INTRODUCTION
Scrub typhus, a rickettsiosis, results from Orientia tsutsugamushi, which is transmitted to humans by the bite of infected larval trombiculid mites [Reference Kelly1]. Patients are exposed to the pathogen in various environments such as field, forest, and scrub, which Orientia-infected mites inhabit [Reference Kawamura, Tanaka and Tamura2]. Farming and outdoor activities are mainly associated with infection [Reference Kim, Kim and Lee3, Reference Tay4]. Fever and rash appear 5–20 days after infection. Eschar is usually observed at the bite site [Reference Kawamura, Tanaka and Tamura2, Reference Kim, Kim and Lee3]. The rickettsiosis is prevalent in the Asia–Pacific region, particularly in eastern Asia and Oceania [Reference Kelly1, Reference Aung5, Reference Derne6]. It is noteworthy that the numbers of scrub typhus cases in eastern Asian countries have increased rapidly during the last two decades [Reference Jeung7, Reference Zhang8].
Early diagnosis and treatment are particularly important for treating scrub typhus. Tetracycline and chloramphenicol antibiotics show marked therapeutic effects and typically lead to a complete cure [Reference Kawamura, Tanaka and Tamura2]. Mortality related to the disease is not so high if patients are diagnosed and treated at an early stage [Reference Kawamura, Tanaka and Tamura2]. Recent studies conducted in Asian countries show that the mortality of scrub typhus with proper antibiotic treatment is about 0–1·2% [Reference Nadjm9–Reference Yasunaga11]. However, a recent systematic review revealed that the untreated mortality of scrub typhus varies widely from 0% to 70% [Reference Taylor, Paris and Newton12]. These facts suggest that early diagnosis and appropriate antibiotic treatment are fundamentally important to avoid a poor prognosis of scrub typhus.
Meteorological factors are known to affect the reproduction, development, behaviour, and population dynamics of arthropods transmitting vector-borne pathogens [Reference Gage13–Reference Haines15]. Recently, the relationship between meteorological factors and the number of scrub typhus cases in several Asian countries has been investigated [Reference Chang16–Reference Yang22]. Although no consensus has been reached, meteorological factors such as temperature and rainfall are generally believed to be associated significantly with the number of scrub typhus cases. These findings indicate that a statistical model incorporating meteorological factors is applicable to predict the number of cases.
Seasonal patterns of scrub typhus can be well explained considering the vector mite species. In Japan, there are two seasonal peaks of the disease: spring–early summer type and autumn–early winter type. In the northern part of Honshu Island, Japan (Tohoku and Hokuriku regions), scrub typhus occurs mainly in spring–early summer [Reference Kawamura, Tanaka and Tamura2, Reference Hashimoto23]. The seasonality of the disease in these regions is related to the life-cycle of Leptotrombidium pallidum, a predominant vector of Karp- and Gilliam-type scrub typhus [Reference Kawamura, Tanaka and Tamura2, Reference Hashimoto23–Reference Seto25]. The larvae of L. pallidum, which become dormant in winter and resume activities the subsequent spring, brings a large numbers of scrub typhus cases in spring–early summer [Reference Takahashi26].
This study was conducted mainly to ascertain the meteorological factors affecting the occurrence of scrub typhus of spring–early summer type in Japan. We analysed the relationship between meteorological factors and the number of spring–early summer cases of scrub typhus collected from Yamagata Prefecture, which is an endemic area of spring–early summer-type scrub typhus.
MATERIALS AND METHODS
Study area
Yamagata Prefecture, Japan, situated between 37° 44′ N to 39° 12′ N and between 139° 31′ E to 140° 38′ E (see Supplementary Fig. S1), has 1·1 million inhabitants. Its population density was 118 people/km2 in 2015. The climate of this prefecture is categorized as peculiar to areas along the Sea of Japan, which experience heavy snowfall in winter. The monthly mean temperatures are 0–25 °C and annual rainfall is typically 900–1400 mm.
Laboratory diagnosis of scrub typhus
Scrub typhus was diagnosed using immunofluorescence assays (IFAs) and polymerase chain reaction (PCR) reactive with six O. tsutsugamushi serotypes: Gilliam, Karp, Kato, Kawasaki, Kuroki, and Shimokoshi [Reference Seto25, Reference Furuya27]. In Yamagata Prefecture, most specimens were examined at the Yamagata Prefectural Institute of Public Health. IFAs were used for diagnosis during 1984–1998. PCR was added after 1999. Some specimens were tested at commercial laboratories (identity of laboratories not available) using IFAs for three serotypes: Gilliam, Karp, and Kato. Diagnostic criteria of scrub typhus were PCR positive or a ⩾fourfold rise of IFA titre. Scrub typhus cases were reported to the Yamagata Infectious Disease Surveillance Center, as established in the Yamagata Prefectural Institute of Public Health, from attending physicians according to the Japanese Law related to infectious diseases.
