INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) infections, once primarily confined to health-care facilities are increasingly recognized as a major cause of skin and soft tissue infections (SSTIs) in the community [Reference Fridkin1–Reference Davis3]. While case definitions vary, community-associated MRSA (CA-MRSA) is typically defined as MRSA occurring in a patient without a history of hospitalization, surgery, dialysis, or long-term care residence in the 6–12 months prior to disease onset; no indwelling line at the time of diagnosis; and culture obtained less than 48 h after hospital admission [Reference Fridkin1–Reference Davis3]. Unlike health-care-associated MRSA (HA-MRSA), CA-MRSA isolates often carry phage-harbouring Panton–Valentine leukocidin (PVL) genes, associated with increased virulence and tissue destruction [Reference Boyle-Vavra and Daum4, Reference Gosbell5]. This toxin apparently accounts for necrotic SSTIs, necrotizing fasciitis, as well as necrotizing pneumonia associated with this pathogen [Reference Boyle-Vavra and Daum4, Reference Gosbell5]. CA-MRSA isolates almost exclusively carry the staphylococcal chromosome cassette (SCC) mec-type IV resistance genes and are typically susceptible to non-β-lactam antibiotics [Reference Boyle-Vavra and Daum4–Reference Daum6].
Outbreaks of CA-MRSA have been reported from correctional facilities [7–Reference Como-Sabette10], institutions for adults with developmental disabilities [Reference Borer11], military ships and barracks [Reference LaMar12–Reference Campbell14], rural or religious communities [Reference Baggett15–Reference Coronado18], and sports teams [Reference Nguyen, Mascola and Bancroft19–Reference Bartlett, Martin and Cahill29]. Persons with CA-MRSA usually have not had chronic health conditions and can be treated with oral antibiotics [Reference Gorwitz30]. In addition to crowding and close personal contact, risk factors for infection include inadequate hygiene, skin trauma, and previous antibiotic use. On the basis of initial evaluation of sports team outbreaks, CDC issued guidelines for preventing staphylococcal infection among sports team members [24]: (1) cover all wounds; (2) encourage good hygiene, including showering and washing with soap after all practices and competitions; (3) ensure availability of soap and water; (4) discourage sharing of towels and personal items; (5) establish routine cleaning schedules for shared equipment; (6) train coaches and athletes in recognition and first aid for wounds that are potentially infected; and (7) encourage athletes to report skin lesions and encourage coaches to assess athletes regularly for skin lesions.
On 6 September 2006, a cluster of four hospitalizations for MRSA SSTIs was reported to the West Virginia Department of Health and Human Resources. All affected persons were collegiate football players on Team A and had required hospitalization for intravenous antibiotic therapy. On further inquiry, investigators learned that an unknown number of additional team members were being treated for SSTIs as outpatients. Because of the unusually high morbidity and the concern for ongoing transmission, an investigation was initiated. By following initial control recommendations based on previous guidelines [Reference Kazakova26], the objectives for this investigation were to characterize the extent of the outbreak, identify risk factors for infection, and recommend additional control measures to prevent further infection.
METHODS
Case ascertainment
Team medical staff identified cases of MRSA infection through clinical examination of football players at the team training facility. Medical and athletic training records were reviewed to identify suspected and confirmed cases. Suspected cases were defined as a clinical diagnosis of cellulitis or staphylococcal infection with two or more signs of inflammation (i.e. redness, swelling, fever, heat, and purulence) and symptom onset during 3 August–21 September 2006. Confirmed cases met the suspected case definition, and the patient had a skin lesion culture-positive for MRSA. The defined outbreak period represents the beginning of training camp to the conclusion of the investigation. Team medical staff were asked to collect culture specimens from any players with suspected infections who had not been already treated. However, the majority of cases were identified before the investigation, limiting the number of culture specimens obtained. The athletic training staff of other athletic teams and the student health centre from the university of Team A were also contacted to identify any additional cases on campus as well as on competing football teams.
