INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) originated in Staphylococcus aureus from acquiring the mecA gene, and got the resistance to methicillin and other β-lactam antimicrobials [Reference Graveland1]. MRSA can cause several diseases, including minor skin and soft tissue infections and severe invasive diseases [Reference Huang2]. MRSA infections are difficult to treat, particularly if the isolates have acquired multiple antimicrobial resistance elements and become multidrug-resistant S. aureus (MDRSA) isolates [Reference Hau3]. MRSA isolates were generally classified into hospital-associated MRSA and community-associated MRSA based on epidemiological characteristics. Recently, a new kind of MRSA was found in animals such as pigs, bovine, and poultry, and was referred to as livestock-associated MRSA (LA-MRSA) [Reference Huijsdens4–Reference Mulders6]. In European countries and North America, the widest spread of LA-MRSA is clonal complex 398 (CC398), while CC9 is predominant in most Asian countries [Reference Chuang and Huang7].
Of note, more and more studies reported the detection of LA-MRSA not only in animals, but also in humans [Reference Cuny8–Reference Garcia-Graells10]. LA-MRSA could be transmitted to humans especially those workers with occupational livestock exposure such as pig-related farmers and veterinarians. Previous studies mainly focused on pig farmers and indicated that the prevalence of MRSA carriage is positively associated with occupational pig exposure [Reference Cuny8, Reference Smith9]. Such occupational exposure may occur at slaughterhouses and retail markets as well as the farm levels. Although some studies have identified LA-MRSA in slaughterhouse workers in Europe [Reference Van Cleef11, Reference Normanno12], there is still few to explore the potential relationship between phenotypic and molecular characteristics to reveal livestock-associated S. aureus in slaughterhouse workers. Therefore, we conducted this study to investigate the prevalence, antimicrobial susceptibility and molecular characteristics of S. aureus and MRSA isolates from slaughterhouse pig-related workers and control workers with no occupational livestock exposure, and tested the hypothesis that pig-related workers may have a higher prevalence of livestock-associated S. aureus (including MRSA) carriage than control workers due to occupational pig exposure.
METHODS
Ethics statement
The study was approved by the Ethics Committee of Guangdong Pharmaceutical University, and was performed in accordance with the approved guidelines. The survey was qualified as involving no risks to participants. Before participating, all participants signed an informed consent form.
Study design and population
A cross-sectional study was conducted in Guangdong Province of China between November 2013 and November 2014. Four cities from 21 cities in Guangdong Province were randomly sampled to enroll representative participants. In each city, slaughterhouses were selected to enroll about 100 pig-related workers with occupational exposure to pigs. Pig butchers were also included in pig-related workers because butchers go to slaughterhouse to kill pigs at 1–5 AM in the morning and go to the meat markets to sell pork during the day. At the same time, factories unrelated to livestock (i.e. hardware factory or biscuit factory) in each city were sampled to enroll about 200 control workers with no occupational livestock exposure. After obtaining informed consent, two nasal swabs were taken from each participant and a face-to-face questionnaire was administered to collect influencing factors of S. aureus carriage, including sex, age (15–30, 31–40, and 41–60 years), education (elementary school, junior high school, senior high school, and above), personal monthly income (⩽¥1000, ¥1001–2000, ¥2001–3000, or ⩾¥3001), occupational pig exposure (yes or no), antimicrobial use in the last month (yes or no), and visit to medical facilities in the last month (yes or no).
Bacterial isolation and identification
Swabs were stored in 5 ml of enrichment broth containing 1% tryptone, 7·5% NaCl, 1% mannitol, and 0·25% yeast extract at 4 °C during transportation and incubated at 35 ± 1 °C for 24 h. Then a loopful of the broth was plated on mannitol salt agar and incubated at 35 ± 1 °C for 24–48 h. Suspected colonies were subcultured to 5% sheep blood agar plates and incubated at 35 ± 1 °C overnight. Initial identification of S. aureus was based on Gram staining, morphology, catalase test, DNase test, and tube coagulase test.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed by the disk diffusion method of the Clinical and Laboratory Standards Institute guidelines [13]. The following antimicrobial disks were used: cefoxitin (30 µg), penicillin (10 units), clindamycin (2 µg), tetracycline (30 µg), erythromycin (15 µg), trimethoprim–sulfamethoxazole (25 µg), chloromycetin (30 µg), ciprofloxacin (5 µg), rifampin (5 µg), nitrofurantoin (300 µg), gentamicin (10 µg), and linezolid (30 µg). The reference strain S. aureus ATCC 25923 was used for quality control and S. aureus ATCC 29213 was used for positive control. S. aureus isolates were classified as MDRSA if they were non-susceptible to ⩾3 classes of antimicrobials or were MRSA isolates [Reference Magiorakos14].
