Introduction
Cervical cancer is the fourth most common cancer in women globally [Reference Vu1], with more than 500 000 new cases and 300 000 deaths worldwide every year [Reference Cohen2]. The burden faced by developing countries is significantly greater than developed countries due to the lack of resources and infrastructure such as organised vaccination and screening programmes for cervical cancer [Reference Vu1]. There are about 130 000 new cases each year in China, accounting for 18.6% of the world's total new cases [Reference Chen3]. Persistent infection of high-risk human papillomavirus (HPV) is the main cause of cervical cancer [Reference Crosbie4]. HPV DNA can be detected in up to 99.7% of cervical cancer patients [Reference Okunade5], making high-risk HPV detection an effective screening method for cervical cancer.
Based on the pathogenicity, the high-risk HPV genotypes were divided into 15 types, which were closely related to cervical cancer and cervical intraepithelial neoplasia (CIN). They were HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66 and 68, respectively [Reference Schiffman6]. Currently, it has been proven that HPV16 and 18 are the most virulent high-risk genotypes, accounting for about 70% of all invasive cervical cancer worldwide [Reference Chan7]. The main HPV screening technology used in China is hybrid capture 2 (HC2), which was the earliest HPV detection technology used in clinics and could detect 13 kinds of high-risk HPV (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68). However, HPV16 and 18 could not be typed [Reference Dong8]. In clinical application, women with high-risk HPV16/18 positive and normal cytology could not get timely vaginoscopy referral [Reference Wang9]. The Cobas HPV test was a diagnostic technique in vitro for cervical cancer screening in recent years, which can detect 14 high-risk HPV subtypes, specifically report the results of high-risk HPV16 and 18 subtypes, and provide pooled results for the 12 other high-risk HPV subtypes (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68) [Reference Abraham and Stenger10]. In this study, we aimed to compare the consistency between the Cobas HPV test and the HC2 test, in order to evaluate the performance of the Cobas HPV test technology in cervical cancer screening.
Methods
Study design and population
This is a cross-sectional analysis, and a total of 7004 women were recruited into the Chinese public welfare project for two types of cancer screening (cervical cancer and breast cancer, 2015) in Guangzhou Women and Children's Medical Center from December 2016 to June 2018. All of the participants were migrant workers, who lack regular cervical cancer screening. Before the HPV test, a questionnaire was conducted on the subjects’ education, marriage, contraceptive methods and cognition of cervical cancer. Participants were included if they met the following inclusion criteria: (1) subjects received both Cobas HPV test and HC2 test; (2) age ⩾20 years and (3) subjects with a history of sexual life, non-pregnancy, no history of cervical surgery. This study was approved by the Institutional Review Board (IRB) of the Guangzhou Women and Children's Medical Center, and written informed consent was obtained from all participants.
Screening procedures
Established files and registers, and provided informed consent and epidemiological investigations for women who met the screening conditions and voluntarily accepted the screening, and carried out Cobas HPV test and HC2 test (the primary screening). The positive results were detected by the liquid-based cytology (LBC) test (the second screening), and those with cytological abnormities or positive Cobas HPV16/18 were referred to colposcopy for examination. If necessary, a cervical biopsy was performed under colposcopy. The pathological diagnosis was performed by qualified pathologists in Guangzhou Women and Children's Medical Center. The accuracy of the screening methods was evaluated by pathologically confirmed cases of CIN2 or above.
HC2 test
HC2 was a nucleic acid hybridisation detection method using microplate chemiluminescence for signal amplification, which could detect 13 types of high-risk HPV DNA (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68) at one time by 96-well plate method, and simultaneously detect the viral load of HPV DNA in the samples. The HC2 test was performed by using the HC2 sample conversion kit (Qiagen) for the PreservCyt medium. If the relative light unit/cutoff ratio of the sample was ⩾1.0, the sample was recorded as positive.
Cobas HPV test
Cobas HPV test used the same specimens as the LBC test. Cobas HPV test is an in-vitro quantitative detection technique for high-risk HPV DNA by polymerase chain reaction amplification, and can detect HPV16, 18 and other 12 types of high-risk HPV types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68), respectively. The Cobas HPV test was carried out according to the manufacturer's protocol [Reference Rao11]. Interpretation of the amplification and detection stage was carried out using software supplied with the Cobas 4800 platform.
LBC test
The diagnostic criteria of the Bethesda System (TBS, 2001) [Reference Solomon12], LBC and sedimentation thin-layer methods were used for the LBC test. The reports were interpreted for negative for intraepithelial lesion or malignancy (NILM), atypical squamous cells of undetermined significance (ASCUS), atypical squamous cells cannot exclude HSIL (ASC-H), low-grade squamous intraepithelial lesion (LSIL) and high-grade squamous intraepithelial lesion (HSIL). The diagnosis result of NILM is considered normal, and the positive result of any one of ASCUS, ASC-H, LSIL and HSIL is considered abnormal.
Colposcopy test and cervical biopsy
Five percent acetic acid solution was applied on the surface of the cervix. After 1 min, the changes in cervix were observed under a colposcope. Preliminary diagnosis was made according to the thickness, extent, surface morphology and turbidity of the acetowhite epithelium. Healthy cervix had no acetowhite epithelium. LSIL included HPV infection and CIN1, which has a light acetowhite epithelium and could be at or outside the junction of squamous column. HSIL included CIN2, CIN3 and cervical carcinoma in situ. HSIL was characterised by thick acetowhite epithelium with clear boundary and was located near the junction of squamous column. Invasive carcinoma of cervix was featured with irregular, thick and brittle masses on the surface of the acetowhite epithelium. Cervical biopsy was performed under a colposcope. If lesions were visible under the colposcope, biopsy was taken directly at the lesion site, and if there were no visible lesions, biopsy was not performed.
Statistical analysis
SAS 9.4 software (SAS Institute, Inc., Cary, North Carolina) was used for statistical analysis. Quantitative variables were tested by the t-test and expressed as mean ± s.d. Categorical variables were analysed by the chi-squared test (χ 2 test) or the Fisher's exact test and displayed as number (n) and percentage (%). The Cohen's kappa was calculated to compare the HC2 test and Cobas HPV test results.
When evaluating the screening efficacy of the Cobas HPV test and LBC test in detecting HSIL, pathological diagnosis was used as the gold standard and CIN2+ and CIN3+ were the disease endpoints respectively. MedCalc 14.8.1 software (Medcalc Software bvba, Ostend, Belgium) was used to calculate the sensitivity, specificity, positive-predictive value, and negative-predictive value (NPV) of Cobas HPV test and LBC test. All statistical analyses were the two-sided test. The 95% confidence intervals (95% CIs) were calculated, and P < 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 3442 women met the inclusion criteria (Fig. 1). The mean age of subjects was 42.23 ± 8.16 years. Of these women, 3270 were the Han nationality (95.00%), 723 were primary school education level or illiterate (21.00%), 2255 graduated from junior middle schools or senior high schools (65.50%) and 464 from junior colleges or above (13.50). Their educational levels were concentrated in the lower educational levels. Among them, 2882 were married (82.00%), 327 were unmarried and had sex (9.5%) and 293 were divorced, separated or widowed (8.5%). In total, 2168 participants had never received a formal gynaecological examination (62.99%), and 585 had received cervical HPV or LBC tests in the past 3 years (17.00%) (Table 1).