Meteorological factors
We selected four meteorological stations located in the highly endemic area of scrub typhus in Yamagata Prefecture (Fig. 1). Meteorological data were obtained, including monthly average temperature (°C), monthly rainfall (mm) and snowfall (cm), and monthly maximum depth of snow cover (cm). We were unable to incorporate humidity into the model because of lack of data. We calculated the mean of monthly meteorological data obtained from four stations and used them as explanatory variables. All meteorological data at four meteorological stations were obtained from the Japan Meteorological Agency (http://www.jma.go.jp/jma/index.html).
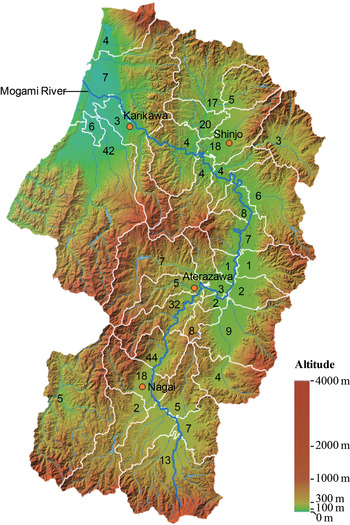
Fig. 1. Geographical distribution of four meteorological stations (red plot) where meteorological data were acquired for this study. The cumulative number of scrub typhus cases in each city of Yamagata Prefecture during 1984–2014 is shown on the map. The colour altitude map was obtained from the Geospatial Information Authority of Japan.
Statistical analysis
Although the effectiveness of deterministic models to estimate the number of cases of vector-borne diseases has been demonstrated elsewhere [Reference Massad28], we selected a statistical model due to small number of scrub typhus cases observed. A negative binomial regression analysis was used to investigate the relationship between meteorological factors and the number of scrub typhus cases. The negative binomial distribution, a mixed distribution of Poisson distribution and gamma distribution, is suitable for analysis of overly dispersed data. We chose a negative binomial distribution model because overdispersion was confirmed in a Poisson regression model using our dataset (data not shown) according to a previous study [Reference Li20].
First, we analysed the relationship between each meteorological factor and the number of scrub typhus cases adjusted by the population for each year. Specifically, we used 23 explanatory variables as follows: monthly average temperature from July of the previous year to March of the following year (nine variables), monthly rainfall during July–November of the previous year (five variables), monthly snowfall from December of the previous year to March of following year (four variables), total snowfall from December of the previous year to March of following year (one variable), and the monthly maximum depth of snow cover from December of the previous year to March of following year (four variables). Meteorological data from July 1983 to March 2014 were included in this study. The population in Yamagata Prefecture (as of 1 January of each year) was obtained from statistical data published by the prefecture. P values <0·01 were inferred as significant.
Second, the optimal negative binomial multivariable model with forced entry of the population variable was determined based on Akaike's Information Criterion (AIC), using significant factors selected as described above. Backward stepwise variable selection was performed to select a final model. Adjusted R 2 for actual and predicted numbers of disease cases from 1984 to 2014 was obtained using regression analysis. All statistical analyses were executed using R v. 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Seasonality of scrub typhus
During 1984–2014, 326 cases of scrub typhus were diagnosed in Yamagata Prefecture. In all, 282 (86·5%) of the cases occurred in spring–early summer (Table 1). Furthermore, scrub typhus of Karp and Gilliam types, which are transmitted by L. pallidum, occupied 213 (98·2%) of 217 in spring–early summer with the exception of samples without serotype information.
Table 1. Number of scrub typhus cases in Yamagata, Japan between 1984 and 2014, according to serotypes of Orientia tsutsugamushi
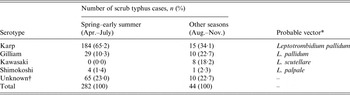
* Kelly et al. [Reference Kelly1]; Seto et al. [Reference Seto25].
† Some results from commercial laboratories were not obtained.
Relationship between meteorological factors and the number of scrub typhus cases
We used a negative binomial regression model to analyse the respective relationships between each of 23 meteorological factors and the number of scrub typhus cases (see Supplementary Table S1). For this study, the number of scrub typhus cases occurring in every spring–early summer time period (1 April–31 July) during 1984–2014 was used as a response variable.
Relationships between meteorological factors and scrub typhus cases are presented along with their significance (P < 0·01) in Figure 2. The average temperature in August of the previous year (T_Aug) was most strongly correlated with the number of scrub typhus cases. The other five factors [average temperature in July of the previous year (T_Jul), cumulative rainfall in September of the previous year (R_Sep), total snowfall from December of the previous year to March of the following year (S_Tot), and maximum depth of snow cover in January (D_Jan) and February (D_Feb)] also showed a positive relationship. However, cumulative rainfall in July of the previous year (R_Jul) showed a negative relationship to the number of scrub typhus cases.