Molecular characterization
All S. aureus isolates were further tested through polymerase chain reaction (PCR) assays for the carriage of 16S rRNA, nuc, and mecA genes [Reference Zhang15]. Multi-locus sequence typing (MLST) was conducted for all S. aureus isolates as previously described [Reference Enright16]. The sequence type (ST) was assigned by comparison with the MLST database (http://www.mlst.net/), and CCs were determined using the eBURST algorithm. The presence of Panton–Valentine leukocidin (pvl) and immune evasion cluster (IEC) genes (including scn, chp, sak, and sea) were determined as previously described [Reference Zhang15, Reference van Wamel17].
Data analysis
All data were entered in duplicate into an EpiData (version 3.1 database) (the EpiData Association, Odense Denmark). Categorical variables were compared by Pearson's chi-squared (χ 2) test or Fisher exact test when appropriate. The relations between pig exposure and S. aureus (including MRSA and MDRSA) carriage were examined using univariable and multivariable logistic regression models. All multivariable models were adjusted for sex, age, education, personal monthly income, antimicrobial use in the last month, and visit to medical facilities in the last month. We defined a two-sided P-value of ⩽0·05 as being of statistical significance. All statistical analyses were conducted using STATA version 14·0 (StataCorp LP, College Station, TX, USA).
RESULTS
Study population
In total, 1190 workers voluntarily participated in this study. The participants consisted of 335 pig-related workers (including 178 slaughterhouse workers and 157 butchers) and 855 control workers. There were statistically significant differences between groups with regard to sex, age, and personal monthly income (P < 0·05, Table 1). Pig-related workers were more males than control workers, and on average, pig-related workers were older than control workers (mean = 39·3 vs. 36·4 years of age; t-test, t′ = −4·45, P < 0·001). There was no significant difference in human distribution according to visit to medical facilities in the last month (P = 0·812) or antimicrobial use in the last month (P = 0·852).
Table 1. Study population characteristics by participant category
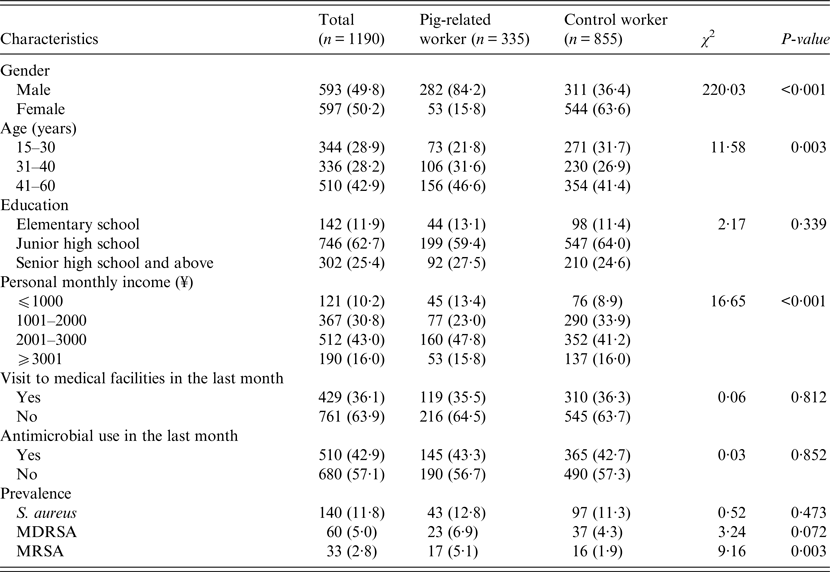
Note. Values are number of participants (proportion of participants surveyed) or as otherwise indicated.
MDRSA, multidrug-resistant S. aureus; MRSA, methicillin-resistant S. aureus.
Group differences in prevalence of S. aureus, MDRSA, and MRSA
The overall prevalence of S. aureus nasal carriage among the study population was 11·8% (140/1190), with similar prevalences between pig-related workers (12·8%, 43/335) and control workers (11·3%, 97/855) (P = 0·473, Table 1). As for MDRSA, the overall prevalence was 5·0% (60/1190, including 6·9% for pig-related workers and 4·3% for controls) and there was no significant difference between the groups (P = 0·072). The overall prevalence of MRSA was 2·8% (33/1190, including 5·1% for pig-related workers and 1·9% for controls) and was significantly higher in pig-related workers than in control workers (P = 0·003).
