Introduction
Urinary tract infection (UTI) in pregnancy represents one of the most prevalent infections in this young, relatively healthy population [Reference Dielubanza and Schaeffer1]. It is well established that asymptomatic bacteriuria (ABU) occurs in 2–10% of pregnancies. Early studies have shown that when left untreated, approximately 30% of women developed pyelonephritis, and that both ABU and pyelonephritis were associated with poor obstetric outcomes, including preterm labour and low birth weight [Reference Smaill and Vazquez2]. Therefore, current guidelines recommend routine urine cultures during pregnancy, and antimicrobial treatment for positive urine cultures [Reference Nicolle3], although this concept is currently being challenged [Reference Kazemier4].
Extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-E) have been a major health concern among hospitals and long-term care facilities, mostly affecting elderly patients with significant co-morbidities. However, they are also increasingly implicated as causes of community-acquired infections. In recently published data, the global prevalence of ESBL-E rectal colonisation among healthy individuals was 14%, varying greatly geographically, with the highest rates in developing countries [Reference Karanika5]. Previously recognised risk factors for ESBL-E included: prior antibiotic use [Reference Ben-Ami6–Reference Reuland9], prior hospitalisation, residence in a long-term care facility, age above 65 years, male gender, [Reference Ben-Ami6, Reference Doi7] prostate disease [Reference Azap8], prior episodes of UTI, proton pump inhibitor use and travel [Reference Reuland9]. Among pregnant women, rectal colonisation of ESBL-E near term was found to be 2.9–18.5%, with the highest rates reported among women in Africa [Reference Rettedal10, Reference Chereau11]. Furthermore, a study done at an antenatal clinic in India found that 45% of Escherichia coli isolates from the urine of both symptomatic and asymptomatic pregnant women had an ESBL phenotype [Reference Sabharwal12].
Currently, there is a paucity of data regarding ESBL-E UTI/ABU during pregnancy. The aim of this study was therefore to assess the risk factors, clinical course and obstetric outcomes of pregnant women with ESBL-E-positive urine cultures, as opposed to pregnant women with non-ESBL-producing Enterobacteriaceae UTI/ABU.
Methods
This retrospective matched case–control study was performed at Soroka Medical Center, a 1100 bed tertiary care hospital in southern Israel. This is the only medical centre providing obstetrics and gynaecology services to over 1 million residents in the Negev region. After obtaining permission and waiver of informed consent from the institutional ethical review board, data were obtained from the microbiology laboratory on all positive urine cultures, taken in the obstetrics and gynaecology wards at our institution, between 1 January 2004 and 30 June 2015 (Fig. 1). Medical files were reviewed, and only women, who were pregnant at the time of specimen collection, were included in the study. Urine cultures were taken by the treating physicians, at their clinical judgement, upon admission to the hospital or during hospitalisation. Files were then reviewed by the authors, and judged to be ABU or symptomatic infection. Cystitis was defined by the presence of frequency, urgency, dysuria or abdominal pain other than contraction, and pyelonephritis was considered when either flank pain, fever or chills were documented. All other clinical scenarios were classified as ABU.
