INTRODUCTION
Salmonellae cause an estimated 1·4 million human cases of gastroenteritis and 600 deaths annually in the United States [Reference Mead1]. Of the more than 33 000 clinical isolates of Salmonella reported in 2003, 530 (1·6%) were serotype Braenderup [2]. S. Braenderup outbreaks previously have been associated with chicken, eggs and jelly used in meat pies [Reference Urfer3]. On 16 July 2004, the Centers for Disease Control and Prevention (CDC) was notified by the Pennsylvania Department of Health (PADOH) of 23 cases of Salmonella serotype Braenderup with onsets of illness over a 1-month period, an increase from the baseline of 1–3 cases per month. Of these, 14 were subtyped and demonstrated indistinguishable pulsed-field gel electrophoresis (PFGE) patterns. At the same time, via PulseNet, the National Molecular Subtyping Network for Foodborne Disease Surveillance, Pennsylvania state health officials received reports of five isolates from Massachusetts and three isolates from Kansas with indistinguishable PFGE patterns. In order to determine the source of this outbreak, an epidemiological investigation was carried out by the CDC in collaboration with state and local health authorities.
METHODS
Case finding, laboratory investigation and hypothesis generation
The database of PulseNet was reviewed to identify isolates with indistinguishable PFGE patterns. All state public health laboratories participating in PulseNet perform Salmonella serotyping and molecular subtyping on clinical isolates, and all were asked to notify the PADOH of case isolates indistinguishable by PFGE from the outbreak strain. A confirmed case was defined as an infection with S. Braenderup, indistinguishable from the outbreak PFGE pattern by one DNA restriction enzyme, XbaI, in a person residing in the United States with illness onset or specimen collection date between 15 June and 31 August 2004. A probable case was defined as diarrhoeal illness with onset between 15 June and 31 August 2004, in a patient who consumed a meal with a confirmed case-patient during the incubation period. No relevant food or environmental samples were available for testing.
Pennsylvania district and local health officials collected demographic information and food histories from a sample of cases using an open-ended food history questionnaire for the week preceding illness. Additional food history information from cases in Massachusetts and Kansas was also used to generate outbreak source hypotheses.
Case-control study, Phase I
A case-control study was performed by the CDC in collaboration with state health departments to test the hypotheses. Study cases were defined as culture-confirmed S. Braenderup infections with a date of illness onset between 15 June 2004 and the end of the study period, 10 August 2004. As no two patients were members of the same family, we did not attempt to exclude secondary cases. Controls were persons who did not report having a diarrhoeal illness between 15 June and the date of interview. Both cases and controls were restricted to being between the ages of 15 and 60 years in order to have a study population with similar food consumption habits. Controls were enrolled between 09:00 and 21:00 hours local time 7 days a week, from 23 July to 10 August.
Because this outbreak involved cases dispersed over many states without an evident link to a single restaurant or supermarket chain, anyone from the involved states may have had a similar risk of illness to that of cases. However, in order to define areas from which controls could be conveniently selected, controls were enrolled using cases' area codes and prefixes through sequential digit dialling. Controls were matched to the cases on this basis, with a goal of enrolling two matched controls for each case. If the person answering the telephone was an adult resident of the household, this person was interviewed; otherwise, we asked to speak to any available adult household resident.
The case questionnaire included questions about consumption of meats, dairy, fruits, vegetables, spices and condiments prepared outside and inside the home in the 5 days before illness onset. We asked about food items related to our hypotheses, as well as food items implicated in previous Salmonella outbreaks. Controls were asked the same questions about the same 5-day period as their matched case. Specific meal and restaurant location information was obtained for both cases and controls.
Analysis was performed using SAS 9.1 (SAS Institute, Cary, NC, USA). χ2 and Cochran–Mantel–Haenszel testing was used for univariate analysis of food exposures. Conditional logistic regression was used for multivariate analysis of food exposures and analysis of frequency of eating at a restaurant. A two-tailed P value of <0·05 was considered significant.
Case-control study, Phase II
The first phase of the case-control study did not implicate a unique food item, but instead narrowed our hypothesis to three specific food items eaten at restaurants. To further investigate these food items, we performed a second case-control study. We included unmatched case-patients and controls who had been interviewed during the study period, but did not fall into matched groups, in order to have sufficient participants in the study. As matching had been done for ease of finding controls and not to control variability, we ignored the match. The matched groups were considered to be exchangeable given that most food exposure information collected more than a few days after an event generally reflects food preferences, and that controls, regardless of location, should have had similar risks of exposure.
