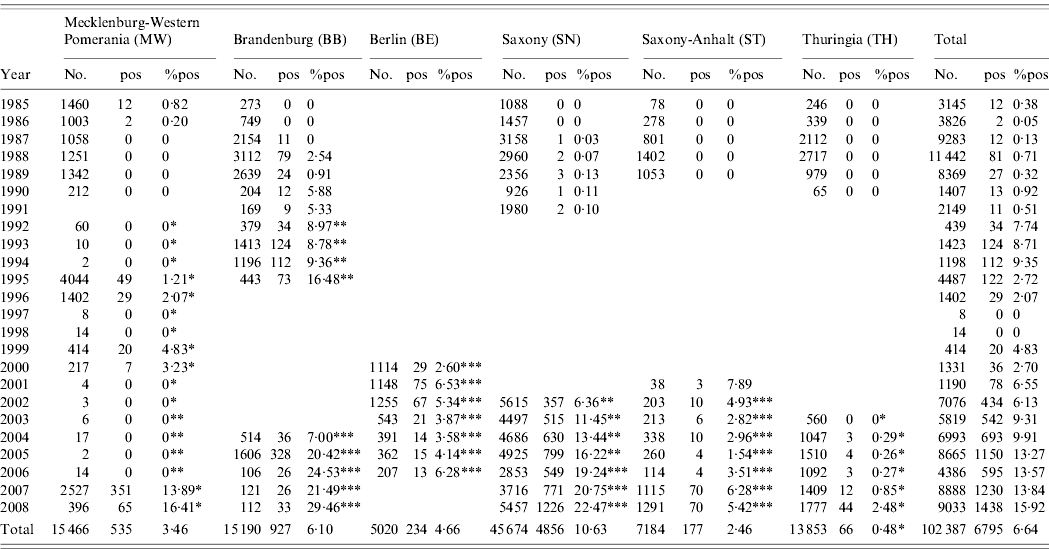
Asterisks represent the brands of different ELISA test kits used:
* SvanovirTM PRV-gB-Ab; ** Chekit® Aujeszky-ELISA; *** HerdCheck Anti-ADVgb.
Data analysis and statistical methods
Serological data on ADV from wild boar for the period 1985–2008 were submitted by the regional veterinary laboratories to the national reference laboratory for AD at the Friedrich-Loeffler-Institute (FLI) for epidemiological analysis. For the years 1985–1990, data were available at the district level, based on the administrative units (n=189) of the former GDR. Data collected in subsequent years were based on the new administrative units (districts, n=87) of the Federal Republic of Germany as of 2009. To take the reorganization of administrative units during the study period into account and to enable comparative epidemiological analyses, serological data obtained during the years 1985–1990 were assigned to the current districts. Data were analysed using Microsoft ExcelTM and the analysis tools of the national disease reporting system (TSN 3.0, FLI). The variables sex or age were examined for statistical associations with the serological results using Fisher's exact test. Associations were considered as statistically significant at P<0·05. Both variables were treated as discrete influential variables. Multiple post-hoc comparisons were conducted with the Bonferroni correction of P values [Reference Sokahl and Rohlf24]. The 95% (P=0·05) confidence interval (CI) limits for the estimation of true seroprevalence were calculated for indefinite populations and presented as whisker plots [Reference Willer25]. Samples sizes of <40 per federal state and year were considered unrepresentative and excluded from statistical analysis. An online software package (http://statpages.org/confint.html) was used to perform all statistical calculations. Regression analysis was performed using R (http://www.R-project.org) to test the hypothesis of a significant linear relationship (P=0·05) between the yearly hunting bag and the seroprevalences of the entire study area. Since estimates of seroprevalence vary in their accuracy due to different sample sizes, a weighted linear regression using the variance of seroprevalence estimate as weights was performed. For geographical presentation, results were plotted on maps using Map Explorer (http://www.bfav.de/kartenexplorer/). Further spatial analysis of seroprevalences for the entire observation period was conducted on the basis of the size of affected districts.
RESULTS
Between 1985 and 2008, a total of 104 181 blood samples from wild boar from East Germany were submitted for testing for ADV-specific antibodies.