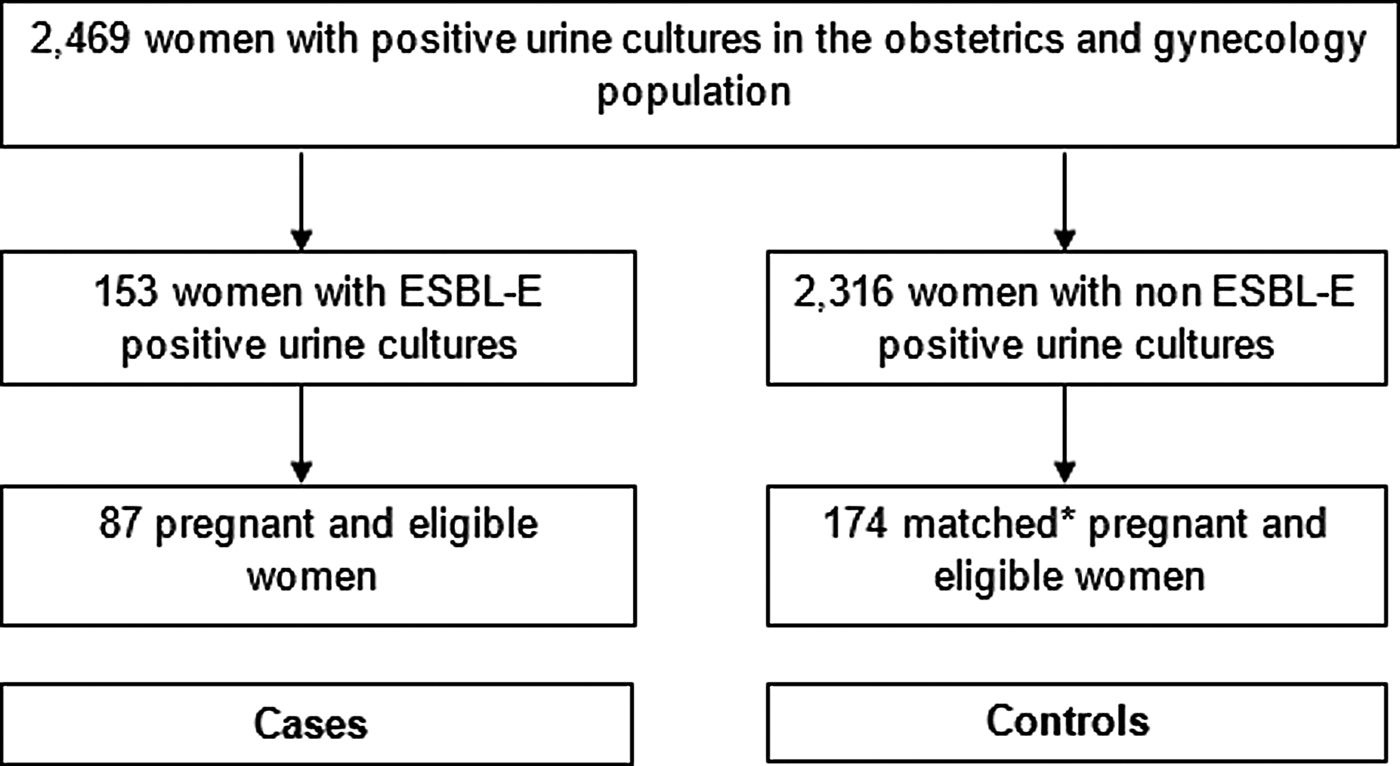
Fig. 1. Selection of study population. *Controls were selected using frequency matching according to the year of the positive urine cultures, the women's age decade, ethically (Bedouin Arab vs. non-Bedouin Arab) and pregnancy trimester.
Identification of an ESBL-E phenotype was performed by disc diffusion tests using ceftazidime/clavulanate 30/10 µg and cefotaxime/clavulanate 30/10 µg combination discs, compared with ceftazidime 30 µg and cefotaxime 30 µg discs alone (Oxoid Ltd, Basingstoke, UK). An increase in a zone diameter >5 mm for either antimicrobial agent in combination with clavulanate vs. the zone diameter of the agent when tested alone defined ESBL-E according to the Clinical and Laboratory Standards Institute (CLSI) antimicrobial susceptibility testing standards [13]. Prior to 2008, the ESBL-E phenotype was defined as resistance to third-generation cephalosporins.