We attempted to telephone managers of all restaurants patronized to ask them about the use of specific kinds of lettuce, tomatoes and cheese in the specific menu items consumed by case-patients and controls on the days of their patronage. We included only case-patients and controls who had reported eating at a restaurant, and for whom we could obtain menu item information for all restaurant visits.
Traceback and environmental investigation
The U.S. Food and Drug Administration (FDA) conducted a traceback investigation in collaboration with state food regulatory agencies and health departments. FDA Center for Food Safety and Applied Nutrition (CFSAN), in conjunction with state and local food regulatory agencies, performed an environmental investigation of a packing facility.
RESULTS
Case finding, laboratory investigation and hypothesis generation
We identified 125 confirmed cases from 16 states (Pennsylvania 40, Massachusetts 22, Virginia 11, New Jersey 10, Ohio 9, Maryland 7, Connecticut 5, Kansas 5, Delaware 3, Iowa 3, New York 3, Missouri 2, Wisconsin 2, Georgia 1, New Hampshire 1, West Virginia 1) and 12 probable cases. The outbreak was centered in the northeastern United States, which had 90% of the cases. Illness onset ranged from 18 June to 21 July (Fig. 1). The median age of patients was 30 years (range 3 months to 88 years); 67% of patients were female (Fig. 2). There were no deaths reported.

Fig. 1. S. Braenderup infections with XbaI outbreak pattern JBPX01.0093 by illness onset date (n=92).

Fig. 2. Age and gender distribution of confirmed outbreak cases (n=110). Only confirmed cases for which gender and age information were available are included. ■, Female; □, male.
A review of the PulseNet database found that the XbaI. S. Braenderup PFGE outbreak pattern (JBPX01.0093) had not been previously recorded among a total of 868 S. Braenderup isolates which demonstrated 98 unique XbaI patterns. Isolates were susceptible to a standard panel of antibiotics.
In Pennsylvania, three patients had eaten at one single outlet of a Mexican restaurant chain between 17 and 30 June. Three other patients had consumed food from one Italian restaurant on 28 and 29 June. In Kansas, three patients had eaten at an Italian restaurant on 25 June. A review of menu items consumed by these groups did not suggest an outbreak vehicle.
Massachusetts state health officials reported 22 confirmed and 11 probable cases. The probable cases, along with one confirmed case, constituted a group associated with a common meal catered by an Italian restaurant on 21 June. The only dish in common for these 12 persons was a chicken caesar salad, containing chicken, mushrooms, mesculun and Roma tomatoes. Separately, a pair of individuals, one of whom was a confirmed case from Massachusetts, reported eating the same meal together at a restaurant, with the exception that the well member of the pair did not eat the Roma tomatoes.
Based on population-based data, produce items are more commonly consumed by women than men [4]. Because there was a predominance of women and because of the foods reportedly consumed by some cases, we hypothesized that a produce item commonly found at Italian and Mexican restaurants might be the outbreak vehicle.
Case-control study, Phase I
We enrolled a total of 32 (26%) of the 125 case-patients. These patients were from Pennsylvania (20), Connecticut (4), Virginia (4), Kansas (2), Ohio (1) and West Virginia (1). Sixty-three matched controls from Pennsylvania (40), Connecticut (8), Virginia (8), Kansas (4), Ohio (1), and West Virginia (2) participated in the case-control study. Reasons for not enrolling patients included identification of cases after the study period, choice by state health departments not to participate in the study, inability to contact patient or find matched controls during the study period, and failure to meet the study case definition. The median age of study patients was 31·5 years, compared to 45 years for controls. Seventy-six per cent of study patients were female, compared to 71% of controls.
All patients reported diarrhoea, with 14 (44%) reporting bloody diarrhoea. Other symptoms included abdominal cramps (91%), fever (86%) and vomiting (50%). As part of their clinical care, 21 (66%) patients received antimicrobial therapy for their illness, and eight (25%) were hospitalized.
Patients ate at a restaurant more frequently than did their matched controls (P=0·04), and the remainder of the analysis was limited to food items eaten at restaurants. The restaurant items most commonly consumed by patients included cheese (72%), lettuce (65%), tomatoes (61%), beef (53%), onion (45%) and chicken (42%). On matched univariate analysis, no items were significantly associated with illness (Table 1). Exposures were grouped by type of restaurant food (meats, dairy, vegetables, produce or spices or condiments); none were associated with illness.
