Methicillin-resistant Staphylococcus aureus (MRSA) infections are being increasingly recognized in persons without exposure to factors traditionally associated with these infections (e.g. hospitalization or nursing-home residence, surgery, dialysis, presence of indwelling catheters, or history of such percutaneous devices as tracheostomy tube, gastrostomy tube, or Foley catheter) [Reference Fridkin1, Reference Naimi2]. These community-associated MRSA (CA-MRSA) infections have been reported in a variety of different populations including prison inmates, Alaskan natives, American Indians, Pacific Islanders, sports participants, and military personnel [3–Reference Gonzalez8]. Studies have reported considerable morbidity and mortality associated with CA-MRSA infections with manifestations similar to those from infections caused by susceptible S. aureus [Reference Naimi2, Reference Kaplan9]. Consistent with what is now commonly observed in different regions, a recent survey demonstrated that the majority of community-associated S. aureus infections were methicillin-resistant, and the predominant site of involvement is skin and soft tissues [Reference Kaplan9].
Persons who are carriers of methicillin-susceptible and methicillin-resistant S. aureus are at risk for subsequent infections with these organisms [Reference Eiff10, Reference Huang and Platt11]. Along with the increasing recognition of symptomatic CA-MRSA infections is the observation that asymptomatic MRSA colonization in persons living in the community is increasing as well. Studies performed in healthy children presenting for health maintenance clinic visits at Nashville, Tennessee, demonstrated a MRSA colonization rate of 9·2% in 2004, compared with 0·8% in 2001 [Reference Creech12]. In the National Health and Nutrition Examination Survey (NHANES) conducted between 2001 and 2002, which is a representative sample of civilian and non-institutionalized US population, the prevalence of MRSA nasal colonization was 0·8% [Reference Kuehnert13]. However, studies that have estimated the prevalence of MRSA colonization have typically included subjects with potential exposure to a healthcare environment (e.g. emergency-department visits, hospitalizations, or employment in healthcare), or have included subjects who have household members with frequent healthcare exposure [Reference Creech12–Reference Salgado, Farr and Calfee15]. As a result, these analyses might overestimate the prevalence of MRSA associated with contact outside the healthcare environment. We sought to determine the prevalence of MRSA colonization in healthy persons who were living in households without recent exposure to a healthcare environment.
During January–June 2005, a descriptive cross-sectional study was implemented in New Orleans, Louisiana. Subjects aged 2–65 years were recruited from selected locations, including a university-based sports recreation centre, a public office building, and two outpatient clinics, including an inner-city paediatric clinic for indigent persons and a group practice serving mainly privately insured adults. Volunteers were excluded if they themselves or someone in whom they were in close contact (defined as a minimum 4 h/day for a minimum of 5 days/week), had within the past 12 months a history of hospitalization, nursing-home residence, in-patient or outpatient surgery, emergency-department visit, or employment in healthcare. Subjects with a history of chronic debilitating illnesses (e.g. cirrhosis, connective tissue disorders, immunosuppression, diabetes mellitus, or burns) and those with a history of dialysis, indwelling intravenous catheters, gastrostomy or tracheostomy tubes, or other surgical devices were also excluded. Persons with asthma or hypertension were not excluded unless they had one of the previously listed exclusion criteria.
After administration of an initial set of screening questions, subjects were invited to participate. Subjects or legal guardians were administered additional questions related to demographics, residence, occupation, insurance, child care, sporting activities, incarceration, illicit drug use, and medical history, including a history of boils, other skin infections, and medications. Subsequently, each participant had the distal part of the anterior nares sampled for culture with the circular movement (five rotations on either side) of a Culturette™ swab (Becton, Dickinson and Company; USA), moistened with sterile saline. Subjects were informed of the results of their culture when positive for MRSA. This study was approved by the Institutional Review Board of Tulane University Health Sciences Center.