Environmental study
Visual inspection of athletic training, practice, and strength and conditioning facilities, as well as interviews with team training staff were performed to identify possible routes of transmission, including shared personal items and equipment; hygiene practices of team members; and disinfection, laundry, and cleaning practices in the facility. Residual chlorine levels in the team whirlpool were measured by using a commercial test strip.
Laboratory study
Sterile swabs of incised abscesses and other potentially colonized skin wounds were cultured on blood agar media. Bacterial identification and antimicrobial susceptibility testing were performed by using the MicroScan Walk-Away® (Dade Behring Inc., West Sacramento, CA, USA) automated microbiology system at a local hospital. Available culture isolates were characterized by pulsed-field gel electrophoresis (PFGE) by using the SmaI restriction enzyme at the Centers for Disease Control and Prevention (CDC) as described previously [Reference McDougal31].
Cohort study
On the basis of interviews with team medical and athletic training staff and results of the environmental assessment, a standardized questionnaire was developed to collect data regarding players' demographics, housing, past medical history during the previous year (including surgeries and outpatient visits to medical facilities), recent medical history, team-related activities, and personal hygiene practices. Questions addressing risk factors identified during previous athletic team outbreaks (e.g. cosmetic body shaving and turf burns, a specific type of skin abrasion) were also included in the questionnaire. Trained health department personnel administered the questionnaire in person at the team training facility on two separate occasions. Multiple attempts were made to interview by telephone those players who were not available to be interviewed in person. Players who were not interviewed were not included in the study. Attack rates were calculated for each variable of interest. Bivariate analyses comparing attack rates among players with and without specific exposures were conducted by Fisher's exact tests by using Epi-Info™ version 3.3 (CDC, Atlanta, GA, USA). Statistical significance was defined as P<0·05. Potential risk factors identified through bivariate analyses were further analysed through multivariate logistic regression and stratification based on playing position. Stratification models were evaluated on the basis of appropriate categorization of playing position and comparison of adjusted and unadjusted risk ratios.
RESULTS
Characteristics of affected players
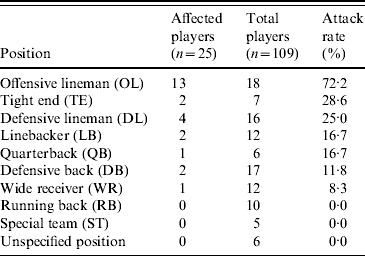
Among the 109 players on the team roster, a total of 25 cases of MRSA infection were identified, including 19 suspected and six confirmed cases, for an overall attack rate of 23%. Although sporadic reports were received, no additional confirmed cases were identified on campus or among competing football teams that were epidemiologically linked to the football players. Illness onset dates ranged from 7 August 2006 to 8 September 2006 (Fig. 1). Intermittent disease onset occurred before the opening game on 2 September, followed by a cluster of 12 cases during the following week. The median age of affected players was 20 years, and all were otherwise healthy males. Nineteen (76%) players had lesions on areas of their skin not covered by practice clothing, including distal arms, distal legs, and neck (Fig. 2). Other sites of lesions included chest, shoulder, axilla, foot, and groin. In total, seven players required incision and drainage, and five were hospitalized. The first player with confirmed illness was initially treated with amoxicillin–clavulanate without improvement, he then received parenteral vancomycin during hospitalization and was discharged on trimethoprim–sulfamethoxazole. All subsequent affected players were treated with trimethoprim–sulfamethoxazole, except one player with a medical contraindication whom was treated with clindamycin. Partly because of a time lag of ⩽4 days between onset of symptoms and presentation to team physicians, four additional players required hospitalization and parenteral vancomycin. As illustrated in Table 1, the attack rate of MRSA infection varied among players in different playing positions. The position most affected by the outbreak was offensive lineman, which included 13 (52%) of the MRSA cases identified and exhibited a position-specific attack rate of 72%. Other positions with elevated attack rates (AR) included tight end (AR 29%) and defensive lineman (AR 25%), which included two (8%) and four (16%) cases, respectively.