Relation of pig exposure with S. aureus, MDRSA, and MRSA carriage
After adjusting for gender, age, education, personal monthly income, visit to medical facilities and antimicrobial use, pig-related workers had significantly higher rates of MRSA carriage (adjusted odd ratio (aOR) 3·70, 95% CI 1·63–8·40) than control workers, whereas no significant difference was found in S. aureus (aOR 1·40, 95% CI 0·90–2·17) or MDRSA (aOR 1·72, 95% CI 0·93–3·18) carriage.
Group differences in phenotypic characteristics
Antimicrobial susceptibility testing of 140 S. aureus isolates revealed that penicillin resistance (89·3%) was the most common phenotype, followed by clindamycin (44·3%), tetracycline (37·9%), erythromycin (36·4%), and cefoxitin (23·6%), while these isolates showed slight resistance to the rest of antimicrobials (Table 2). Notably, among 140 S. aureus isolates, the proportions of tetracycline-resistant S. aureus and trimethoprim–sulfamethoxazole-resistant S. aureus were higher in pig-related workers than in control workers (51·2% vs. 32·0%, P = 0·031 and 16·3% vs. 3·1%, P = 0·015, respectively, Table 2). Additionally, the proportions of clindamycin-resistant MRSA, erythromycin-resistant MRSA, tetracycline-resistant MRSA and chloromycetin-resistant MRSA were significantly higher in pig-related workers than in control workers (Table 2).
Table 2. Group differences in phenotypic and molecular characteristics of S. aureus and MRSA carriage among study participants, in Guangdong, China

Note. Values are number of S. aureus or MRSA isolates with specific phenotype or genotype (proportion of these isolates among all S. aureus isolates) or as otherwise indicated.
MRSA, methicillin-resistant S. aureus; R, resistance; FOX, Cefoxitin; PEN, Penicillin; CLI, Clindamycin; ERY, Erythromycin; TET, Tetracycline; CHL, Chloromycetin; CIP, Ciprofloxacin; SXT, Trimethoprim–sulfamethoxazole; RIF, Rifampin; GEN, Gentamicin; NIT, Nitrofurantoin; LZD, Linezolid.
Among 60 MDRSA isolates from the pig-related workers and control workers, the dominant multidrug-resistant pattern was similar and mainly non-susceptible to clindamycin, erythromycin and tetracycline (Fig. 1). For MRSA isolates, multidrug-resistant pattern was also similar in the two groups (Fig. 1).
