INTRODUCTION
Wild boars (Sus scrofa) have the potential to harbour a wide range of foodborne pathogens that can cause serious illness in humans [Reference Al1–Reference Sánchez6]. Of these, Salmonella spp., Yersinia enterocolitica, Y. pseudotuberculosis and Escherichia coli O157:H7 (enterohaemorrhagic E. coli; EHEC) are of particular significance.
Salmonella spp. is the pathogen most commonly associated with outbreaks of food poisoning in Europe, with the most frequently reported serovars being Salmonella enterica subspecies (subsp.) enterica serovar Enteritidis and serovar Typhimurium, comprising 45·0% and 22·4% of the isolates, respectively [7]. Sweden, together with Norway and Finland, has a unique situation with a low number of domestic cases of salmonellosis reported in humans and with serovar Typhimurium as the most commonly isolated serovar [8]. Salmonella spp. has recently been demonstrated in European wild boars, e.g. in Switzerland and Germany [Reference Wacheck3, Reference Methner, Heller and Bocklisch9].
Another serious illness is yersiniosis, where all strains of Y. pseudotuberculosis and the biotypes 1B and 2–5 with serotypes O:3, O:5,27, O:9, O:8 of Y. enterocolitica have the potential to cause yersiniosis in humans [Reference Fredriksson-Ahomaa, Lindström, Korkeala, Jujena and Sofos10]. The majority (91%) of confirmed cases are caused by Y. enterocolitica [7]. One recent Swiss study showed that Y. enterocolitica and Y. pseudotuberculosis are also commonly found in wild boars, with a higher prevalence in younger individuals [Reference Fredriksson-Ahomaa2].
Confirmed cases of enterohaemorrhagic E. coli O157:H7 (EHEC) are generally associated with direct or indirect contact with cattle or the consumption of unpasteurized milk [Reference Aspan and Eriksson11]. However, in 2006, a large outbreak of EHEC in North America was linked to fresh baby spinach, and wild boars were suggested as the source of contamination [Reference Jay12]. Further, faeces from wild boars in Europe have tested positive for E. coli O157:H7 [Reference Sánchez6, Reference Wahlström13].
The Swedish wild boar population was established in the 1970s, when animals held for hunting and meat production escaped into the wild [Reference Thurfjell14]. The population size in the 2009/2010 hunting season was estimated to be over 150 000 and calculations based on hunting harvest, reproduction studies and population growth indicate an annual biological wild boar population growth of 48%, hunting excluded [Reference Magnusson15]. The spread and possible accumulation of pathogens within a wild boar population might be enhanced by the common use of artificial feeding places.
Hunted animals are slaughtered under various hygiene conditions, ranging from government-controlled wild game-handling establishments that sell meat on an open market, to sheds and garages at the homes of hunters. Wild boar offal and carcasses destined for the market are inspected by a veterinarian and examined for the presence of Trichinella spp., whereas meat consumed privately in the homes of hunters usually does not undergo such inspection. Hence, zoonotic pathogens that do not induce macroscopically visible lesions may go undetected, with the risk of spread to humans through consumption or handling of wild boar meat. Few studies have evaluated the occurrence of such pathogens in wild boars in Sweden [Reference Wahlström13].
The objective of the present study was thus to investigate the occurrence of the foodborne pathogens Salmonella spp., enteropathogenic Y. enterocolitica, Y. pseudotuberculosis and E. coli O157:H7 in the Swedish wild boar population.
MATERIALS AND METHODS
The study was approved by the ethical committee of Uppsala, Sweden.
Animals and sampling
The sampled material originated from an evaluation study on wild boar restraining traps. Between January 2010 and April 2011, a total of 80 wild boars were captured in traps placed close to existing artificial feeding areas for free-living wild boars on two different hunting estates in central Sweden. Further, three control animals were shot and euthanized in close proximity to the traps in the evaluation study. The trapped animals were euthanized using a small calibre rifle and then transported to the National Veterinary Institute (NVI), where they underwent necropsy in accordance with a standard protocol [16], selected specimens were collected and frozen at −20°C. In addition, a gilt submitted to the NVI for necropsy and four animals shot close to a farm with free-range domestic pigs infected with S. Derby were included. Although the farm was put under restrictive measures, it was sanitized and declared free of infection when the sampled wild boars were shot. Selected specimens from these four pigs were taken by the hunters.