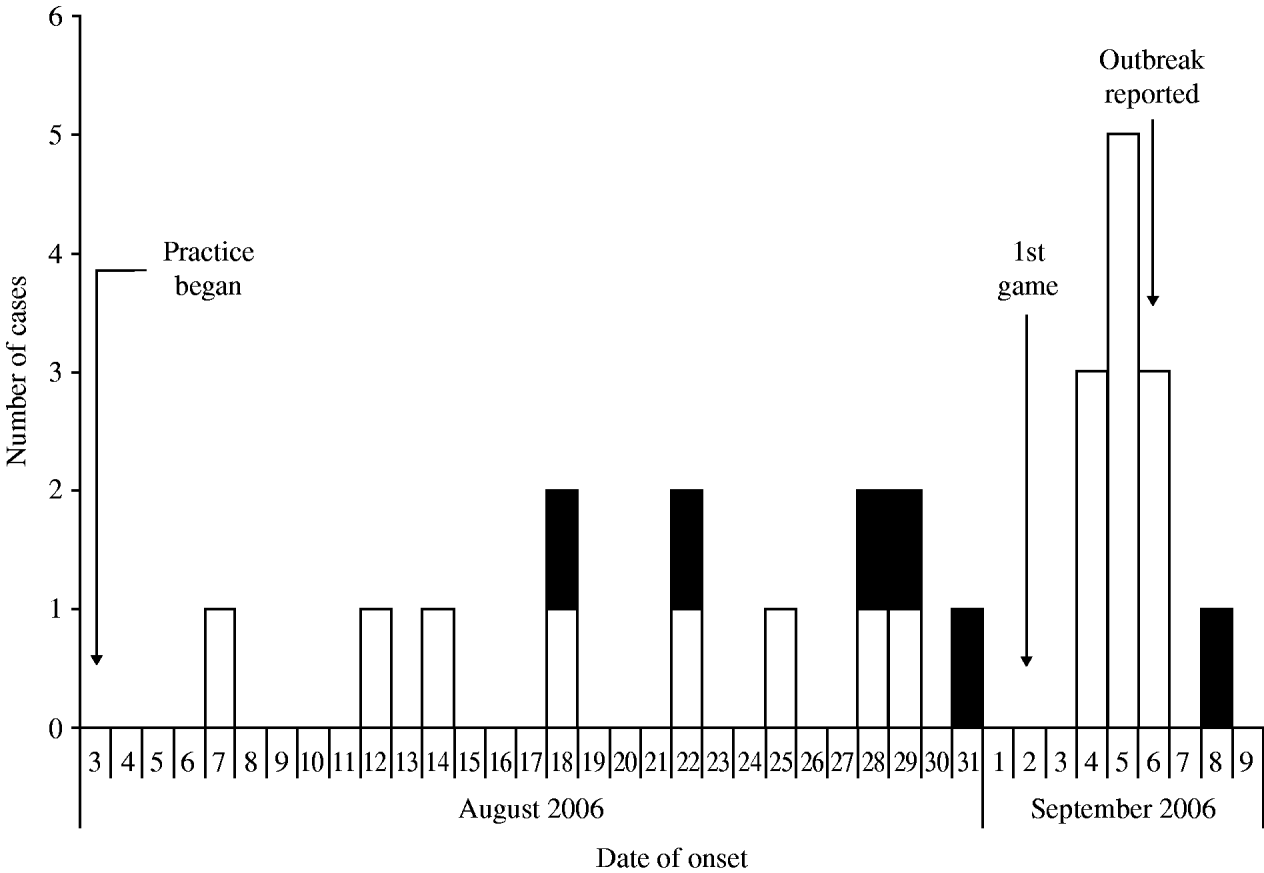
Fig. 1. Epidemic curve of suspected* (□) and confirmed† (■) methicillin-resistant Staphylococcus aureus (MRSA) infections among players on a college football team by date of onset, West Virginia, 2006. [* Suspected cases were defined as clinical diagnosis of cellulitis or staphylococcal infection with two or more signs of inflammation (i.e. redness, swelling, fever, heat, and purulence) and symptom onset during August 3–September 21, 2006. † Confirmed cases met the suspected case definition and had a skin lesion culture-positive for MRSA.]