After obtaining information on pregnant patients with an ESBL-E urine isolate (cases), two pregnant patients with a non-ESBL-E urine isolate were matched as (controls) according to the year of the positive cultures, age decade, ethnicity (Bedouin Arab vs. non Bedouin) and pregnancy trimester. All other information collected was unmatched. These parameters were chosen, as both age and infection in late pregnancy, may pose a risk for adverse pregnancy outcomes [Reference Luke and Brown14, Reference Wing, Fassett and Getahun15], and there are discrepancies in access to medical care within different ethnic groups in our community. Matching was done using frequency matching in the controls, in order to get the same distribution of variable as in the cases and to minimise time differences effects [Reference Wacholder16]. After defining our groups according to the urine culture taken within the hospital, data were extracted both from medical records at our institution, and a computer database system, which included comprehensive information on all cultures taken in both the ambulatory and inpatient settings, and information on all hospital admissions, nationwide. Previous isolation of ESBL-E was defined as any prior positive culture with this phenotype. Other details, such as underlying medical conditions, obstetric history and antibiotics prescribed by the treating physician in the prior 6 months, were also recorded. Hospital-acquired UTI/ABU was defined as urine cultures obtained 72 h or more following hospitalisation, or from patients who were hospitalised within the previous 2 weeks.
Statistical analysis was performed using IBM Statistics SPSS (version 22). Univariate analysis was used to describe the groups. Bivariate analysis was used to compare baseline characteristics, risk factors for the development of ESBL-E infections, clinical course and obstetric outcomes for categorical variables by Pearson's χ 2 test. Normally distributed quantitative variables were analysed with either Student's t test or one-way analysis of variance test as appropriate. Abnormally distributed quantitative variables were processed using Mann–Whitney test or Kruskal–Wallis test. We also analysed the association between prior UTI/ABU episodes, previous isolation of ESBL-E in urine cultures and prior antibiotic exposure and ESBL-E-positive urine culture, using logistic regression analysis. ESBL-E-positive urine culture was defined as the dependent variable. Prior UTI/ABU episodes, previous isolation of ESBL-E in urine cultures and prior antibiotic exposure were defined as the independent variables. We employed two-tailed tests with P = 0.05 for statistical significance.
Results
We identified 2469 hospitalised women with positive urine cultures, and of these, 153 cultures were ESBL-E-positive. After reviewing the medical charts, excluding 48 non-pregnant women, and 18 women for whom data were unavailable, 87 cases were included and randomly matched to 174 pregnant controls (Fig. 1).
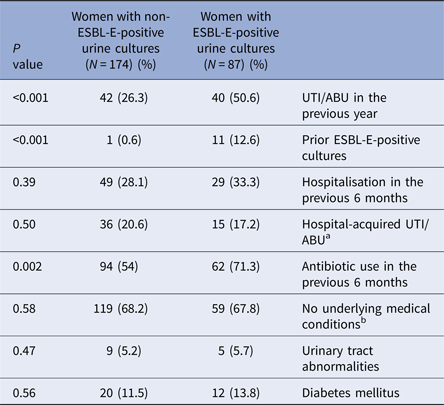
Baseline characteristics of the study groups (Table 1) were similar overall in both groups. There was also no significant difference in various risk factors for adverse pregnancy outcomes including the proportions of nulliparity (34.5% vs. 33.9%, P = 0.77), pregnancy after fertility treatments (6.9% vs. 6.3%, P = 0.85) and most notably high-risk pregnancy (36.8% vs. 44.3%, P = 0.28) defined by standard obstetric criteria [Reference Riley and Stark17]. The majority of the ESBL-E group were E. coli (64.4%), but a significantly higher proportion of Klebsiella spp. isolates was evident in the ESBL-E group than in the non-ESBL-E group (31.1% vs. 17.8%, P = 0.01). Comparison of risk factors for ESBL-E between cases and controls showed a significant difference in: prior UTI/ABU episodes (50.6% vs. 26.3%, P < 0.001), previous isolation of ESBL-E in urine cultures (12.6% vs. 0.6%, P < 0.001) and prior antibiotic exposure in the last 6 months (71.3% vs. 54%, P = 0.002) (Table 2). No significant difference was found in other parameters including diabetes mellitus (13.8% vs. 11.5%, P = 0.56), urinary tract abnormalities (5.7% vs. 5.2%, P = 0.47) and previous hospitalisation (33.3% vs. 28.1%, P = 0.39). We observed hospital-acquired infections in 17.2% of cases, and 20.6% of controls (P = 0.50). Multivariate analysis identified previous isolation of ESBL-E in urine cultures (P < 0.001) and prior UTI/ABU episodes (P = 0.03) as statistically significant predictors of ESBL-E-positive urine culture during pregnancy.