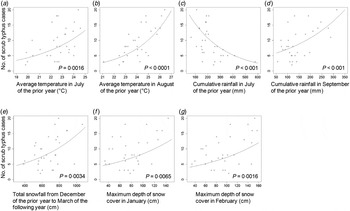
Fig. 2. Scatter plot between the number of scrub typhus cases and meteorological factors, which were significant (P < 0·01) for each negative binomial regression adjusted by population, in Yamagata Prefecture, Japan, 1984–2014. A regression curve was drawn with the population fixed to 1·13 million, representing the population in 2014. The average temperature in August of the previous year showed the greatest association to the number of scrub typhus cases. Six of seven meteorological factors showed a positive association to the number of the cases.
Selection of an optimal negative binomial multivariable model
We selected the optimal negative binomial multivariable model for predicting the number of scrub typhus cases. Specifically, the model with minimal AIC was selected using seven meteorological factors that showed an association with the number of scrub typhus cases, with forced entry of a population variable. Finally, the model was constructed using five variables as follows: population (P), T_Jul, T_Aug, R_Jul, S_Tot (Table 2). Multicollinearity was not confirmed in the model because all variance inflation factors (VIFs) were <2 [Reference Belsley29]. Therefore, the equation for prediction of the number of scrub typhus cases in spring–early summer in Yamagata Prefecture (Sc_Yg) was determined as shown below:
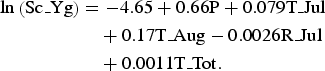 $$\eqalign{{\rm ln}\left( {{\rm Sc}\_{\rm Yg}} \right) & = - 4.65 + 0.66{\rm P} + 0.079{\rm T}\_{\rm Jul} \cr & \quad + 0.17{\rm T}\_{\rm Aug} - 0.0026{\rm R}\_{\rm Jul} \cr & \quad + 0.0011{\rm T}\_{\rm Tot}.}$$
$$\eqalign{{\rm ln}\left( {{\rm Sc}\_{\rm Yg}} \right) & = - 4.65 + 0.66{\rm P} + 0.079{\rm T}\_{\rm Jul} \cr & \quad + 0.17{\rm T}\_{\rm Aug} - 0.0026{\rm R}\_{\rm Jul} \cr & \quad + 0.0011{\rm T}\_{\rm Tot}.}$$
Table 2. Negative binomial multivariate model with minimal AIC using statistically significant meteorological factors

s.e., Standard error; VIF, variance inflation factor; AIC, Akaike's Information Criterion.
Validation of the determined model
To validate the negative binomial multivariable model, actual and predicted numbers of scrub typhus cases during 1984–2014 were compared (Fig. 3). The adjusted R 2 value between actual and predicted numbers of scrub typhus cases was 0·74. All actual numbers of the disease were included within the prediction interval (Fig. 4). Residuals between actual and predicted numbers of scrub typhus cases were −4·0 to 7·1. Moreover, the predicted value of scrub typhus in 2015, for which meteorological data were not included in the model, was 8·7 (prediction interval 2·0–19·0). The actual number of reported cases in 2015 was four, which was included in the prediction interval, but was about half of the predicted value.
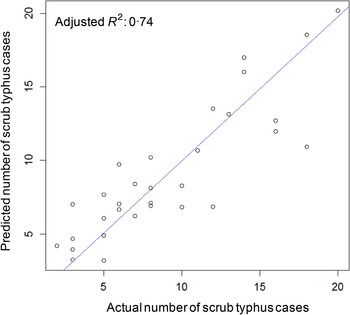
Fig. 3. Scatter plot showing actual and predicted numbers of scrub typhus cases in spring–early summer in Yamagata Prefecture, Japan, 1984–2014. Values of the two groups showed good fitness, with adjusted R 2 = 0·74.
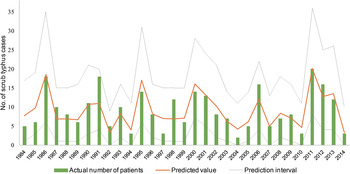
Fig. 4. Results of actual and predicted numbers of scrub typhus cases in spring–early summer in Yamagata Prefecture, Japan, during 1984–2014. All actual numbers of cases were included within the prediction interval.
DISCUSSION
For this study, we first identified the meteorological factors related to the occurrence of scrub typhus in spring–early summer in Japan. Our findings might be applicable to other regions of the northern part of Japan, where seasonal occurrence of scrub typhus results from the bite of infected L. pallidum chiggers.