Fig. 2. Resolving lesions on the forearm (a) and distal leg (b) of two players infected with methicillin-resistant Staphylococcus aureus, West Virginia, 2006. Before hospitalization and administration of intravenous antibiotics, both of these players had severe swelling of the affected limbs that substantially limited mobility and function.
Table 1. Attack rates of methicillin-resistant Staphylococcus aureus (MRSA) infections by playing position among players on a football team, West Virginia, 2006
Environmental results
On the basis of interviews with team coaching and athletic training staff, we determined that the football practice and athletic training facilities had been used exclusively by the football team, with rare exception. Dispensers with appropriate antimicrobial liquid soap were present in the shower facilities of the team locker room. Each player was assigned a personal towel embroidered with the player's jersey number, and he was responsible for submitting his towel for daily team-provided laundry services. Lockers were individually assigned and grouped by position, except team captains who occupied the lockers in the centre of the room. Team management cleaned protective shoulder pads and helmets after each practice and game with a Lysol® disinfectant spray (Reckitt Benckiser Inc., Slough, Berkshire, UK).
The athletic training room was used for medical treatments and physical therapy. Communal equipment in the training room was disinfected sporadically with a commercially available isopropyl alcohol solution, Iso-Quin® (Cramer Products Inc., Gardner, KS, USA). Players did not use barriers (e.g. towels) when using most equipment. Hydrocollator packs used for muscle and soft tissue therapy were covered with terrycloth sleeves before direct skin application; these hydrocollator covers were hung to dry between uses and laundered weekly by team equipment management staff (Fig. 3). The team hot whirlpool was typically used by more than one player at a time, causing substantial water displacement. Water disinfection was achieved by using chlorine disks, and a spot analysis of the whirlpool water taken between players' use revealed adequate residual chlorination. A cold water tub available for players' use was drained and refilled daily, but chlorination or other disinfection had not been used.

Fig. 3. Terrycloth covers of therapeutic hydrocollator packs used during outbreak of methicillin-resistant Staphylococcus aureus infections on a football team, West Virginia, 2006. Before implementation of control measures, hydrocollator covers were hung to dry between uses by players, as illustrated, and laundered weekly.
Laboratory results
Isolates from the six culture-confirmed cases underwent antimicrobial susceptibility testing. Isolates were resistant to β-lactam and macrolide antibiotics, but sensitive to clindamycin, vancomycin, and trimethoprim–sulfamethoxazole (Table 2). Four (66%) isolates were susceptible to ciprofloxacin. Three (50%) of the six isolates were available at the time of the investigation for further characterization by PFGE. Each of the isolates exhibited a unique banding pattern, corresponding to the following PFGE subtypes of MRSA: USA300.0047, USA300.0114, and USA800.0058 (Fig. 4). The two USA300 strains were 96% similar to one another and might represent an intra-outbreak variation of the same parent strain. However, the USA800 strain was 70% similar to the two USA300 strains and represents a unique lineage. Testing for the PVL toxin gene was not performed.

Fig. 4. Dendrogram and pulsed-field gel electrophoresis (PFGE) subtype of methicillin-resistant Staphylococcus aureus isolates from three football players, West Virginia, 2006.