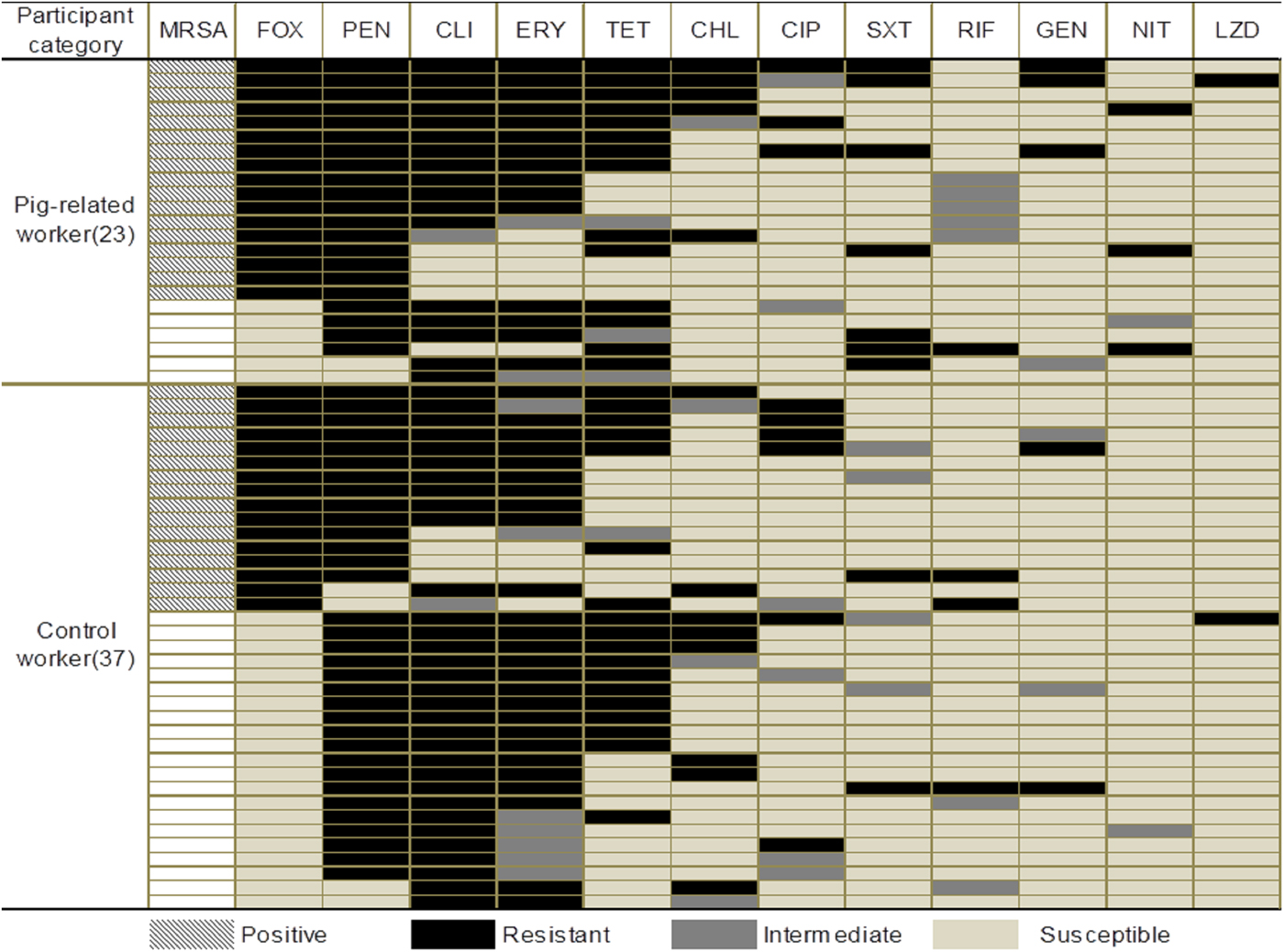
Fig. 1. Antimicrobial resistance profile of MDRSA isolates from the pig-related workers and control workers. Note. FOX, Cefoxitin; PEN, Penicillin; CLI, Clindamycin; ERY, Erythromycin; TET, Tetracycline; CHL, Chloromycetin; CIP, Ciprofloxacin; SXT, Trimethoprim–sulfamethoxazole; RIF, Rifampin; GEN, Gentamicin; NIT, Nitrofurantoin; LZD, Linezolid.
Group differences in molecular characteristics
Diversity of CC types was observed among 140 S. aureus isolates, the predominant CC types in pig-related workers were CC7 (nine isolates), CC59 (six isolates), CC6 (six isolates), and CC188 (four isolates), followed by CC9 (three isolates), CC15 (three isolates), CC45 (three isolates), and CC1 (two isolates). Similarly, CC7 (24 isolates), CC59 (13 isolates), CC6 (12 isolates), and CC188 (11 isolates) were common among S. aureus isolates from control workers (Table S1).
The pvl gene was only found in three S. aureus isolates from pig-related workers and was absent from all control workers. As to IEC genes, there were no significant differences in IEC(scn, chp, sak, sea)-negative S. aureus proportions between two groups (Table 2), while pig-related workers showed greater proportions of sak-negative MRSA and sea-negative MRSA carriage as compared with the control workers (P = 0·039 and P < 0·001, respectively, Table 2).
Overlap in phenotypic and molecular characteristics of livestock-associated CC9
For S. aureus CC9, presence of the livestock-associated characteristics (tetracycline resistance and IEC-negative) was more prevalent in pig-related workers than in control workers (7·0% vs. 0·0%, P = 0·028). All CC9 isolates observed in pig-related workers were MRSA and also resistant to tetracycline, clindamycin, erythromycin, trimethoprim–sulfamethoxazole and gentamicin, whereas the CC9 strain isolated from a control worker was resistant only to penicillin (Table S1). Moreover, all CC9 isolates from pig-related workers were IEC (scn, chp, sak, and sea)-negative, while the CC9 from control workers carried the IEC genes (scn, chp, and sak).