Of these samples, 1794 were not suitable for analysis and therefore excluded. About 40% of the serum samples were obtained during the years 1985 and 1991. Between 1986 and 1989 the number of submitted samples was high. Due to political changes after the unification of the two former German states, the serological ADV monitoring of wild boar ceased in all federal states except MW and BB between 1992 and 1999, where wild boar sera were still obtained and tested, although considerably reduced in sample size. Since 2000, sample sizes increased but never reached the yearly spatial-temporal coverage of the 1980s (Table 1). If the City of Berlin (BE) is excluded, 19 428 serum samples were investigated per federal state during the observation period, on average. In Berlin, comprising 889 km2, more than 5000 serum samples were investigated from 2000 to 2006 (Table 1).
Of the 102 387, serum samples assayed by SNT or ELISA between 1985 and 2008, 6795 (6·6%) yielded positive reactions. While 158 (0·4%) of the 39 621 wild boar sera tested by SNT between 1985 and 1991 had virus-neutralizing antibodies, 6637 (10·6%) wild boar sera obtained during the years 1992–2008 showed ADV-specific antibodies either in commercial gB or full ADV antigen ELISAs. A spatial-temporal analysis revealed that the true seroprevalence against ADV in East Germany ranged within very narrow CI limits except for the years 1995–2000. The estimated prevalence increased from 0·4% in 1985 to 15·9% in 2008, depending on the serological test used (Fig. 2). At the regional level, however, the seroprevalence differed considerably, especially from 2004 to 2008. In 2004, SN had a statistically significantly higher seroprevalence on average compared to all other federal states in the study area, followed by BB and ST (P<0·05). In contrast, in 2008, BB and SN had similar seroprevalences of 29% and 22%, respectively (P>0·05). These were statistically significantly higher compared to the remaining study area, with MW still having a statistically significantly higher seroprevalence than ST and TH. In TH, the first serologically positive results were obtained in 2004. This federal state always had the lowest seroprevalence throughout the observation period (Fig. 3).
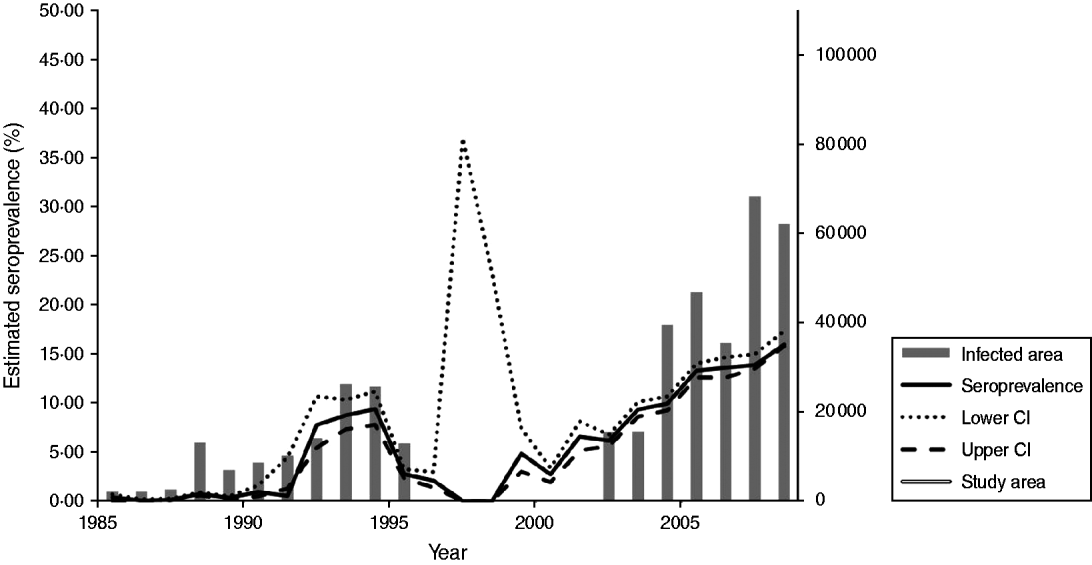
Fig. 2. Overall Aujeszky's disease virus (ADV) seroprevalence of the study area as determined by seroneutralization test (1985–1990) and ELISA (1991–2008) and development of the size of the ADV-affected area (district level) in East Germany. Estimated seroprevalences (%) and 95% confidence intervals (CI) of the true seroprevalence in the wild boar population are indicated. The differences in regional sampling (as shown in Table 1) should be taken into account in interpreting this figure.