Table 2. Antimicrobial susceptibility pattern and pulsed-field gel electrophoresis (PFGE) subtype of Staphylococcus aureus isolates obtained from skin lesions (e.g. abscess) of affected football players, West Virginia, 2006
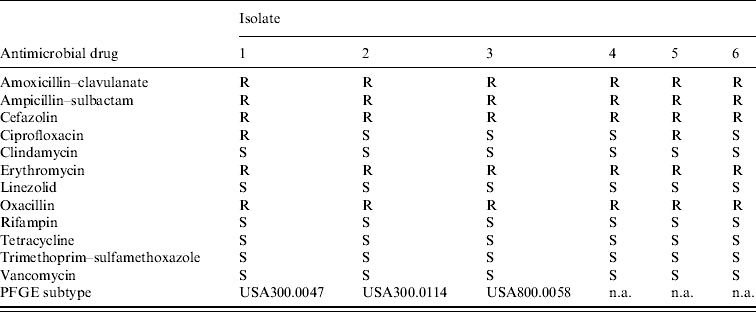
S, susceptible; R, resistant; n.a., not available.
Cohort study results
Interviews were conducted with 88 (81%) of the 109 players on the team roster. Bivariate analyses identified multiple statistically significant risk factors for CA-MRSA infection (Table 3). Stratification and multivariate logistic regression models were developed to further address these risk factors; however, inadequate cell sizes limited interpretation, and only bivariate analyses are presented here. During the previous year, in-patient or outpatient surgery [risk ratio (RR) 2·0, 95% confidence interval (CI) 1·0–3·8] and unspecified previous skin infection (RR 2·6, 95% CI 1·3–5·1) were risk factors for MRSA infection during this outbreak. Four players were identified as having had MRSA infection during the preceding year, three of whom were also infected during this outbreak. During the previous month, recent skin trauma, including lacerations and abrasions were also associated with an increased risk for infection (RR 1·9, 95% CI 1·0–3·7). However, abrasions specifically caused by contact with the playing surface (i.e. turf burns) were not a significant risk factor.
Table 3. Predictors of infection caused by methicillin-resistant Staphylococcus aureus (MRSA) among 88 football players included in cohort investigation, West Virginia, 2006
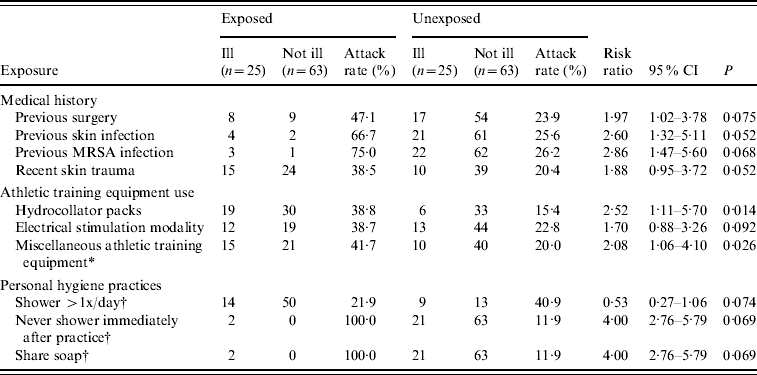
CI, Confidence interval; P, P value based on Fisher's exact test.
* No response given by two players who did not become ill; Examples of miscellaneous athletic training equipment include elastic bands, exercise balls, and weight belts.
† No response given by two players who became ill.
Team-related activities, including the use of equipment in the training facility, were evaluated. Using hydrocollator packs was the only specific item associated with MRSA infection (RR 2·5, 95% CI 1·1–5·7). Using miscellaneous equipment (e.g. elastic bands, exercise balls, and weight belts) was also a significant risk factor (RR 2·1, 95% CI 1·1–4·1). The use of therapeutic electrical stimulation, ultrasound, compression, massage, and hot paraffin modalities available in the athletic training facility were not associated with infection. The three team positions with elevated attack rates (offensive linemen, defensive linemen, and tight ends) were classified as high-risk positions, and attack rates were evaluated relative to players in other positions. This group had a significantly greater risk for MRSA infection, compared with the other players on the team (RR 5·2, 95% CI 2·3–11·5). Body mass index >30 was also associated with an increased risk for infection (RR 2·6, 95% CI 1·3–5·1), probably as a proxy for positions of offensive and defensive linemen and tight ends, because the largest players were in the high-risk positions.