Table 1. Comparison of demographic and obstetric characteristics in the study groups
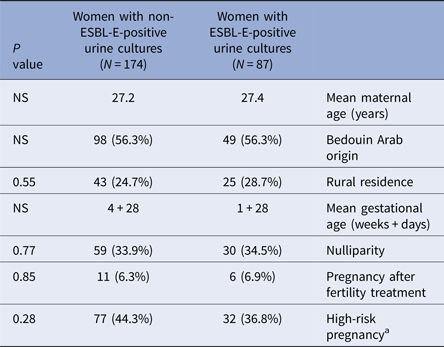
a Defined as the presence of one or more of the following: age ⩾35 years, chronic medical conditions (e.g. diabetes mellitus, hypertension, epilepsy), multiple gestation pregnancy, cervical insufficiency, history of late abortion or preterm labour.
Table 2. Comparison of risk factors for the development of ESBL-E infections in the study groups
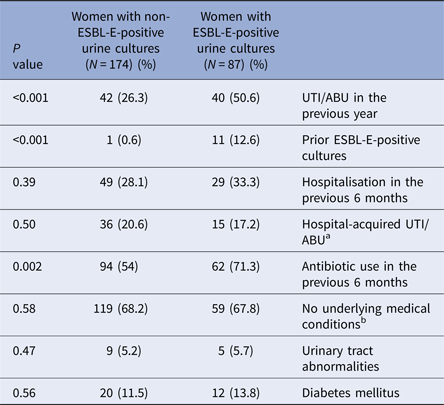
a Defined as urine cultures obtained 72 h or more following hospitalisation, or from patients who were hospitalised within the previous 2 weeks.
b Defined as any chronic medical condition other than anaemia, including: hepatitis, Antiphospholipid syndrome, epilepsy, psychiatric problems, hypertension, thyroid disease, asthma and chronic steroid treatment.
Clinical manifestations were similar in both study groups. The majority of cases and controls were classified as ABU (47.1% vs. 43.1%, P = 0.70). Presentation as cystitis was observed in 28.7% of cases and 30.5% of controls (P = 0.70), and 23% of both groups presented with pyelonephritis (P = 1). Complications in both groups were uncommon; bacteraemia occurred in four cases and three controls (4.6% vs. 1.7%, P = 0.30), and three women, one case and two controls, were admitted to intensive care unit. Creatinine levels were within normal range in all women.
Obstetric outcomes are presented in Table 3. No significant difference was found between case and control groups in any of the various adverse maternal and neonatal outcomes assessed, including preterm labour (25.3% vs. 29.9%, P = 0.51) and low birth weight (20.7% vs. 26.4%, P = 0.48).
Table 3. Comparison of obstetric outcomes in the study groups
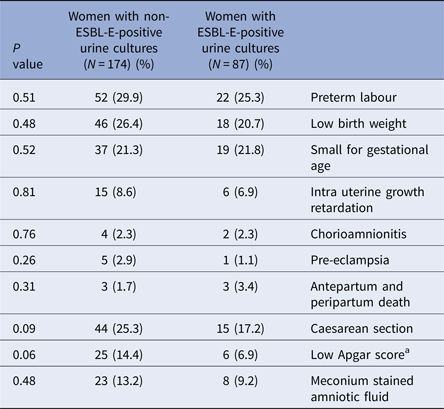
a Apgar score was defined as low if either the 1 or 5 min score was <7.
