INTRODUCTION
In recent years, two devastating diseases, human immunodeficiency virus (HIV) and hepatitis viral infections, have received substantial global attention. More than 30 million people are living with HIV according to UNAIDS (http://www.unaids.org/globalreport/default.htm). The pandemic strain of HIV-1 group M has at least nine genetically distinct subtypes (or clades), including A, B, and C. Occasionally, two different subtypes yield a recombinant virus known as a circulating recombinant form (CRF), such as CRF01_AE. In Japan, the number of people living with HIV and AIDS has continued to increase, although the figures are relatively small compared to those from other countries. The precise rate of HIV prevalence in high-risk populations (HRPs) has not been assessed in Japan.
Over 350 million people worldwide are chronically infected with hepatitis B virus (HBV) according to the World Health Organization (WHO; http://www.who.int/mediacentre/factsheets/fs204/en/) [Reference Lee1]. HBV is classified into 10 genotypes (A–J). The global distribution of HBV genotypes indicates geographical divergence [Reference Arauz-Ruiz2–Reference Kato6]. In Japan, it is estimated that one million people are carriers of HBV [Reference Tanaka7]. The major route of HBV infection has been mother-to-child transmission [Reference Yoshikawa8]. However, virtually no cases of mother-to-child HBV infection have been reported since nationwide measures were introduced to prevent mother-to-child transmission in 1986 [Reference Yoshikawa8]. In addition, the incidence of nosocomial infections has rarely been reported due to programmes operated by the Japanese Red Cross Society, which maximize the safety of blood transfusion products, and an HBV vaccination programme for health professionals [Reference Mitsui9]. However, the vaccination programme has not been standardized. Furthermore, no preventive measures have been taken to combat sexually transmitted HBV infection, which may accelerate the spread of HBV in young people.
HBV and HIV share similar transmission routes, so it is not surprising that there is a high frequency of co-infection. HIV/HBV co-infected individuals develop chronic hepatitis B at a higher rate than HBV mono-infected individuals and they have a high mortality rate [Reference Bodsworth, Cooper and Donovan10, Reference Bonacini11]. Furthermore, the approved anti-retroviral drugs emtricitabine (FTC), lamivudine (3TC), and tenofovir (TDF) also have antiviral activity against HBV [Reference Menne12]. It has been noted that the early diagnosis of HIV and HBV infection should improve treatment outcome, and potentially HIV-infected individuals are encouraged to undergo HBV testing to ensure that they receive appropriate medical care.
The number of HIV-infected individuals continues to rise in Japan. This study aimed primarily at assessing the prevalence and the latest epidemiological trend of HIV and HBV infections in HRPs attending primary sexually transmitted infection (STI) clinics in Japan, which remain uncharacterized. Viral genotype analysis can provide insights into the epidemiology of viral infection. Thus, a molecular epidemiological study of HIV and HBV infections was conducted to determine the traits of HIV and HBV prevalence in HRPs, as well as an analysis of Treponema pallidum (TP) seroprevalence. We focused on HBV, rather than HCV, because the co-infection rate of HBV was significantly higher than that of HCV in HIV-positive individuals in Japan [Reference Gatanaga13]. Moreover, individuals with blood clotting disorders treated with contaminated non-heated blood products, i.e. the majority of HCV/HIV co-infected individuals in Japan, were not included in this study [Reference Koike14]. Interestingly, HBV genotype analysis was more informative than the HIV subtype analysis for understanding the epidemiology of HRPs.