Inadequate personal hygiene was also associated with infection. Showering more than once per day was associated with a risk ratio of 0·5 (95% CI 0·3–1·1), indicating that this practice might have been protective. Conversely, never showering immediately after practice was a statistically significant risk factor (RR 4·0, 95% CI 2·8–5·8). Sharing of personal items (e.g. towels, deodorants, and razors) was not associated with MRSA infection; however, sharing personal soap was a risk factor (RR 4·0, 95% CI 2·8–5·8). That only two players reported having these last two significant risk factors, which therefore does not explain the extent of the disease affecting the team, is noteworthy. Other potential risk factors that were not associated with an increased risk for infection included whirlpool use, body shaving, previous antibiotic use, on-campus housing, and shared use of other personal items or equipment (e.g. deodorant, razor, hair clipper, gloves, tape cutter, knee brace, sandals, etc.).
DISCUSSION
This report documents a multiclonal outbreak of MRSA SSTIs among an athletic team, which has rarely been reported previously [Reference Müller-Premru20]. Although all isolates from affected players had similar antimicrobial susceptibility patterns, at least two distinct MRSA genotypes were identified. Four players were identified with self-reported MRSA infections during the preceding year, providing evidence of multiple potential sources for these outbreak strains. Furthermore, three of four previously infected players experienced illness during this outbreak, indicating continued colonization and recurrent infection. Recurrent MRSA infections among football team members during consecutive seasons have been previously reported [Reference Nguyen, Mascola and Bancroft19]. Isolates from all players clinically infected during that outbreak yielded indistinguishable PFGE patterns, although a nasal carriage study identified two additional MRSA strains. An outbreak among a football team, which was reported in Germany, identified two different PFGE patterns from patient isolates [Reference Müller-Premru20]. However, other MRSA outbreaks reported among competitive sports participants have typically identified a single clone with indistinguishable PFGE patterns among isolates from affected persons [Reference Lindenmayer21–Reference Romano, Lu and Holtom27]. The convergence of multiple strains of MRSA and possible recurrent infections during this outbreak highlight the difficulties in eradicating MRSA infections among populations at high risk and the continued widespread emergence of this pathogen in the community.
The elevated risk of MRSA infection observed among offensive and defensive linemen and tight ends is likely the result of the frequent and intense skin-to-skin contact in which these players engage through their usual team activities, which is analogous to hand-to-hand combat techniques that have been associated with MRSA infection in military settings [Reference Zinderman13]. This type of contact has also been associated with MRSA outbreaks among other competitive sports participants, including wrestlers [Reference Lindenmayer21] and rugby players [Reference Stacey22]. The offensive and defensive players in these positions also have frequent direct contact with one another during scrimmages and drills, providing further opportunity for spread. These players are usually the largest members of the team, and increased body mass index was identified as a risk factor for infection during this outbreak. These findings are consistent with previous outbreaks on football teams, which identified linemen as being at elevated risk [Reference Nguyen, Mascola and Bancroft19, 25–Reference Romano, Lu and Holtom27], transmission between corresponding offensive and defensive players (e.g. wide receivers and defensive backs) [Reference Begier23], and increased body mass index associated with MRSA infection [Reference Kazakova26].