Fig. 1. Flowchart of the screening profile.
Table 1. Baseline characteristics of the subjects

Comparison of Cobas HPV test and HC2 test (the primary screening)
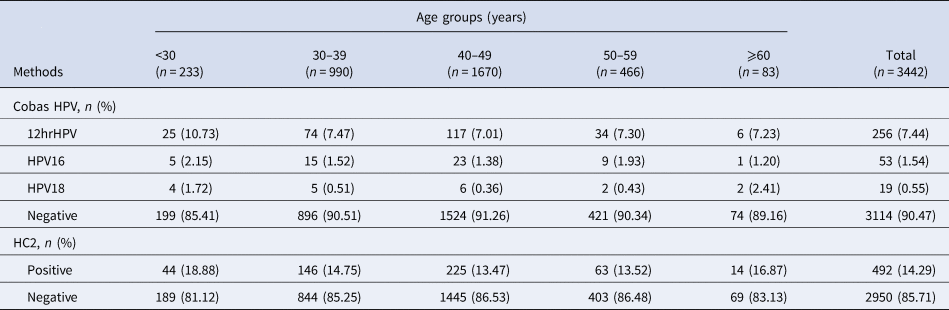
Totally, 328 cases were Cobas HPV positive, and the positive rate was 9.53% (95% CI 8.50–10.53). The positive rate of HPV16, HPV18 and other 12 types of high-risk HPV were 1.54% (95% CI 1.12–1.95), 0.55% (95% CI 0.30–0.80) and 7.44% (95% CI 6.56–8.32), respectively. A total of 492 cases were found to be HC2 positive, and the positive rate was 14.29% (95% CI 13.09–15.51). The coincidence rate of Cobas HPV test and HC2 test was 90% (95% CI 89.00–91.00), which is medium-high consistency and statistically significant (Kappa = 0.526, P < 0.001) (Tables 2 and 3).
Table 2. Screening results of the Cobas HPV test and HC2 test in different age groups