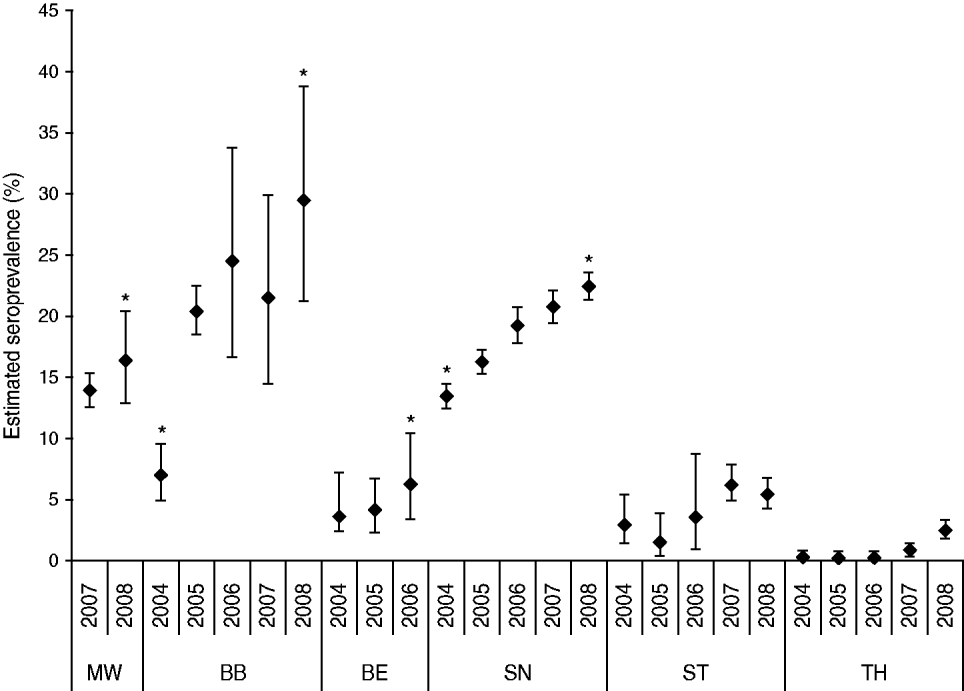
Fig. 3. Regional differences in estimated and true (95% confidence intervals) ADV seroprevalence between the six federal states of the study area for the years 2004–2008. Asterisks indicate significant differences in annual seroprevalence compared to other federal states.
Data on sex and age of the animals were only available for 3987 and 4019 animals from MW and BB, respectively. While seroprevalences in the sample size tested from MW and BB did not differ between sexes, there was a statistically significant association (P<0·05) with the age of the animals. The seroprevalence in animals aged <12 months and 12- to <24 months was determined as 4·7% and 10·1%, respectively. Seroprevalence (16·7%) was highest in adults (⩾24 months).
Although the hunting bag for wild boar was still relatively stable during the 1980s and 1990s, the hunting statistics almost doubled in 2008 compared to 1985. As a result, the wild boar population continuously increased in all federal states of the study area from 1998 as shown by the HIPD, ranging on average, from 1·09 to 2·43 wild boar shot/km2 in 1985 and 2008, respectively. During the entire observation period, the territorial federal states of MW and BB always had the highest hunting bags. They were 1·34–2·14 and 1·46–1·88 times higher, respectively, than those of TH, SN and ST. In 2008, MW and BB had the highest HIPDs with 3·23 and 2·72 followed by ST, TH and SN with 1·74, 1·85 and 1·55 wild boar shot/km2, respectively, with the latter values reaching HIPDs recorded in MW and BB 24 years earlier (Fig. 4 a). An increase in hunting statistics was also seen in the City of Berlin (BE), although on a low level but resulting in the highest HIPD in East Germany in 2008. When the development of the overall hunting bag was compared with the seroprevalence for the entire study area, there was a significant (P<0·05) association of the yearly hunting bag with the estimated annual seroprevalence. Thirty percent of the variation in estimated seroprevalence could be attributed to the development of the hunting bag during the observation period (Fig. 4 b).