Similar in phenotypic and molecular characteristics of human-associated CCs
For S. aureus CC7, there was no significant difference in IEC profile between two groups (P = 0·344 for scn; P = 0·784 for chp; P = 0·175 for sak; P = 0·718 for sea) and in antimicrobial resistance between two groups (P = 0·722 for cefoxitin; P = 0·924 for penicillin; P = 0·149 for clindamycin; P = 0·344 for erythromycin; P = 0·176 for tetracycline; P = 1·000 for chloromycetin and gentamicin; P = 0·533 for ciprofloxacin; P = 0·586 for trimethoprim–sulfamethoxazole; P = 0·522 for rifampin; P = 0·093 for nitrofurantoin; all CC7 isolates were susceptible to linezolid).
As to S. aureus CC59, there was similar in IEC profile between two groups (P = 1·000 for scn and chp; P = 0·538 for sak; P = 0·930 for sea) and in antimicrobial resistance between two groups (P = 0·133 for cefoxitin; P = 0·570 for penicillin; P = 0·554 for clindamycin; P = 0·550 for erythromycin; P = 0·058 for tetracycline; P = 0·701 for chloromycetin; P = 0·307 for ciprofloxacin and nitrofurantoin; all CC59 isolates were susceptible to trimethoprim–sulfamethoxazole, gentamicin, rifampin, and linezolid).
Similarly, CC6 S. aureus exhibited similar characteristics in the IEC profile between two groups (P = 0·169 for scn; P = 0·762 for chp; P = 0·643 for sak; P = 0·701 for sea) and in antimicrobial resistance between two groups (P = 1·000 for cefoxitin and rifampin; P = 0·776 for penicillin; P = 0·371 for clindamycin; P = 0·223 for erythromycin and tetracycline; P = 0·522 for ciprofloxacin and trimethoprim–sulfamethoxazole; all CC6 isolates were susceptible to chloromycetin, gentamicin, nitrofurantoin, and linezolid).
CC188 S. aureus also showed similar characteristics in IEC profile between two groups (P = 0·553 for scn; P = 1·000 for chp, sak, and sea) and in antimicrobial resistance between two groups (P = 0·307 for cefoxitin; P = 1·000 for penicillin, tetracycline, chloromycetin, and nitrofurantoin; all CC188 isolates were susceptible to clindamycin, erythromycin, trimethoprim–sulfamethoxazole, ciprofloxacin, rifampin, gentamicin, and linezolid).
DISCUSSION
This observational study showed that human MRSA carriage is associated with occupational pig exposure. The most striking finding from this study was that the overlap of livestock-associated characteristics (tetracycline-resistant and IEC-negative) was observed only in MRSA CC9 isolated from pig-related workers and human-associated CCs had similar IEC profile and antimicrobial resistance in both pig-related and control workers.
The prevalence of MRSA carriage in pig-related workers (5·1%) in this study was comparable with data found in slaughterhouse workers and butchers in the Netherlands (5·6%) [Reference Van Cleef11], Korea (6·9%) [Reference Moon18], Hong Kong (5·6%) [Reference Boost19], and the USA (3·6%) [Reference Leibler20], but lower than prevalence among pig farm workers (farm workers in Germany [Reference Cuny8], 86%; veterinarians in Germany [Reference Cuny8], 45%; swine workers in America [Reference Smith9], 45%). Additionally, our study indicates that the prevalence of MRSA carriage is substantially higher in pig-related workers than in control workers and the prevalence is positively associated with occupational pig exposure after adjusting for other covariates, which is consistent with findings from previous studies [Reference Oppliger21, Reference Neyra22]. Notably, our recent study also has revealed that there are significant frequency-risk and duration-risk relations between occupational livestock (pig) exposure and MRSA carriage, suggesting the probability of LA-MRSA spread via animal contact, a scenario demonstrated for LA-MRSA transmission in Europe and Asia [Reference Ye23].
Since substantial amount of antimicrobials are used in livestock farming for growth promotion, prophylactic or therapeutic, increased resistance to multiple antimicrobials among MRSA isolates has become a matter of concern. A recent study conducted in Taiwan pig farms reported that multidrug resistance was prevalent, with >80% porcine MRSA isolates resistant to erythromycin, ciprofloxacin, gentamicin, tetracycline and clindamycin [Reference Lo24]. Another study in China observed that all MRSA from pig and pig farmers were resistant to cefoxitin, ciprofloxacin, clindamycin and tetracycline [Reference Cui25]. In Korea slaughterhouse, porcine MRSA were resistant to tetracycline, erythromycin, clindamycin and ciprofloxacin [Reference Moon18]. In our study, the predominant phenotype of S. aureus isolates from pig-related workers were resistant to penicillin, clindamycin, tetracycline and erythromycin, and this finding is in agreement with previous studies on pigs and related workers [Reference Moon18, Reference Lo24, Reference Cui25]. Note that tetracycline antimicrobials are commonly used in food animal production in Asian livestock farming, and high rates of resistance to tetracycline among MRSA isolates in pig and related workers have been reported [Reference Moon18, Reference Lo24, Reference Cui25]. Consistent with knowledge about the use of tetracycline in China, our study indicates that the proportion of tetracycline-resistant S. aureus (including MRSA) is significantly higher in pig-related workers than in control workers. These observations highlight the need for further surveillance data (including prevalence and antimicrobial susceptibility) to better understand the epidemiology and transmission of LA-MRSA between animals and humans.