To elucidate the relationship between meteorological factors and scrub typhus, the ecology of trombiculid mites, which transmit the pathogen, must be understood. Fortunately, a life-cycle of L. pallidum, which is the main vector of scrub typhus in our study, has been well investigated by Takahashi et al. using their semi-natural experimental rearing system [Reference Kawamura, Tanaka and Tamura2, Reference Takahashi26]. In brief, the adult mites lay eggs in July and August. From the eggs larvae hatch predominantly during September and October. Then, unfed larvae lie dormant in winter, subsequently resuming activities to attach to hosts the following spring. Experimental evidence indicates a favourable atmospheric temperature range for oviposition: female mites do not lay eggs at temperatures <20 °C or >30 °C [Reference Takahashi26]. Given that the number of scrub typhus cases correlates with the number of mites in the environment, knowledge related to the life-cycle of the vector mite can support discussion of how some meteorological factors can affect the number of scrub typhus cases. For example, our data show that the rise of average temperatures in July and August is related to the number of scrub typhus cases (Fig. 2). Considering that the respective means of average temperatures in July and August in our study periods (31 years) were 22·5 °C and 24·1 °C and that the temperature of moist soil in shade, which trombiculid mites inhabit, is normally lower than the air temperature, then higher temperatures in July and August might be a favoured condition for L. pallidum oviposition (>20 °C). This notion shows agreement with that of Wharton & Fuller in 1952, i.e. the limiting factors of the reproductive period of the mites are probably temperature in temperate climates (including Japan) and humidity in tropical climates [Reference Wharton and Fuller30]. In contrast, too much rainfall in July might interfere with fertilization by washout of male mites or spermatophores around the females. Rainfall in September is thought to contribute to the viability of L. pallidum eggs by giving proper humidity [Reference Takahashi26, Reference de Vis, de Moraes and Bellini31]. In addition, because trombiculid mites are sensitive to dryness [Reference Kawamura, Tanaka and Tamura2, Reference Takahashi26, Reference Rubio and Simonetti32], snowfall might serve to maintain dormancy through humidification of unfed larvae that lurk in the soil.
The meteorological factors identified in this study cannot be directly applicable to explain circumstances in other endemic areas because scrub typhus results from various vectors having different life-cycles [Reference Kelly1, Reference Takahashi33]. Moreover, weather differs greatly in endemic areas. Recent studies conducted in Asian countries have demonstrated that meteorological factors are statistically related to the occurrence of scrub typhus [Reference Chang16–Reference Yang22]. Specifically, temperature, rainfall, humidity, sunshine, atmospheric pressure, surface temperature, and wind speed are indicated as relevant factors in reports of earlier studies. However, these studies only slightly address the relationship between the meteorological factors and the detailed life-cycle of vector trombiculid mites. Some relationship between meteorological factors and the occurrence of scrub typhus might be elucidated by consideration of the life-cycle of vector mites living in the respective areas.
Using meteorological data that are accessible to the public, we proposed a negative binomial multivariable model to predict the number of scrub typhus cases in Yamagata Prefecture. The model shows a certain level of prediction potential (adjusted R 2 = 0·74). Our model is useful in that it can predict the number of scrub typhus cases using meteorological data that can be collected before the endemic season starts. The predicted result might be used most effectively when the predicted value is high because public health agencies can promote educational activities to prevent scrub typhus (e.g. use of tick repellent and protective clothing). Consequently, it is expected that the number of patients, complications, and deaths can be reduced.
A potential limitation of our study is that we did not consider the socioeconomic activities of local residents. For example, after reporting severe fever with thrombocytopenia syndrome cases in Japan in 2013 [Reference Takahashi34], the mass media devoted a great deal of attention to tick-borne disease prevention, which might have increased people's awareness of personal hygiene regarding outside activities and might have contributed to the reduction of exposure to the mites. Another limitation is that the model might have incorporated underestimated numbers of cases as a response variable because undiagnosed cases of scrub typhus might occur [Reference Taylor, Paris and Newton12]. However, because the frequency of undiagnosed cases does not seem to change markedly over the study period, we believe that the findings of the present study are more or less reliable.
In conclusion, by conducting a retrospective study in Yamagata Prefecture, we identified meteorological factors related to the occurrence of scrub typhus in spring–early summer in Japan. Relationships between significant meteorological factors and the number of scrub typhus cases can be explained partially by the life-cycle of the vector trombiculid mites. These findings might be applicable to establish a scrub typhus early warning system for northern Japan. A similar approach might be useful to identify other important meteorological factors in other endemic areas with different weather conditions. These efforts might ultimately lead to the elucidation of epidemic patterns of scrub typhus worldwide.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268816002430.
ACKNOWLEDGMENTS
The authors thank Tomohiro Nakamura, PhD, for his invaluable advice.
DECLARATION OF INTEREST
None.