The pigs consisted of 42 females and 46 males, with body weight ranging from 4·6 to 110 kg, and an estimated age of a few months up to 3 years. The weight of the animals was correlated to the presence of pathogens using Pearson's χ 2 test. For the first 36 individuals, both tonsils (with one exception) were removed and stored separately and 5–20 g faeces were collected. For the remaining 44 individuals and for the eight animals not caught in traps, the sampling also included the ileocaecal lymph nodes. The samples collected from the four animals shot close to the farm infected with S. Derby were frozen at −20°C and transported on ice to the laboratory for further analysis. All samples were stored at −20°C prior to analysis.
Sample preparation
The frozen samples were thawed, minced and homogenized for 60 s in buffered peptone water (BPW) at a dilution of 1:10 (w/w) (Kern & Sohn GmbH, Germany) before incubation at 30°C for 18 ± 2 h. Following incubation, 10 μl of the suspension was plated on selective agar plates. Yersinia spp. was cultivated on CIN agar at 30°C for 24 ± 3 h. To detect Salmonella spp., Brilliant Green (BG) and xylose lysine decarboxylase (XLD) were used, and to detect E. coli O157:H7 cefixime tellurite sorbitol MacConkey (CT-SMAC) was used. These plates were incubated at 37°C for 24 ± 3 h. From each plate, colonies with typical morphology were collected and suspended in 500 μl distilled water, lysed by boiling for 10 min, cooled on ice and centrifuged at 15 800 g for 10 min in an Eppendorf centrifuge 5424 (Eppendorf AG, Germany). Thereafter, 400 μl of the supernatant was transferred to a new microtube and stored at −20°C prior to polymerase chain reaction (PCR) analysis.
PCR analysis
All PCR analyses were performed in a 7500 Fast Real-Time PCR system (Applied Biosystems, USA). The analyses for Y. enterocolitica and Y. pseudotuberculosis targeted the chromosomally encoded attachment and invasion (ail) gene [Reference Jourdan, Johnson and Wesley17–Reference Thisted19]. A real-time PCR protocol was applied according to Lambertz et al. [Reference Lambertz18, Reference Lambertz, Nilsson and Hallanvuo20] with primers and a TaqMan MGB probe manufactured at Eurofins MWG Operon, Germany (Table 1). The PCR mixture consisted of 12·5 μl TaqMan universal PCR master mix (Applied Biosystems), 900 nm of each primer, 200 nm of the probe and 5 μl template, in a total volume of 25 μl. The PCR cycling conditions consisted of an initial denaturation of the template DNA at 95°C for 10 min, followed by 45 cycles at 95°C for 15 s and at 60°C for 60 s. An internal positive control (IPC) was added to every PCR run using a commercially available TaqMan exogenous IPC kit (Life Technologies, USA). The reagent kit included primers, a VIC probe, IPC target DNA and blocking solution. The kit was diluted ×50 to enable detection of inhibition below a cycle threshold (Ct) value of 32. To the diluted reagent kit, 6 μl ddH2O and 5 μl template were added. For Y. pseudotuberculosis, the fluorophore of the probe was identical to that of the commercial IPC probe, and therefore the IPC was added to separate wells in each run. The expected Ct value for the IPC was within the range 35–38. If the IPC Ct value was >38 and no Ct value was detected for the bacterial target, the template was diluted 1:10 with ddH2O and subjected to a second PCR.