Discussion
Significant associations were found between prior episodes of UTI/ABU and ESBL-E bacteriuria during pregnancy, as well as prior history of ESBL-E in urine cultures and prior antibiotic use. As opposed to previously published studies [Reference Ben-Ami6, Reference Doi7], underlying medical conditions and prior hospitalisation were not found to be associated with an increased risk for ESBL-E bacteriuria. The association between antibiotic exposure and the risk for harbouring an ESBL-producing isolate, especially in community-acquired infection/carriage, is well described in recent years, in keeping with the persistent rise in the prevalence of these infections [Reference Karanika5, Reference Azap8, Reference Reuland9]. Our data imply that even in hospitalised pregnant women, the role of factors associated with community-acquired infection, such as prior antibiotic use, is of greater importance than prior health care exposure, making ESBL-E infection/colonisation an important public health issue. This may help clinicians who face the need to choose an empiric therapy for pregnant women with a UTI, select an appropriate regimen, especially in areas with a high prevalence of ESBL-E pathogens.
The effect of ESBL-E on the clinical course in controversial. Most studies show a trend towards higher morbidity and mortality in patients with ESBL-E infections in the non-pregnant general population [Reference Schwaber and Carmeli18–Reference Rottier, Ammerlaan and Bonten20]. However, Denis et al. [Reference Denis21] showed no significant difference in the clinical outcomes of adults with ESBL E. coli bacteraemia. Most of the patients described in these studies were bacteraemic, with differences in empiric treatment. In our study, in which 75% of patients presented with a mild infection or ABU, we found a similar clinical course and a relatively low complication rate. This can probably be attributed to the low rate of severe infections, but it is important to point out that that in our specific setting, these pathogens on their own were not associated with a worse clinical outcome.
When examining wider maternal and neonatal outcomes, including those associated with UTI/ABU such as low birth weight and preterm labour [Reference Smaill and Vazquez2], we found no significant differences between cases and controls. Of note, as our studied population was composed of hospitalised women, we had a high, and yet similar, rate of pregnancies defined as ‘high-risk’ (36.8% vs 44.3%) according to standard obstetric criteria [Reference Riley and Stark17]. As such, the two groups were relatively homogeneous in their risk factors for adverse pregnancy outcomes, other than their ESBL-E status. The high rates of preterm labour (25.3% vs. 29.9%), noticed in our study, corresponds with the prevalence of high-risk pregnancies, and did not differ between groups. Having a higher prevalence of a somewhat rare outcome in our study makes the statistical power of similarity stronger, but makes it less applicable to the general healthy population. As for the contribution of the infection itself to the high rate of preterm labours, our study was not designed to answer this question.
As discussed above, the proportion of severe infections (e.g. pyelonephritis, ICU patients) was relatively low in our study group, representing real-life experience of the clinical presentation of bacteriuria in pregnant women, with the majority presenting with mild to no clinical symptoms. Evidently, this is both an advantage of our work, being relevant to daily practice, but also less applicable for a scenario of the severely infected patient.
The main limitation of our study was its retrospective nature. As such, the isolates were not available for further molecular testing and typing or identifying mechanisms or resistance. Our study population were hospitalised pregnant women with higher rates of complicated pregnancies, which limits the generalisation of the results.
In conclusion, our study examined the risk factors and outcomes of a widening and yet not extensively described phenomenon of ESBL-E bacteriuria in pregnant women. In an era of shifting trends in risk factors for infection with resistant bacteria, we found that prior antibiotic exposure was associated with ESBL-E bacteriuria; however, we did not find ESBL-E bacteriuria to be associated with a more severe clinical course or adverse pregnancy outcomes, as compared with non-ESBL-E bacteriuria.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.