Table 3. Comparison between Cobas HPV test and HC2 test

The restricted cubic spline was used to develop a model and visualise the relationship between age and Cobas HPV positive rate and HC2 positive rate. The results showed that there was a non-linear relationship between age and Cobas HPV positive rate (χ 2 = 4.240, P = 0.040). A ‘U-shaped’ distribution was shown in the relationship between age and Cobas HPV positive rate, and there were two peak age groups, 20–29 years old and 60+ years old respectively. There may be a non-linear relationship between age and HC2 positive rate, but the non-linear test was not statistically significant (P = 0.143) (Fig. 2).

Fig. 2. Relationship between age and Cobas HPV positive rate and HC2 positive rate. (a) The Cobas HPV positive rate and age and (b) The HC2 positive rate and age.
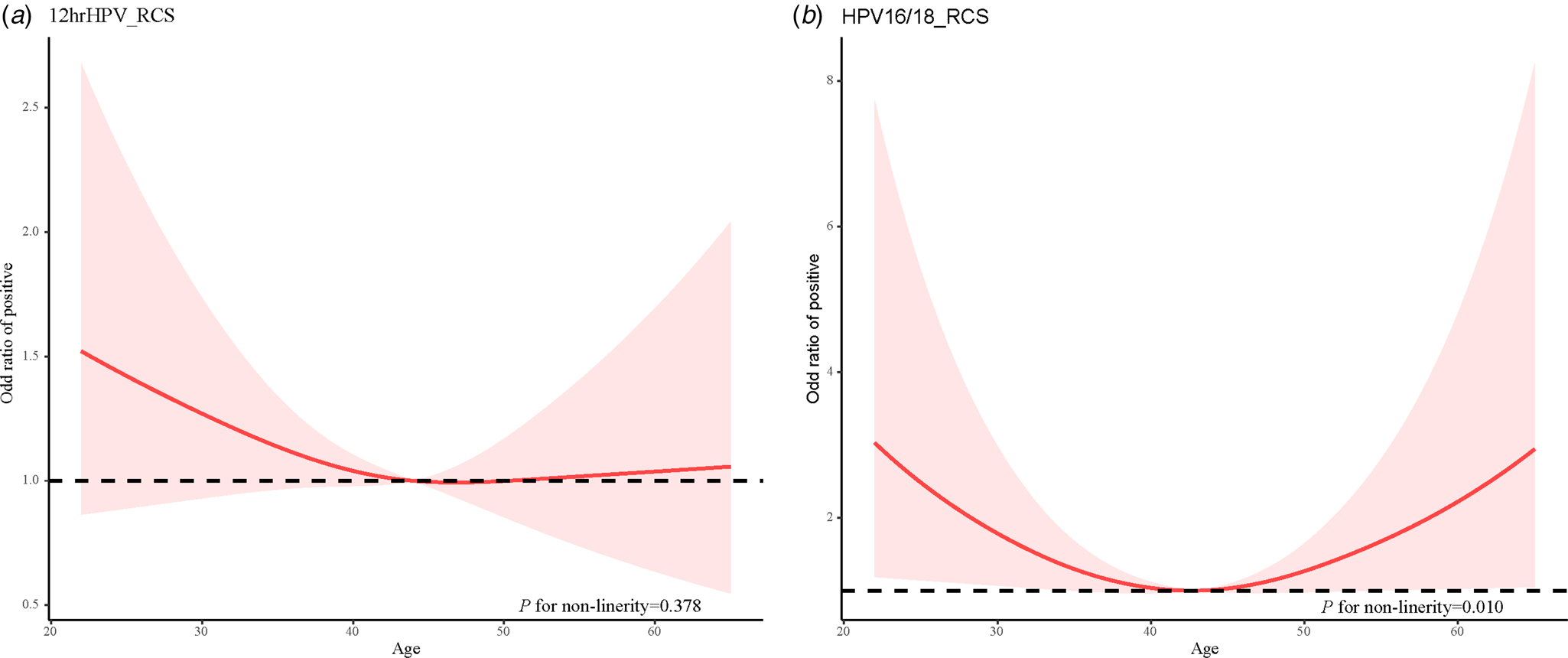
Similarly, the restricted cubic spline was used to develop a model and visualise the relationship between age and Cobas HPV typing positive rate. There was a non-linear relationship between age and HPV16/18 typing positive rate (χ 2 = 6.610, P = 0.010). The age and HPV16/18 typing positive rate was a ‘U-shaped’ distribution, and there were two peak age groups, 20–29 years old and 60+ years old respectively. In addition, there may be a non-linear relationship between age and other 12 types of high-risk HPV typing positive rate, but the non-linear test was not statistically significant (P = 0.378) (Fig. 3).