CASES AND METHODS
Specimens
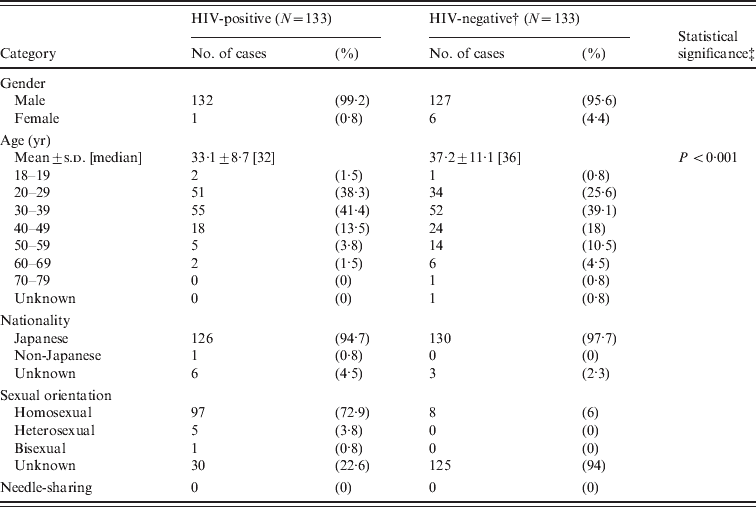
During 2006–2011, 7898 cases were examined (5120 men, 2773 women, and five of unknown sex; mean age±s.d. = 33·3 ± 10·0 years; median 31 years). All attended primary STI clinics in Osaka province, Japan. Osaka has the second highest number of new HIV-infected individuals and AIDS patients (http://idsc.nih.go.jp/iasr/32/380/tpc380.html). The current study included individuals who were considered to be engaged in sexual activities carrying a high risk of HIV infection. We considered the following as high-risk behaviours: unprotected sex, having sex with multiple partners, men who have sex with men (MSM), and needle-sharing. None of the subjects reported a blood clotting disorder as a present medical history. For comparison purposes, an equal number of HIV-negative and HIV-positive cases was selected from the cases where medical professionals recommended HIV testing. The mean age±s.d. of this HIV-negative group was 37·2 ± 11·1 (Table 1).
Table 1. Summary of HIV-positive and HIV-negative populations investigated in the current study*

* Total number of cases studied was 7898. See the Cases and Methods section for details.
† An equal number of HIV-negative and HIV-positive cases were selected for comparison.
‡ Fisher's exact test between HIV-positive and HIV-negative groups.
Serology
An HIV screening test was performed using the Genedia HIV-1/2 mix particle agglutination (PA) and Serodia HIV-1/2 PA anti-HIV assay method (Fujirebio Inc., Japan). After screening for HIV-positive cases, confirmatory tests were performed using LAV Blot 1 and LAV Blot 2 (Bio-Rad, USA). Pepti-LAV 1/2 (Bio-Rad) was used for differentiation of HIV-1 and HIV-2 antibodies, if necessary. There were no cases of HIV-2 infection identified in this study. HIV antibody-negative cases were tested further by nucleic acid amplification testing (Amplicor HIV-1 Monitor test or COBAS® TaqMan® HIV-1 test, Roche Diagnostic Systems, Japan). HBV serological examinations were performed for the HBs antigen (Ag) using the Espline HBsAg kit (Fujirebio Inc.), anti-HBs antibody (Ab) using the Serodia anti-HBs PA kit (Fujirebio Inc.) and anti-HBcAb using the Mycell anti-HBc kit (Institute of Immunology Co. Ltd, Japan). TP Ab was tested by the Serodia TP PA test (Fujirebio Inc.).
Genetic analysis
After extracting RNA from HIV-positive serum using Isogen LS (Nippon Gene, Japan) or QIAamp UltraSens Virus kit (Qiagen, Germany), the env-C2V3 region of HIV-1 was amplified by RT–PCR using a one-step RNA PCR kit (TaKaRa, Japan) to determine the nucleic acid sequence as described previously [Reference Kojima15]. Multiple alignment of the nucleic acid sequences from samples and reference strains (http://www.hiv.lanl.gov/content/sequence/HIV/mainpage.html) was performed using CLUSTAL W (DDBJ: DNA Data Bank of Japan). A phylogenetic analysis was performed to determine the HIV subtypes using the Genetyx-Mac v. 14 (Genetyx, Japan).
DNA was extracted from HBsAg-positive serum using a DNA Extractor kit (Wako Pure Chemical Industries Inc., Japan) or a QIAamp UltraSens Virus kit (Qiagen). Then the core gene of the HBV genome was amplified by nested PCR as described previously [Reference Vitale16]. In brief, an initial PCR was performed to amplify a 617-bp fragment using the Core-Out-For and the Core-Out-Rev primers, followed by a second PCR that amplified a 459-bp fragment using the 1868 and AS2330 primers. If these primers failed to amplify the fragment, the Core-Inn-For and Core-Inn-Rev primers were used in the initial PCR. The HBV genotypes were determined by phylogenetic analysis using the genomic sequences of reference strains (http://jose.med.kuleuven.be/genotypetool/html/subtypeprocesshbv.html). In the HBV genetic analysis, we examined HIV-negative cases that were not included in the HIV-negative comparison group because the number of suitable HBV-positive cases was low.