Table 1. Primers and probes used for PCR analysis and the genes targeted
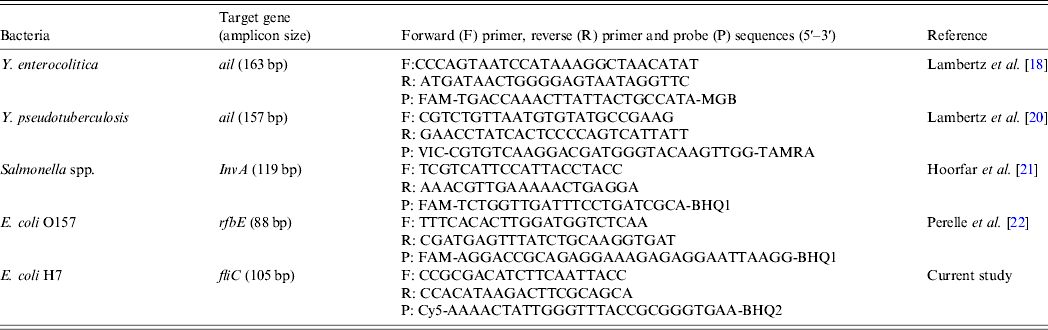
In the analyses of Salmonella spp., primers and a TaqMan probe targeting the invasion (invA) gene were used [Reference Hoorfar, Ahrens and Rådström21]. In the analyses of E. coli O157:H7, primers targeting the rfbE gene that encodes the O157 antigen [Reference Perelle22] and an in-house constructed real-time PCR for the H7 antigen targeting the fliC gene were used. All primers and probes were manufactured by Thermo Scientific Biopolymers, Germany (Table 1). Probes were labelled with 6-carboxyfluorescein (FAM) and Black Hole Quencher-1 (BHQ-1). A modified protocol based on the work of Hoorfar et al. [Reference Hoorfar, Ahrens and Rådström21] and Perelle et al. [Reference Perelle22] was used, with a PCR mixture that consisted of 7·5 μl Perfecta Q-PCR toughmix Low-ROX (Quanta Biosciences, USA), 500 nm of each primer, 100 nm of the probe, 1·5 μl of 10 × EXO IPC/VIC mix, 0·3 μl 1 × EXO IPC DNA (Life Technologies) and 2 μl template in a total volume of 15 μl. The PCR cycling conditions consisted of an initial denaturation at 95°C for 3 min, followed by 45 cycles at 95°C for 3 s and at 60°C for 30 s.
For all bacteria, a Ct value ⩽40 was considered a positive result [Reference Lambertz18].
Samples that tested PCR positive for Salmonella spp. were subjected to further cultivation according to the ISO standard [23].
RESULTS
A total of 319 samples from the 88 wild boars were analysed by PCR. These comprised 175 tonsil samples, 88 faecal samples and 56 ileocaecal lymph node samples. Eighteen (20·5%) of the sampled individuals tested positive for Y. enterocolitica, 17 (19·3%) tested positive for Y. pseudotuberculosis and nine (10·2%) tested positive for Salmonella spp. (Table 2). None of the samples from wild boars shot close to a farm with free-range domestic pigs was positive for Salmonella spp.
Table 2. Number of samples testing PCR-positive for Yersinia enterocolitica, Y. pseudotuberculosis and Salmonella in tonsil, ileocaecal lymph node and faecal samples collected from Swedish wild boars
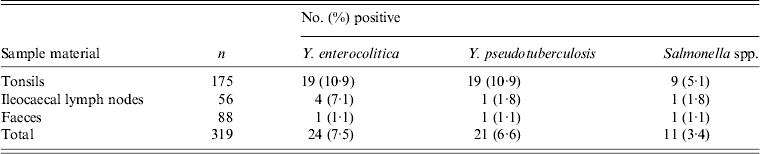
All specimens tested negative for E. coli O157:H7 (Table 2).
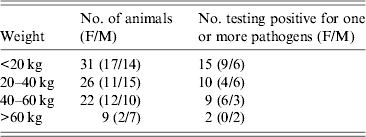
No statistical differences were observed in the occurrence of the various pathogens related to the weight of the animals (Table 3).
Table 3. Incidence of Yersinia enterocolitica, Y. pseudotuberculosis and Salmonella spp. in wild boars of different weight groups
Of the 88 wild boars sampled, 36 (40·9%) tested positive for at least one of the three pathogens detected in the analyses. One individual tested positive for all three pathogens, while six individuals tested positive for two pathogens. All three pathogens could be detected in all of the three different sample materials, with tonsils being the most commonly infected (Table 2). Four of the nine individuals that tested positive for Salmonella spp. also tested positive for one or both of the other pathogens, one for Y. pseudotuberculosis, two for Y. enterocolitica and one for Y. pseudotuberculosis and/or Y. enterocolitica. Eight individuals tested positive for the same pathogen in both tonsils. Four individuals tested positive for both Y. enterocolitica and Y. pseudotuberculosis.
The 11 samples that tested positive for Salmonella spp. were subjected to further cultivation and six isolates were obtained (Table 4).