Samples were transported on BD BBL™ CultureSwab™ Liquid Stuart Transport system (Becton, Dickinson and Company). Remel's mannitol salt agar (Remel, USA) with 4 μg/ml oxacillin was used as a selective and differential medium for the recovery of staphylococci from primary cultures. Swabs were rolled over a small area of the agar surface and streaked for isolation. Plates were incubated aerobically at 35–37°C for 24–48 h and observed for characteristic colony morphology and colour change. A coagulase test was performed to differentiate S. aureus from coagulase-negative staphylococci (CNS). The oxacillin salt-agar screening plate procedure was used for detection of MRSA. The test was performed by inoculating a S. aureus isolate onto Mueller–Hinton agar that had been supplemented with NaCl (4% w/v, 0·68% mol/l) and that contained 6 μg oxacillin/ml. The agar was inoculated from a direct colony suspension equivalent to a 0·5 McFarland standard by using either a 1-μl loop or a swab. By using a 1-μl loop, the inoculum was spread in an area 10–15 mm in diameter. The plate was incubated at temperatures no higher than 35°C for 24 h and examined carefully with transmitted light for evidence of small colonies (i.e. >1 colony) or a light film of growth indicating oxacillin resistance. Etest® (AB Biodisk, Sweden) was used for antibiotic susceptibility testing of MRSA. Clinical Laboratory Standards Institute (CLSI; formerly known as NCCLS) interpretive criteria for susceptibility categorization were used.
Pulsed-field gel electrophoresis (PFGE) was performed at the Louisiana Office of Public Health microbiology laboratory, by following the recommendations of the Centers for Disease Control and Prevention (CDC) [16]. The banding patterns of chromosomal DNA fragments resulting from restriction endonuclease cleavage were compared to determine the relatedness of MRSA [Reference Tenover17].
A total of 490 subjects volunteered to be screened for the study and 259 (52·8%) were enrolled. The remainder (47·2%) met one of the exclusion criteria as noted below. The majority of the subjects who were screened (233/490, 47·5%) and enrolled (133/259, 51·3%) were interviewed at the student recreation centre. The remainder of subjects screened included 160 (32·6%) subjects screened at the public office building, and 97 (19·8%) at outpatient clinics. Of the 259 subjects enrolled in the study, 83 (32%) were enrolled at the public office building and 43 (16·6%) at the outpatient clinics. The most common personal reason for exclusion from the study was having had an emergency-department visit (43·9%), followed by employment in healthcare (16·3%), chronic illness (14·2%), hospital admission (13·7%), surgical procedure (11·6%), indwelling medical device (4·7%), and nursing-home admission (1·2%), all during the 12 months before enrolment. Overall, 21% of the excluded subjects had more than one personal reason for exclusion; 26% had no personal reason for exclusion but were excluded because of contact with a household member with a healthcare exposure.
The subjects' baseline characteristics are detailed in Table 1. The majority of the subjects were female (54·4%), white (62·2%), aged 18–65 years (87·3%), and medically insured (92·7%). The most common occupation was student (42·9%). Approximately one third had taken an antibiotic, and 7·7% reported having had boils or sores during the previous year. Three subjects reported having used illicit intravenous (i.v.) drugs, two within the previous year, and two had been incarcerated in prison. The majority had visited a doctor, and one third reported having had an infection during the previous year. Approximately one fifth had participated in team sports at least once a week.
Table 1. Baseline characteristics of subjects (n=259), New Orleans, Louisiana, 2005
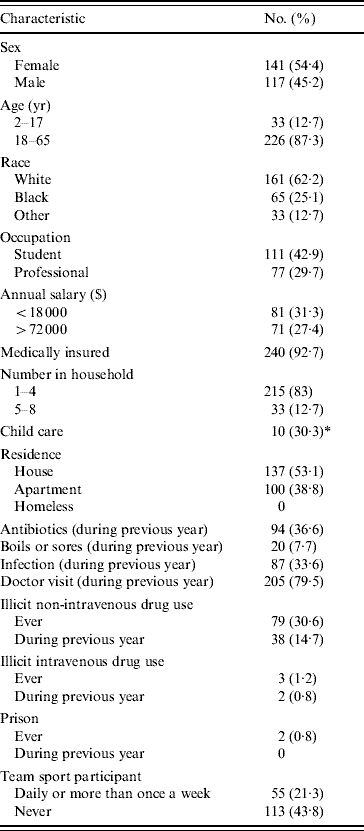
* Percentage of paediatric subjects (n=33).