Statistical analysis
Statistical analyses were performed using StatMate III (ATMS Co. Ltd, Japan). The mean ages of cases with HIV and HBV infections were compared using Fisher's exact test. Student's t test was used if the variances were homogenous, whereas Welch's t test or Cochran–Cox's t test were used if the variances were not homogenous. The rates of HBV and TP infections, and the correlation of HBV genotypes were analysed by χ2 test.
Ethical considerations
Blood sampling was performed after obtaining informed consent following an explanation of the study. The identity of cases and some of the data were anonymized (age, sex, nationality, risk of transmission). The study was approved by the Ethical Review Board of Osaka Prefectural Institute of Public Health (approval number 0810-4 and 0810-5-2).
RESULTS
Analysis of HIV infection
Of the 7898 cases examined, 133 (1·7%) were confirmed as HIV-1 positive (Table 1). All were males with one exception. The mean age±s.d. of the HIV-positive cases was 33·1 ± 8·7 years (median 32 years). About 93% were aged 20–40 years and the number aged in their 20 s, 30 s and 40 s was 51 (38·3%), 55 (41·4%) and 18 (13·5%), respectively. Compared to the HIV-negative group, the age of the HIV-positive group was significantly lower (P < 0·001, Fisher's exact test). The major risk factor was MSM (97/103 cases, 94·2%) according to the available risk data. Five cases were heterosexual and one case was bisexual. Most were Japanese, with the exception of one French subject and two of unidentified nationality. It was reported that the rate of HIV positivity was 0·0019% (1·9 per 0·1 million) during 2006–2011 according to the Japanese Red Cross. The rate of positive HIV testing in public health centres throughout Japan was 0·27% (277/103,007) in 2010 (http://idsc.nih.go.jp/iasr/32/380/tpc380.html). Thus, the positive HIV rate in HRPs in the current study was significantly higher than previously reported (P < 0·001, χ2 test).
Phylogenetic analysis of the HIV-1 env-C2V3 region showed that 118 cases (88·7%) were subtype B, three cases (2·3%) were CRF01_AE, and one case (0·8%) was subtype C. In Japan, 75–89·9% of new HIV-infected cases between 2003 and 2007 were subtype B, 6·1–13·9% were CRF01_AE, and 0·5–2·1% were subtype C (http://idsc.nih.go.jp/iasr/31/366/tpc366.html). These results suggested that the HRP viral subtype profile was indistinguishable from the national profile and that the HIV subtypes of HRPs are not unique in Japan.
Analysis of HBV infection
The number of past or present HBV infections in the HIV-positive group was 84 (84/133, 63·2%), whereas that in the HIV-negative group was 34 (34/133, 25·6%, Table 2). Overall, the positive HBV infection rate was significantly higher in the HIV-positive group than in the HIV-negative group (P < 0·001, χ2 test). Of the HIV-positive cases, the number of HBsAg-positive cases was 15 (15/84, 17·9%). The positive HBsAg and HBcAb rates in the HIV-positive cases were 11·3% and 55·6%, respectively, while those in the HIV-negative cases were 1·5% and 21·1%, respectively. By contrast, the rates in blood donors during 2006–2011 were 0·073% and 0·23%, respectively, according to the Red Cross Blood Centre, which highlights the prevalence of HBV in the study population; this was significantly higher than that in healthy individuals, irrespective of HIV status (P < 0·001, χ2 test). It was notable that the age of HIV-positive/HBV-positive cases (33·5 ± 8·8) was significantly lower than that of HIV-negative/HBV-positive cases (42·2 ± 12·1, P < 0·01, Fisher's exact test). These data suggest that co-infection with HIV and HBV occurred relatively early in life in HRPs.