Table 4. Serovar distribution of cultivated samples for Salmonella spp. for which isolates were obtained

DISCUSSION
This is the first study to examine the presence of these four major foodborne pathogens in the Swedish wild boar population. Overall, few previous studies have focused on wild boars as a reservoir for presumptive zoonoses in a ‘one-health’ perspective. In the present study, more than one-third of the wild boars examined carried Salmonella spp., Y. enterocolitica and/or Y. pseudotuberculosis, thereby constituting a risk for the transmission of these pathogens to humans through consumption or handling of wild boar meat. These results are consistent with results from other studies and highlight the importance of accurate information to personnel in wild-game handling establishments and veterinary officials on the presence of these pathogens and the need for good hygiene practices. Targeted recommendations for hunters on the slaughter of wild boars not destined for the market would also be useful.
The majority of cases of human yersiniosis reported in Europe are caused by Y. enterocolitica and only a few have been attributed to Y. pseudotuberculosis [Reference Lambertz24]. This might indicate that the presence of Y. pseudotuberculosis in the food chain is low. However, some cases of yersiniosis may go undetected and thus fail to be reported. In Finland, the situation is somewhat different, with occasional outbreaks of yersiniosis caused by Y. pseudotuberculosis, often in conjunction with contaminated vegetables. The source of this contamination has not been identified [Reference Rimhanen-Finne25], but wildlife has been suggested [Reference Kangas26]. Yersinia spp. have been found in 12·8% of sampled migratory birds [Reference Niskanen27] and in rodents trapped close to pig farms; both pathogenic Y. enterocolitica and Y. pseudotuberculosis have been detected [Reference Backhans, Fellström and Lambertz28]. Although Sweden has a uniquely low prevalence of Salmonella spp. in domestic pigs and cattle, our results show that wild boars may act as a reservoir for Salmonella spp. and thus constitute a risk in the food chain by spreading the infection to free-range domestic pigs, grazing cattle and sheep.
Isolates were obtained from 6/11 samples that tested positive for Salmonella spp. and originated from nine different individuals (Table 4). Serotyping revealed that two of these isolates belonged to subsp. IIIb (diarizonae) and four isolates to subsp. I (enterica), one of these being further serotyped as Typhimurium. It was not possible to fully identify the other three isolates by conventional serotyping, but the antigens expressed showed a match to isolates previously only obtained from woodpeckers collected within the Swedish wild game monitoring programme. Neither could the two isolates of subsp. IIIb be fully subtyped (Table 4). These isolates originated from two different animals caught at two separate locations 130 km apart, while the three isolates of the woodpecker-associated subsp. I originated from two juvenile pigs caught simultaneously in two traps at the same artificial feeding place. However, further studies are needed to evaluate the potential pathogenicity of these strains.
Wild boar has a good reproductive performance and, with a favourable climate, the proportion of naive, young individuals tends to be high [Reference Fernández-Llario and Mateos-Quesada29]. Hunters tend to prefer juveniles for shooting due to their superior meat quality and due to sows generally being spared for ethical reasons, hence the hunting bag is dominated by young animals. Fredriksson-Ahomaa et al. [Reference Fredriksson-Ahomaa2] indicated that there was a significantly higher risk of wild boars of <20 kg live weight being carriers of enteropathogenic Yersinia spp. However, in the present study no significant correlation to weight was noted (Table 3).
The spread and possible accumulation of pathogens within a wild boar population might be enhanced by the common use of artificial feeding places. These may also attract rodents, foxes, birds and other ungulates and this large variety of animals eating and defecating at the same feeding place might pose a risk for the spread of pathogens within and between species. The effect on food safety has yet to be evaluated and further studies are needed.
CONCLUSIONS
This study confirms that the wild boar population carry pathogens with the potential to cause serious illness in humans. The associated increase of the wild boar population and the consumption of wild boar meat warrants further studies in order to assess the public health risks.
ACKNOWLEDGEMENTS
We especially thank the hunters for providing the sample material. This study was financially supported by Wildtech project (EU 7th Framework Programme for Research and Technological Development, grant agreement no. 222633) and the Ivar and Elsa Sandberg Foundation.
DECLARATION OF INTEREST
None.