Table 1. Analysis of food items consumed by >40% of cases
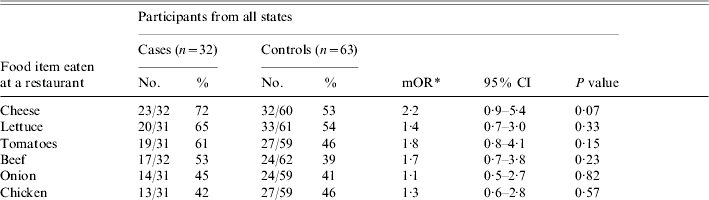
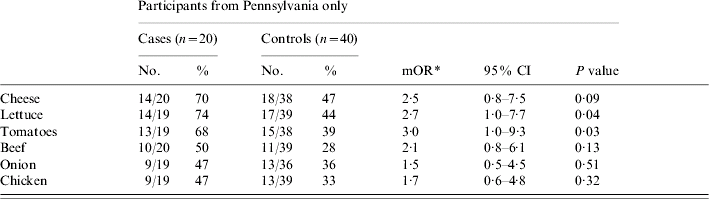
mOR, Matched odds ratio; CI, confidence interval.
* mOR and two-tailed P values calculated using the Cochran–Mantel–Haenszel method.
The 20 patients and 40 matched controls from Pennsylvania were then analysed separately, as this appeared to be a more homogenous subset in regards to both data collection and exposures. The odds of becoming ill were greater for eating lettuce [matched odds ratio (mOR) 2·7, 95% confidence interval (CI) 1·0–7·7, P=0·04], tomato (mOR 3·0, 95% CI 1·0–9·3, P=0·03) and cheese (mOR 2·5, 95% CI 0·8–7·5, P=0·09), although for cheese, this association was not statistically significant (Table 1). All three of these food items were consumed by ⩾68% of patients. In multivariate analysis, with the inclusion of each of these three food items individually, neither of the two remaining food items were associated with illness.
Case-control study, Phase II
Although the findings of Phase I of the case-control study did not implicate a single food item, they did provide a narrower hypothesis: lettuce, cheese or tomatoes eaten at a restaurant could be the outbreak vehicle. All of these food items were consistent with the original hypothesis, and were commonly consumed foods available in a wide variety, which would be difficult to distinguish by patrons.
In the second phase of the case-control study, information on meal ingredients was obtained from telephone surveys of restaurant managers, for 27 cases and 29 controls, totaling 84 meals at 79 restaurants. The median age of study patients was 31 years, compared to 45 years for controls; 71% of patients were female compared to 66% of controls.
Cheese was eliminated as a potential source of this outbreak. Eleven different varieties of cheese were consumed by patients and no specific cheese type was consumed by more than 28% of patients. None of the cheeses were reported to be made with unpasteurized milk.
Iceberg lettuce was consumed by 67% of patients, while romaine lettuce was consumed by only 18% of patients. Red round tomatoes and Roma tomatoes were consumed by 44% and 41% of patients respectively (Table 2). All of the 31 meals with tomatoes, except for one with red round tomatoes, were uncooked. Roma tomatoes were the only food item significantly associated with illness (crude OR 4·3, 95% CI 1·2–15·9, P=0·02).
Table 2. Analysis of food items consumed by >40% of cases, Phase II of case-control study

OR, Odds ratio; CI, confidence interval.
* Unmatched odds ratios and two-tailed P values calculated using the χ2 test.
A matched analysis using 19 cases and the 23 matched controls was performed to assess the validity of ignoring the match. The results were consistent; no items were significantly associated with illness, but Roma tomatoes had the highest odds ratio (mOR 3·0, 95% CI 0·6–16·3, P=0·20). As an additional validation measure, the converse was done for Phase I. An unmatched analysis of Phase I Pennsylvania data, including 21 cases and 47 controls, revealed results consistent with those of the matched analysis presented, indicating that tomatoes (crude OR 2·9, 95% CI 0·9–8·8, P=0·06), cheese (crude OR 2·4, 95% CI 0·8–7·2, P=0·12) and lettuce (crude OR 3·0, 95% CI 0·9–9·6, P=0·06) appeared to be associated with illness. Also, after returning from the field, all analyses described above were performed using exact statistical tests, and results were again consistent.
Managers of three Pennsylvania restaurants where nine patients had consumed Roma tomatoes were contacted to learn more about their tomato handling practices. Each of these managers reported receiving whole Roma tomatoes only, storing them for a maximum of 3 days, and processing them by washing and slicing on the day of serving.