Table 2. Summary of serological markers for HBV infection in HIV-positive and HIV-negative groups
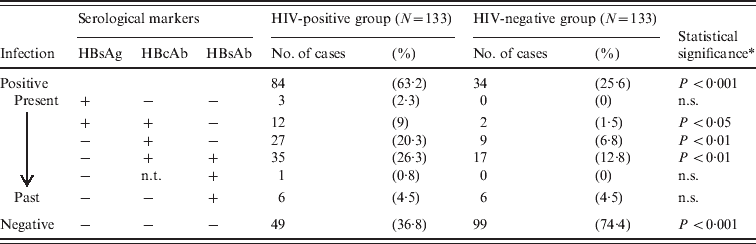
n.s., Not significant; n.t., not tested.
* χ2 test between HIV-positive and HIV-negative groups.
The genotype analyses revealed that of the 15 HIV-positive/HBsAg-positive cases, the majority of the HBV genotypes were Ae (9/15, 60·0%), followed by G (3/15, 20·0%), and C (3/15, 20·0%, Table 3). By contrast, the major HBV genotypes in the HIV-negative group were C (6/13, 46·2%) and Ae (5/13, 38·5%). Similarly, frequent detection of HBV genotype A in the Japanese HIV-1-positive MSM population was reported in Nagoya district although the study was not conducted at the primary STI clinic [Reference Kojima15]. However, it should be noted that the previous study did not identify HBV genotype G in HIV-infected individuals [Reference Fujisaki17]. It was previously reported that the nationwide prevalence of HBV/A, B, C, D and mixed genotypes during chronic hepatitis B in Japan was 1·7%, 12·2%, 84·7%, 0·4% and 1·0%, respectively [Reference Ozasa18]. This indicates that the overall HBV genotype profile of HRPs was distinct from other HBV-positive populations (P < 0·001, χ2 test).
Table 3. Analysis of HBV genotypes between HIV-positive and HIV-negative groups
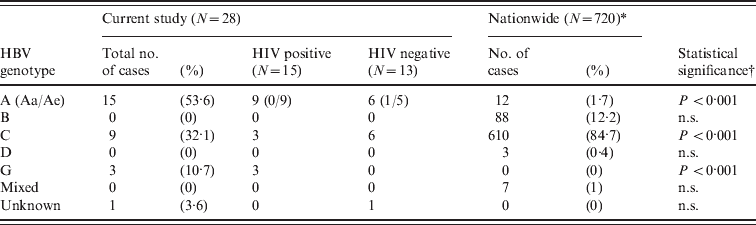
n.s., Not significant.
* Orito et al. [Reference Orito29].
† χ2 test between current study (total) and nationwide survey.
Analysis of TP infection
We also investigated the prevalence of TP to determine whether the low HBV prevalence was unique to HIV-negative subjects. The results of the TP Ab test showed that 44·4% of HIV-positive (59/133) and 48·9% of HIV-negative (65/133) cases were TP Ab-positive (Table 4). These values were significantly higher than the Red Cross Blood Centre's data for 2006–2011 (0·13%, P < 0·001, χ2 test), indicating that the target population of our study was indeed at high risk of STIs. It was interesting to observe that, unlike HBV infections, the rate of TP Ab positivity was not significantly different between the HIV-positive and HIV-negative groups, suggesting that the transmission of HIV is correlated with HBV, but not with TP.
Table 4. Analysis of HBV and TP infection rates between HIV-positive and HIV-negative groups
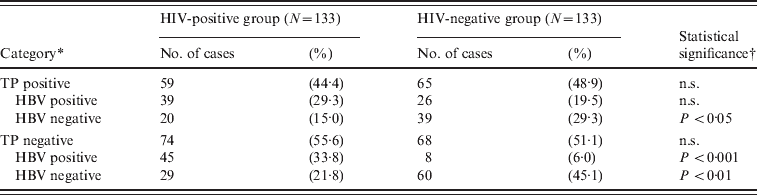
TP, Treponema pallidum; n.s., not significant.
* HBV-positive cases include both present and past infection (please see Table 2).
† χ2 test between HIV-positive and HIV-negative groups.