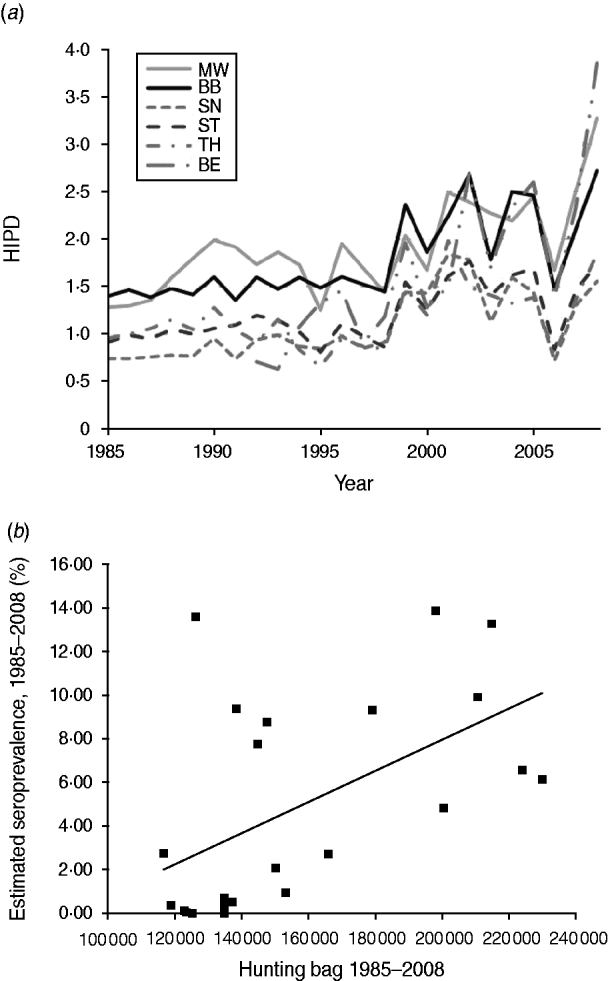
Fig. 4. Development of the hunting index of population density (HIPD) of the six federal states (a) for the period 1985–2008 and correlation between the cumulative yearly hunting bag (all federal states) and the estimated Aujeszky's disease virus seroprevalence for the entire study area based on the weighted linear regression using the variance of seroprevalence estimate as weights (b).
The temporal increase in ADV seroprevalence during the observation period was also reflected by a continuous spread of the infection in a western direction, especially in recent years. The size of the affected territories increased from 1985 to 2008 (Fig. 2). The first serologically ADV-positive wild boar in the study area were detected in a district (2085 km2) close to the town of Wismar in MW in 1985 and 1986, followed by districts in BB and SN, where a focus established along the border with Poland in subsequent years. The largest expansion of ADV infections was observed for the period 2001–2008 with the Elbe and Saale rivers as the assumed northwestern and southwestern borders of the endemic area. Except for the westernmost parts of TH seropositive findings were exclusively found in territories to the east of both rivers. Between 2006 and 2008, 66/87 districts of the study area were affected, with 12, 9, 11 and 34 districts having had ADV seroprevalences of >30%, 15–30%, 5–15% and <5%, respectively (Fig. 1). In those years, the endemic area comprised of 68 300 km2, i.e. 63·15% of the study area (Fig. 2).
DISCUSSION
Previous studies in BB and MW suggested that ADV infections in wild boar had been endemic in certain parts of East Germany for a long period of time possibly also affecting other neighbouring regions [Reference Oslage13–Reference Kaden17]. To our knowledge, with more than 100 000 wild boar sera tested over a 24-year period, this is the most comprehensive serological study ever conducted in Europe giving valuable insights into the evolution, spread and dynamics of ADV in wild boar. Within 24 years, the seroprevalence against ADV in wild boar populations of East Germany increased from 0·4% to 16·5% (Table 1, Fig. 2) resembling ADV infections in feral swine populations in the USA, where the overall seroprevalence was estimated as 19% nationwide [Reference Nettles26]. ADV seroprevalence, however, differed considerably at the local level (Table 1, Fig. 3), especially in core endemic areas in the easternmost parts of the study area, where the local ADV seroprevalence in wild boar could be as high as 45% (Fig. 1). This is in accord with findings from Spain (11–61%) [Reference Vicente27, Reference Ruiz-Fons28], Slovenia (26–31%) [Reference Vengust, Valencak and Bidovec9, Reference Vengust, Valencak and Bidovec29]), Croatia (55%) [Reference Zupancic7, Reference Gagrcin, Cirkovic and Orlic30], Romania (55%) [Reference Vuta31], and Russia (39%) [Reference Shcherbakov10]. TH is the only federal state in East Germany that still had a low ADV seroprevalence (<5%) as reported for The Netherlands and Switzerland [Reference Leuenberger11, Reference Cromwijk32–Reference Köppel34]. Our data confirm that seroprevalence is age-dependent rather than sex-dependent with significantly higher percentages found in adult wild boar followed by juveniles and piglets [Reference Müller15, Reference Lutz23, Reference van der Leek35–Reference Lari37]. However, only in Spain, a temporarily higher seroprevalence was found in sows [Reference Vicente38]. In the first two quarters of a year, serologically positive findings in wild boar piglets may be due to the presence of maternal antibodies, which can be detected up to 27 weeks postpartum by ELISA in consecutive generations of offspring born of infected sows [Reference Müller39].