Traceback and environmental investigation
Roma tomatoes from two restaurants, each with at least two associated cases, were traced back through two distributors to a common packing house in Florida. Environmental investigation and sampling of this packing house during December 2004 did not reveal a source of contamination. The farm which was suspected to have supplied these tomatoes was not in operation during the environmental investigation. The packing house appeared to be following food safety guidelines, including routine inline chlorination of dump tank water and monitoring the water temperature to keep it at or above that of the tomatoes.
DISCUSSION
This large multistate outbreak of S. Braenderup infections was associated with exposure to Roma tomatoes in restaurants. An initial questionnaire did not implicate a specific food category. However, after identifying more specific exposures in restaurants, a strong association between infections with S. Braenderup and consumption of Roma tomatoes outside the home was demonstrated. Given the additional information from patient cohorts and traceback investigation, Roma tomatoes were implicated as the likely source of this outbreak. Illnesses among patients who were not known to have eaten Roma tomatoes at a restaurant may be explained by poor recall, cross-contamination, secondary infections or home Roma tomato consumption. This is the first S. Braenderup outbreak we are aware of to have implicated a produce item as the vehicle of infection.
Efforts to increase the epidemiological ‘signal-to-noise ratio’ can be applied for both disease and exposure. The use of pathogen subtype testing, such as PFGE in this outbreak, has allowed more accurate identification of outbreak-related cases for certain pathogens, distinguishing them from unrelated background cases. However, an equally specific identification of exposure is a persistent challenge. In this case, evidence of suspicious exposures (cheese, lettuce and tomatoes) and information about the time, date, location and context (meal) of these exposures, combined with a reliable source of further information (restaurant managers), allowed exposure ‘subtyping’ sufficient to implicate a specific food item. In situations where many restaurants are involved, telephoning restaurant managers to ask a limited set of questions is a method that has the advantages of being simple, rapid, inexpensive and easily replicable.
Efforts to subtype exposure and reduce misclassification may be useful in other field investigations. When outbreaks do not occur in a single location or event, or the outbreak vehicle is an easily forgotten or an unapparent item to the consumer, it may not be possible to initially generate focused hypotheses. More narrow hypotheses generated from an initial analytical study can be used to perform a second analytical study if sufficient contextual information is available.
Although we used this method for food items, it could be used for any type of risk factor that can be divided into epidemiologically relevant, more useful categories. For example, after an initial case-control study, investigators of Salmonella outbreaks in 1990 and 1993 suspected tomatoes as the source, but could not exclude lettuce [Reference Hedberg5]. Tomatoes and lettuce were then traced back to their packing house of origin, and odds ratios were calculated based on the proportions of ill and well persons who consumed tomatoes from different packing houses, implicating one specific tomato packing house.
This method of obtaining more specific exposure data through a second analytical study can be used with a matched or unmatched study design; however, it is easier to apply in an unmatched setting. In a matched design the inability to obtain additional exposure information for some individuals can result in the loss of entire strata. Ignoring the match is not ideal because of the potential introduction of bias if matched groups are not exchangeable.
Salmonella and tomatoes
Most of the estimated 1·4 million Salmonella infections that occur each year in the United States are caused by contaminated foods [Reference Mead1]. Foods of animal origin are traditionally associated with Salmonella outbreaks. However, fresh produce is responsible for a growing proportion of outbreaks, increasing from 0·7% of all foodborne outbreaks with a known food item in the 1970s, to 6% in the 1990s [Reference Sivapalasingam6]. Unlike meats, eggs and dairy products, produce is often eaten raw, and preventing produce-related infections is a unique challenge. Tomatoes in particular are popular worldwide; five billion pounds of fresh market tomatoes, mostly uncooked, are consumed annually in the United States [7]. In response to the increase in produce-associated foodborne disease outbreaks, the FDA has initiated a focused strategy directed at produce production and processing practices in order to prevent produce-related outbreaks [8].
Salmonella outbreaks have been linked to tomatoes since 1990, and have since increased in frequency and magnitude. Nine tomato-associated outbreaks of Salmonella were reported in North America from 1990 to 2004 [Reference Hedberg5, 9–Reference Gupta and Crowe13], causing a total of 1616 illnesses (Table 3). Considering that approximately 1 of every 38 cases of salmonellosis is reported to public health authorities, we estimate that tomatoes have caused approximately 61 000 outbreak-related Salmonella illnesses since 1990 [Reference Voetsch14]. All but two of these outbreaks were multistate, indicating contamination occurred before the tomatoes were distributed. In one single state outbreak, contamination of tomatoes was probably amplified at a processing plant that diced tomatoes before they were supplied to a large tourist resort; cases were recognized in nine states [Reference Srikantiah12].