Fig. 3. Relationship between age and Cobas HPV typing positive rate. (a) The 12hrHPV positive rate and age and (b) The HPV16/18 positive rate and age.
The results of LBC test (the second screening)
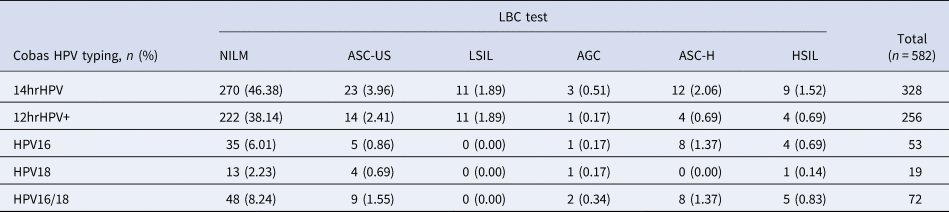
Of these 582 positive cases in the first screening, 66 cases were also positive in the LBC test (11.34%; 95% CI 8.76–13.92). The positive rate of ASC-US, LSIL, AGC, ASC-H and HSIL in the LBS test were 4.46% (95% CI 2.78–6.14), 1.89% (95% CI 0.78–3.00), 1.03% (95% CI 0.21–1.85), 2.03% (95% CI 0.88–3.18) and 1.72% (95% CI 0.66–2.78), respectively. The distribution of Cobas HPV positive rate in NILM, ASC-US, LSIL, AGC, ASC-H and HSIL were 46.38% (95% CI 42.33–50.43), 3.96% (95% CI 2.38–5.54), 1.89% (95% CI 0.78–3.00), 0.51% (95% CI 0.0–1.09), 2.06% (95% CI 0.91–3.21) and 1.52% (95% CI 0.53–2.51), respectively. The detailed results were shown in Tables 4 and 5.
Table 4. Screening results of the LBC test in different age groups

Table 5. Positive rate of Cobas HPV and Cobas HPV typing in different LBC test indicators

The results of colposcopy test and cervical biopsy (the pathological diagnosis)
Among 54 positive cases after two screenings, 20 cases were positive (37.04%; 95% CI, 24.16–49.92) according to the pathological diagnosis. The incidence rates of CIN1, CIN2, CIN3 and squamous/adenocarcinoma were 5.56% (95% CI 0.0–11.73), 12.96% (95% CI 4.00–21.92), 11.12% (95% CI 2.73–19.51) and 7.40% (95% CI 0.42–14.38), respectively (Table 6).
Table 6. Results of the pathological diagnosis in different age groups

Comparison of the efficacy of Cobas HPV test and LBC test in detecting HSIL
When CIN2+ is used as the disease endpoint to compare the efficacy of the two tests, the sensitivity ((76.5%; 95% CI 50.1–93.2) vs. (29.4%; 95% CI 10.3–56.0), P = 0.002) and NPV ((85.2%; 95% CI 70.2–93.4) vs. (75.0%; 95% CI 68.7–80.4), P = 0.042) were higher in the Cobas HPV test than those in the LBC test. Similarly, when CIN3+ is used as the disease endpoint, compared with the LBC test, the sensitivity ((80.0%; 95% CI 44.4–97.5) vs. (30.0%; 95% CI 6.7–65.2), P = 0.009) and NPV ((92.6%; 95% CI 77.9–97.8) vs. (85.4%; 95% CI 79.5–89.9), P = 0.315) were also higher in the Cobas HPV test. The Cobas HPV test is more effective at detecting CIN2+ and above than the LBC test (Tables 7 and 8).
Table 7. Distribution of CIN2+ and CIN3+ detected by the Cobas HPV test and the LBC test

Table 8. Comparison of the efficacy of Cobas HPV test and LBC test in detecting CIN2+ and CIN3+