Recently some studies have attempted to differentiate livestock-associated S. aureus from human-associated S. aureus based on the phenotypic and molecular characteristics [Reference Price26, Reference Uhlemann27]. MRSA CC9 is usually isolated from pig and related workers in most Asian countries [Reference Chuang and Huang7], which has been referred to as the molecular markers of livestock-association. In addition, there is increasing evidence of universal tetracycline resistance among MRSA isolates from livestock, related workers and environmental samples [Reference Lo24, Reference Cui25, Reference Narvaez-Bravo28], indicating that tetracycline resistance may be an important phenotypic marker for livestock-associated S. aureus. In this study, all S. aureus CC9 found in pig-related workers were MRSA and resistant to tetracycline, while the isolate from control workers was MSSA and susceptible to tetracycline. Additionally, recent evidence has revealed that the bacteriophage-encoded IEC genes are associated with human specificity and less common in livestock-associated S. aureus [Reference Spoor29, Reference McCarthy and Lindsay30], suggesting that these characteristics (IEC-negative) may be useful to define livestock-associated S. aureus. Consistent with previous studies, we found that all MRSA CC9 from pig-related workers were absent of all IEC genes, but the S. aureus CC9 from control workers carried the IEC genes (scn, chp, and sak).
Our latest study on humans with no occupational livestock exposure revealed a potential relation between S. aureus CCs and IEC genes and antibiotic resistance patterns in defining livestock-associated S. aureus in humans [Reference Fan31]. Now, we build on previous literature to compare the overlap of livestock-associated characteristics of S. aureus in pig-related workers with those in control workers. We found that the overlap of livestock-associated characteristics was only observed in MRSA CC9 from pig-related workers, indicating a potential risk for livestock-to-human LA-MRSA transmission by occupational pig exposure. Note that CC7, CC59, CC6, and CC188 were the predominant CC types among S. aureus isolates from hospitalized patients [Reference Liu32, Reference Yu33] and healthy workers in this study, indicating that these CC types probably belonged to human association. We found there was no significant difference in IEC profile or antimicrobial resistance for human-associated CCs between the groups, revealing the potential human-to-human transmission of human-associated MRSA. Future studies should direct more attention to exploring the exact transmission routes and distinguishing livestock-associated S. aureus from human-associated S. aureus according to the specific phenotypic and molecular characteristics.
This study contributed additionally to the literature by comparing the phenotypic and molecular characteristics of S. aureus in pig-related workers with control workers to explore the potential transmission routes of MRSA. However, this study also has potential limitations that cannot be ignored. Firstly, we identified MRSA from S. aureus through the detection of mecA gene and didn't detect the novel mecA homolog mecC gene, which should be improved in the further study. Secondly, although MRSA CC9 isolates were only detected in pig-related workers, we cannot draw a causal conclusion but only an association between occupational pig exposure and MRSA CC9 carriage due to the cross-sectional design. Results from this study need to be confirmed in a longitudinal study.
In conclusion, this study reports a detection of LA-MRSA CC9 isolates in pig slaughterhouse workers in China, and a significant overlap of livestock-associated characteristics occurring in LA-MRSA CC9 from pig-related workers, but not in isolates from control workers. In addition to CC9, we also found typical human-associated CCs, which have the similar IEC profile and antimicrobial resistance in both pig-related and control workers. This suggests that there may be a potential risk for livestock-to-human transmission of LA-MRSA and human-to-human transmission of human-associated MRSA.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268817000085
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (Grant No. 81602901) and the Innovation and Strong School Project of Guangdong Pharmaceutical University (Grant No. 2014KQNCX138). The funders played no role in the study design or data collection, analysis and interpretation.
DECLARATION OF INTEREST
None.