Table 3. Tomato-associated Salmonella outbreaks in North America, 1990–2004

The contamination event in this outbreak must also have occurred before the tomatoes were distributed, but the actual point of contamination was not identified. Contamination could occur at several steps along the path from farm to table. Tomato seedlings have been shown to absorb some Salmonella serotypes from hydroponic fluids, although whether this contamination persists to the adult plant has not been demonstrated [Reference Guo15]. Contamination of seedlings in the greenhouse was not investigated in this outbreak. Tomato flowers inoculated experimentally with Salmonella produce contaminated fruit [Reference Guo16]; this could occur in the tomato field, from either irrigation or ground water. Contamination may also be introduced, or existing contamination could be amplified, during processing at the packing house, where thousands of pounds of tomatoes pass through a common water bath [Reference Rushing, Angulo and Beuchat17]. This was the suspected mechanism of contamination in several earlier outbreaks [Reference Hedberg5]. Fresh tomatoes placed in water colder than the tomatoes themselves have been shown to draw water into their interior, along with bacteria present in the water [Reference Bartz and Showalter18]. Maintaining appropriate water temperature, pH and chlorine levels in the water bath decreases but may not eliminate the risk of contamination [Reference Zhuang, Beuchat and Angulo19]. As these procedures were reportedly in place, water bath contamination does not readily explain this outbreak.
Inactivation of Salmonella on the tomato itself is extremely difficult without cooking, even if the tomato is treated with highly concentrated chlorine solution [Reference Zhuang, Beuchat and Angulo19], and such interventions would have no effect on Salmonella inside the tomato. Therefore, interventions should focus on preventing tomato contamination, cross-contamination and amplification from occurring. Pre-slicing of tomatoes has been suspected to amplify pre-existing contamination [Reference Cummings10, Reference Srikantiah12] and Salmonella can multiply on cut surfaces of a tomato at ambient temperature [Reference Lin and Wei20], but the possible contribution to outbreaks has not been established.
CONCLUSIONS
Two practical issues with this field investigation may limit our conclusions. A concurrent but separate outbreak associated with Roma tomatoes and caused by S. Javiana and other serotypes complicated this investigation, as it received media attention and may have caused confusion among some patients and controls [9]. Moreover, the second phase of the case-control study was performed on a subset of patients and controls, and the process of repeated hypothesis generation may have inflated the importance of the P value. However, the epidemiological investigation in combination with information from patient cohorts and traceback investigation provided sufficient evidence for public health action in an outbreak setting.
Exposure ‘subtyping’ through an iterative approach was critical to this investigation, and may be useful in a variety of types of outbreak investigations. Public health officials should keep this method in mind when in the field designing epidemiological studies to investigate outbreaks.
With the use of molecular subtyping strategies, such as PFGE, multistate outbreaks are more readily identified; however, carrying out investigations across multiple jurisdictions leads to other challenges. The difficulty encountered in enrolling sufficient cases in this outbreak illustrates the need for a standardized approach to outbreak investigation, and as well as the need for establishing a mechanism for efficient decision-making among multiple state and local health departments.
Public health professionals should be aware of tomatoes as a possible vehicle when investigating Salmonella outbreaks. Current knowledge of mechanisms of tomato contamination and methods of elimination of Salmonella on or in fruit are inadequate to define interventions that will assure produce safety and prevent similar outbreaks. Rigorous studies of these issues should be a priority for the agricultural community, food safety agencies and public health agencies.
ACKNOWLEDGEMENTS
We gratefully acknowledge E. Kirner, V. Kistler and B. Marks of the Allentown, Pennsylvania Health Bureau; L. Brink of the Allegheny County Health Department; V. Dato, L. Meloro, W. Miller, M. Moll, K. Waller, K. Warren, A. Weltman and C. Yozviak from the Pennsylvania Department of Health; and C. Braden, M. Lynch and R. V. Tauxe from the Foodborne and Diarrheal Diseases Branch, CDC, for their invaluable support with the investigation. We are also indebted to public health, food regulatory and laboratory personnel from the affected states, our US FDA partners, and the study participants for their assistance.
DECLARATION OF INTEREST
None.