As estimating the absolute population density of wild boar in a given area is usually difficult, yearly hunting statistics expressed as HIPD are used. Although, HIPD is influenced by regional and temporal hunting intensities, it is considered to provide a reliable estimate for areas >1000 km2 [Reference Bögel20]. Wild boar populations have drastically increased in Europe in recent decades with average densities for Central Europe believed to range between 2 and 6 animals/km2 as observed for MW and BB in 2008 (Fig. 4 a) [Reference Briedermann40]. However, it can be assumed that here the wild boar density might be even considerably higher as those areas represent optimal habitat for wild boar in Germany [Reference Briedermann40]. Apparently, the progressive development of the wild boar population significantly favoured the spatial-temporal spread of ADV infections in the study area during the 24-year observation period (Fig. 4 b). However, in recent years a relationship between HIPD and seroprevalence at a regional (district) level could no longer be observed (data not shown). Hence, it seems that in certain regions, especially in the easternmost parts of the study area, a ‘steady state’ may have been reached at a high prevalence level.
Although first serological findings in wild boar in MW, BB and SN in the mid-1980s were assumed to be directly related to AD outbreaks in domestic swine [Reference Dedek41], the available data appear to make it impossible to trace the original source for the current ADV infections in wild boar from the study area. Taking into account that early serological investigations were based on less-sensitive SNTs [Reference Müller15], the seroprevalence for the period 1985–1990 might have been underestimated. Therefore, undetected, serologically positive wild boar could have existed before 1985. This is supported by reports of sporadic serological findings from the study area at the beginning of the 1980s [Reference Dedek41].
ADV infections in the wild boar population of the study area seem to spread in a western direction [Reference Thulke, Selhorst and Müller16]. While only a small area of 2085 km2 was affected in 1985, it took 24 years until an area comprising 68 300 km2 was endemically infected. This spread is aligned with a gradient increase in regional average ADV seroprevalence, which is characteristic of 2008 (BB 30%, SN 22%, MW 16%, ST 10%, TH 2%) when the highest seroprevalences were observed in regions along the border river (River Odra) to Poland. Here, seroprevalences increased by 20–30% compared to the 1990s [Reference Müller15]. It appears that the westward spread is at least temporarily restricted by natural barriers. This view is supported by the absence of serological findings in wild boar on the Isles of Rügen and Usedom in MW in the Baltic Sea and also by the current border between endemic and non-endemic regions marked by the rivers Elbe and Saale in ST and TH. In MW, which is the only federal state covering the entire east–west stretch of the former GDR no such barriers exist. Here the River Elbe forms the southwestern border and serological findings can be found much more west compared to regions at the same longitude in ST and TH. Although it appears that ADV infections have already crossed the rivers in ST and TH (Fig. 1), seropositive animals were exclusively found to the east of both rivers (data not shown). However, over time infection pressure may overcome these natural barriers as can be witnessed in SN, where ADV managed to cross the River Elbe upstream in the 1990s. There is reason to believe that ADV infections might have crossed the River Odra in both directions far earlier because ADV is endemic in wild boar populations throughout Poland [Reference Lipowski42]. The fact that seropositive wild boar were also found in two districts in the westernmost parts of TH might be a result of infection pressure from neighbouring Hesse rather than from the Eastern endemic area (Fig. 1). It has been shown previously that there is no steady spatial-temporal spread of ADV infections in wild boar from initial hot-spots as the locations with greatest seroprevalence may vary over time [Reference Thulke, Selhorst and Müller16]. Analysis of data from BB disclosed a dynamic ‘emergence and dissolution of seats of infection’, i.e. a local cyclical accumulation of the infection with forwarding into non-infected areas followed by a rapid decrease in seroprevalence [Reference Thulke, Selhorst and Müller16].