PPV, positive predictive value; NPV, negative predictive value.
Discussion
In this study, we evaluated the performance of Cobas HPV test for cervical cancer screening in female migrant workers. The positive rate detected by the Cobas HPV test was 9.53%, and the coincidence rate of the Cobas HPV test and HC2 test was 90% in the primary screening. The Cobas HPV positive rate and HPV16/18 typing positive rate had a non-linear relationship with age. A ‘U-shaped’ distribution was shown in the relationship between age and Cobas HPV positive rate and HPV16/18 typing positive rate, and there were two peak age groups, 20–29 years old and 60+ years old respectively. Compared with the LBC test, the Cobas HPV test had higher sensitivity and NPV when detecting patients with CIN2+ and CIN3+.
Persistent high-risk HPV infection is one of the leading causes of cervical carcinoma and related deaths in women worldwide. It was reported that HPV16 and HPV18 in high-risk HPV types could cause about 70% of cervical squamous cell carcinomas and about 80% of cervical adenocarcinomas [Reference Marth13]. In China, HPV16 and HPV18 accounted for 76.7% and 7.8%, respectively [Reference Wang and Qiao14]. In April 2014, FDA approved Roche's Cobas HPV test for first-line primary screening of cervical carcinoma [Reference Abraham and Stenger10]. However, the Cobas HPV test is not included in the routine first-line screening programme for cervical cancer in China. Our study compared the performance of Cobas HPV test and HC2 test in primary screening of cervical cancer. The results showed that the coincidence rate of the Cobas HPV test and HC2 test was 90% in the primary screening for cervical cancer. Similar results were performed in the researches of other scholars using Cobas HPV and HC2 test [Reference Bottari15, Reference Huijsmans16]. In addition, the Cobas HPV positive rate and HPV16/18 typing positive rate had a U-shaped distribution with age were presented in our study, and there were two peak age groups, 20–29 years old and 60+ years old. Several studies have analysed the U-shaped functions in biological characteristics [Reference Rao17–Reference Hunt and Bowman19]. In HPV infection, some studies have found that there was a U-shaped distribution between HPV infection risk and age [Reference Rudolph20–Reference Zhang22]. The results of these studies were consistent with ours. A more detailed study displayed that those women at the age of 20–29 years old had the highest prevalence of HPV infection and a second peak was observed at the age of ⩾60 years old [Reference Liu23]. The highest incidence and prevalence of infection with high-risk HPV types usually is observed in women aged <25 years and decreases with age [Reference Fontham24, Reference Stoler25]. In addition, approximately one in five new cases of cervical cancer are diagnosed in women ⩾65 years, this phenomenon is largely attributable to a lack of screening [Reference Castle26, Reference Dinkelspiel27]. The U-shaped distribution between HPV infection risk and age suggested that cervical cancer screening may focus on people aged 20–29 and over 60.
Our study also compared the performance of Cobas HPV test and LBC test in detecting HSIL. The Cobas HPV test had higher sensitivity and NPV than the LBC test when detecting CIN2+ and CIN3+ patients. A prospective study performed that the Cobas HPV test had higher sensitivity than the LBC test in detecting CIN2+ and CIN3+ lesions, and the combination use of Cobas HPV test and LBC test can improve specificity [Reference Andersen28]. Dreyer et al. showed that the best outcomes for detection of disease were seen using the Cobas HPV test compared with the LBC test [Reference Dreyer, Maske and Stander29].
In this study, most women who participated in cervical cancer screening were married women who made a living by labour work in the city. Most of them were in the service industry and lacked the awareness of active physical examinations [Reference Holt30]. In addition, their compliance with medical advice was low, and the lost to follow-up rate was very high. Therefore, to obtain the most information from one tissue sample was a better screening plan. Cobas HPV screening using LBC specimens could achieve simultaneous detection of virus and cells in one sample, reducing sampling frequencies and the difficulty of follow-up. Besides, the samples of Cobas HPV test can last for 1 month without losing test performance compared to the LBC sample [Reference Lin31]. Furthermore, Cobas HPV16/18 positive patients can be referred to colposcopy as soon as possible [Reference Wentzensen32], which can reduce the number of return visits and avoid the possibility of missed diagnosis caused by negative results of LBC.
Conclusion
The Cobas HPV test was more sensitive than LBC in the application of cervical cancer screening, especially in a large-scale population with a high potential infection rate. Compared with the HC2 test, Cobas HPV reported the results of HPV16 and 18 while detecting the high-risk HPV, which is helpful for doctors to refer high-risk groups to colposcopy in time, and to detect and treat patients with high risks of pathological changes earlier.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards and consent to participate
This study was approved by the Institutional Review Board (IRB) of the Guangzhou Women and Children's Medical Center, and written informed consent was obtained from all participants.
Data availability statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.