DISCUSSION
This is the first laboratory test-based surveillance study to determine the rate of HIV positivity and HIV/HBV co-infection in HRPs at primary STI clinics in Japan. The prevalence of HIV in HRPs attending primary STI clinics during 2006–2011 in Osaka province was 1·7%, which was surprisingly higher than we expected. The seroprevalence of HBV in HIV-positive individuals was 63·2%. HBV genotype analyses suggested that HIV was transmitted within a specific community, mainly the MSM population, in which distinct HBV genotypes were prevalent. HIV transmission was linked closely with HBV, whereas TP infection was not strongly associated with HIV prevalence. These epidemiological traits of HIV infection were identified by analysing HBV genotype. It has been suggested that healthcare workers should encourage individuals who engage in high-risk behaviors to have their HIV serostatus tested at primary STI clinics. However, the effect of this advice has been difficult to evaluate. It was notable that 32/133 HIV-positive cases (24·1%) received HIV testing based on the recommendations of health professionals, which demonstrates that active consultation with healthcare workers can facilitate earlier diagnosis of HIV infection. This makes it possible to diagnose HIV infection during the asymptomatic phase, which provides an opportunity for appropriate treatment and the prevention of unknowingly transmitting HIV/HBV to sex partners.
Syphilis is a STI that is caused by TP, which is distributed globally. TP and HIV share similar transmission routes and syphilis may increase the risk of HIV transmission by 2–5 times [Reference Reynolds19]. The clinical symptoms, diagnosis, and management of syphilis differ for HIV-infected and HIV-uninfected patients [Reference Hicks20–Reference Augenbraun23]. In the USA, the rates of primary and secondary syphilis have been increasing steadily since 2001 in the MSM population [24]. In addition, 20–60% of new syphilis cases involved co-infection with HIV [25–Reference Kerani28]. Thus, it is recommended that all syphilis cases undergo HIV testing in the USA. In Japan, it is reported that around 50% of HIV-infected individuals are TP seropositive, which is consistent with the findings of the current study. However, in contrast to the findings in the USA, the rates of TP positivity were similar in HIV-positive and HIV-negative groups. This is probably because the basal rate of TP infection was sufficiently high in this HRP to mask the TP-HIV linkage. It remains an open question whether the prevalence of TP in the HRPs examined in our study has unique characteristics that contributed to these observations.
HBV genotypes B and C are the two main types of chronic hepatitis B in Japan (96·9%) [Reference Orito29]. However, it was reported recently that HBV genotype Ae is increasing gradually, particularly in urban areas, due to homosexual intercourse [Reference Ozasa18, Reference Orito29–Reference Kobayashi32]. An analysis of the responses to questionnaires sent to 372 hospitals across Japan in 2006 suggested that 70·8% of HIV-positive individuals were co-infected with HBV during homosexual intercourse [Reference Koike14]. Thus, it is likely that the subjects involved in the current study might be responsible for this increase in HBV genotype Ae.
Many studies have suggested that HBV genotypes can influence the clinical course, the response to interferon and nucleotide analogue therapy, and the rate of becoming fulminant [Reference Ozasa18, Reference Suzuki30, Reference Yuen33–Reference Kobayashi36]. HBV genotype Ae infections elicit a lower immune response because of their slow viral dynamics, which means that these infections appear to be more persistent, and more likely to become chronic, than infection with other genotypes [Reference Ozasa18, Reference Suzuki30]. Genotype G is an extremely rare genotype, which usually appears as a co-infection with genotype A, although little is known about its pathogenicity [Reference Kato6, Reference Janssen35, Reference Stuyver37–Reference Osiowy40]. It would be beneficial to investigate the clinical course of HBV infection further, particularly genotypes Ae and G, in HIV-infected individuals. Low HBV-prevalence countries, including Japan, have not yet introduced universal hepatitis B vaccinations. A ‘selective’ vaccination policy may be considered where a vaccination could be provided to HRPs. It may be possible to advise individuals with high-risk behaviours about STIs at the same time they receive HBV vaccinations.
ACKNOWLEDGMENTS
This research was supported in part by a grant-in-aid from the Ministry of Health, Labour, and Welfare of Japan and Daido Life Welfare Foundation, Local health-and-welfare research support. We thank Dr Toru Otake for critical reading of the manuscript.
DECLARATION OF INTEREST
None.