Interestingly, ADV isolated from wild boar and hunting dogs from BB (n=4), ST (n=3) and ST (n=2), in 1995, 1996, 2003, 2005 and 2009 were shown to exhibit the same BamHI restriction enzyme DNA pattern (5–10/5–12 double fusion strains) and to display 100% sequence identity within the 5′ non-coding region of the gC gene and part of the open reading frame encoding the N-terminal 223 amino acids of gC [Reference Müller43, Reference Müller44]. These ADV isolates form one homogenous group which is different from ADV isolates circulating elsewhere, thus suggesting that a single ADV variant has been circulating for years and might have triggered the epidemic in the wild boar population in East Germany. Therefore, there is reason to believe that in parallel to the successful elimination of AD in domestic pigs, wild boar in East Germany have provided an ecological niche for this ADV strain [Reference Müller, Conraths and Hahn4] but do not represent a historical reservoir for the virus. In contrast to feral swine populations in the USA, both venereal and respiratory transmission appear to be the main routes of infection [Reference Müller43, Reference Romero45]. Experimental studies with this particular ADV strain suggest the virus is of low virulence resulting in more latent or subclinical infections, although mild and reversible disease could be induced [Reference Müller46]. The fact that clinical AD is extremely rarely observed in wild boar supports the hypothesis of host adaptation. Thus far, few clinical ADV cases in wild boar have been described, apparently associated with combinatory effects of age, genetic disposition, immune status and other factors [Reference Gortazar, Gibbs and Bokma8, Reference Schulze47].
Whereas nationwide elimination of AD in domestic pigs in Germany and recognition as an AD-free country was achieved in 2003 (2003/130/EEC [Reference Müller3]), East Germany had been officially recognized as free from AD since 1985. Why the ADV epidemic in wild boar appears to have started in the same year, still remains elusive. In general, in most regions the real extent of clinical disease and prevalence of latent ADV infections in wild boar are unknown [Reference van der Leek35]. If it holds true that ADV can be transmitted by aerosol over several miles [Reference Christensen48], infected wild boar may thus represent a potential source of infection for domestic pigs, jeopardize eradication programmes and threaten AD-free status [Reference Müller15, Reference Creswell49, Reference Ormiston50]. Between 1990 and 1997, a total of 337 AD outbreaks in domestic pigs were reported from all federal states in East Germany except the city state of BE. Trace-back revealed trading with infected pigs from West Germany, where AD was highly endemic at the time, as the only source of infection [Reference Müller14]. It is of note that despite an expanding epidemic in wild boar and evidence that domestic pigs are susceptible to ADV strains of wild boar origin [Reference Müller46] no spillover infections have been reported in East Germany. Hence, the infectious cycle in wild boar appears to be independent, favoured by the high host adaptation and extremely low virulence of the circulating ADV strain [Reference Müller14, Reference Müller46]. However, the absence of wild boar-triggered spillovers might also indicate that the measures implemented to avoid transmission of ADV from wild boar to domestic pigs as required in the Terrestrial Animal Health Code [51] and laid down in national legislation are effective. Nevertheless, as spillovers cannot completely be ruled out, free-range pigs, in particular, might be at risk if preventive measures are disregarded [Reference Heinritzi52]. Therefore, involvement of free-range pigs in the serological sampling scheme for testing of pig herds in order to maintain AD-free status in Germany is strongly suggested [Reference Teuffert53]. Furthermore, the epidemiological situation in wild boar should be periodically traced by establishing a nationwide, cost-effective monitoring programme in combination with the monitoring of other economically important pig diseases.
ACKNOWLEDGEMENTS
The authors thank numerous previous and current staff members of the regional veterinary laboratories in East Germany for skilful technical assistance with serological investigations during the past decades. The help of Anke Kliemt and Doris Junghans is also very much acknowledged.
DECLARATION OF INTEREST
None.