Results of this investigation demonstrate that transmission and subsequent infection was facilitated by other factors than playing position. Using communal equipment contributed to MRSA spread, probably because of inadequate disinfection of this equipment between players' use. Hydrocollator packs were specifically identified through both the environmental investigation and the cohort study as problematic. The hydrocollator covers were not routinely laundered after each use and remained damp, providing an opportunity for bacteria to persist and the covers to serve as fomites for MRSA transmission. While little is known about the potential for environmental persistence of CA-MRSA, in hospital settings MRSA has been demonstrated to persist on blood pressure cuffs [Reference Walker, Gupta and Cheesbrough32], tourniquets [Reference Ormerod33], health-care providers' ink pens [Reference French34], and moist mattress padding [Reference Ndawula and Brown35], all of which can serve as vehicles of transmission. Even if allowed to desiccate, MRSA can be viable in the environment (e.g. on cotton blankets) for ⩽9 weeks and has been reported experimentally to survive on plastic laminate surfaces for ⩽2 days [Reference Hota36–Reference Duckworth and Jordens38]. During this outbreak, the alcohol-based disinfectant used to clean equipment in the training room was inadequate, and consistent use of a U.S. Environmental Protection Agency-registered disinfectant was recommended [Reference Sehulster39, 40].
On the basis of results of the environmental investigation and cohort study, personal hygiene practices also might have contributed to this outbreak. Increased frequency of showering and showering immediately after practice both tended to be protective against infection. Although antimicrobial liquid soap was available in the locker room showers and the majority of players reported using it, our findings provide evidence that the sharing of bar soap among players might also have been a risk factor for MRSA infection, as previously reported [Reference Nguyen, Mascola and Bancroft19, Reference Begier23]. After being colonized through the multiple routes described previously, traumatic interruption of the skin barrier (e.g. lacerations and abrasions) provided an opportunity for infection, as noted in other MRSA outbreaks among athletes [Reference Nguyen, Mascola and Bancroft19, Reference Begier23, Reference Sosin28, Reference Bartlett, Martin and Cahill29]. Although abrasions specifically designated as turf burns have been associated with MRSA infection in other athletic team outbreaks [Reference Begier23, 24, Reference Kazakova26], this was not the case during this investigation. The array of risk factors for transmission and subsequent infection documented in this study all contributed to an outbreak with a high attack rate and substantial morbidity.
This study is subject to multiple limitations. Selection bias might have been introduced given that patients were overrepresented in the interviewed cohort. Interview responses also might have been influenced by the team announcement and distribution of educational materials before the cohort investigation. Similarly, the interview technique might have biased responses to culturally sensitive questions (e.g. personal hygiene practices). Cultures were performed for only six cases, limiting the number of confirmed cases identified. Environmental and equipment contamination with MRSA could not be documented during this investigation. Finally, the study and infection control measures were limited by delayed recognition of the outbreak, which occurred in part because key players did not immediately report infections for fear they would be excluded from the opening game of the season. This provided the opportunity for continued colonization and propagation of the outbreak. The cluster of infections that occurred after the opening game might have been prevented by more timely intervention.
This report highlights the continuing challenges in preventing and controlling MRSA outbreaks among athletic teams and the emergence of a multiclonal outbreak in a single cohort at high risk. Coaches and athletic trainers should be educated about the importance of early recognition and treatment. Similarly, players should be counselled to report illness, injury, and skin trauma promptly, as delays in reporting probably facilitated transmission during this outbreak. Covering open wounds, employing safe hygiene practices, and not sharing personal items are other essential prevention measures that should be emphasized. During this outbreak, use of therapeutic hydrocollator packs was associated with infection, probably because hydrocollator covers were laundered only once per week. Team management and training staff should be informed of the potential for spreading infection through fomites and adhere to strict disinfection practices of all communal equipment. As MRSA continues to emerge as a common cause of illness in community settings, heightened awareness among groups at high risk and strict adherence to infection control practices are the cornerstones of disease prevention and control.
ACKNOWLEDGEMENTS
The authors gratefully recognize Diana Bensyl, Ph.D. and Anindya De, Ph.D. for their insight and technical support; Ross Patton, M.D. and Harry Tweel, M.D. for logistical collaboration; the staff of the state and local health departments for their enthusiastic field work; and the physicians, trainers, coaches, and players from Team A for their participation in this study.
DECLARATION OF INTEREST
None.