Only 3/259 subjects (1·2%, 95% confidence interval 0·2–3·3) were colonized with MRSA. All three were enrolled at the student recreation centre. One subject was a male aged 20 years who had used illicit i.v. and non-i.v. drugs during the previous year and had boils and sores during the previous 6 months. Another subject reported having taken antibiotics during the previous year. The third had no identifiable risk factor. Seventy-two (27·8%) subjects were colonized with methicillin-resistant CNS.
All three MRSA isolates were sensitive to vancomycin, linezolid, clindamycin, and trimethoprim–sulfamethoxazole, and two of three were sensitive to erythromycin. PFGE patterns of the three isolates revealed that they were unrelated.
CA-MRSA infections have emerged worldwide with noticeable differences from hospital-associated MRSA infections with respect to the majority of common clinical presentations, antibiotic susceptibilities, and molecular analyses [Reference Fridkin1, Reference Vandenesch18]. This has alerted public health professionals and infection-control practitioners to assess the prevalence of these infections in the community. A recently published population-based study conducted to assess the burden of CA-MRSA disease reported an annual incidence of 25·7 cases/100 000 population in Atlanta, Georgia, and 18·0 cases/100 000 in Baltimore, Maryland [Reference Fridkin1]. Children aged <2 years at both geographic locations and blacks in Atlanta had substantially higher incidence of disease, compared with persons aged >2 years and whites, respectively.
In the majority of the studies conducted to assess asymptomatic carriage of MRSA in otherwise healthy persons, cultures were not obtained from sites outside the healthcare environment, possibly because of the lack of a clear definition of what should be labelled community-associated or community-acquired [Reference Creech12–Reference Salgado, Farr and Calfee15]. A pooled analysis of the studies that performed surveillance cultures for community-acquired MRSA carriage demonstrated that persons from whom samples had been obtained within the healthcare environment (e.g. emergency departments, clinics, or hospitals) were 2·35 times more likely to be colonized with MRSA, compared with those from whom cultures had been obtained outside the healthcare environment [Reference Salgado, Farr and Calfee15].
In this study, we identified a low overall prevalence of 1·2% in these subjects, which is contrary to the common belief among healthcare practitioners. Because of the detection of nasal carriage in only three subjects, we were unable to identify any risk factors for colonization with MRSA in this study. Interestingly, however, MRSA colonization was detected in one of the two subjects who reported i.v. drug use during the previous year. Injecting drug use has been cited as a risk factor for both colonization as well as disease with CA-MRSA [Reference Charlebois14].
We explored multiple factors that have been associated with colonization or infection with CA-MRSA. Investigators have examined previous antibiotic use and have identified conflicting results. Multiple studies have reported previous antibiotic use as a risk factor for MRSA colonization and infection [Reference Baggett19]. Conversely, others have identified no association with prior antibiotic use [Reference Gonzalez8]. Approximately one third of our subjects reported having taken an antibiotic in the previous year. In the worldwide dissemination of CA-MRSA, the relative significance of prior antibiotic therapy might be minimal.
Outbreaks of MRSA infections have occurred in persons participating in competitive and team sports, and team members have been identified as having asymptomatic colonization with MRSA [Reference Ellis7]. Approximately half of the subjects in this study were enrolled at the sports recreation centre, and one fifth had participated in team sports at least once a week. Of the 55 subjects who reported participating in team sports at least once a week, two (3·6%) were colonized with MRSA, a rate lower than the 8% that had been reported in a previous study of professional football players [Reference Ellis7]. Possibly, the close clustering and specific practices of sharing utensils did not exist in our population, who would be expected to participate in team sports in a less structured environment.
Using a stringent screening criterion enabled us to estimate with greater authority the prevalence of MRSA that might have been acquired with contact in the community. Conversely, we might have underestimated the true prevalence of CA-MRSA or community-acquired MRSA by excluding approximately half of the subjects in this study.
Another explanation for a low prevalence of MRSA in our study might be inappropriate culture technique employed in the study. Broth enrichment along with agar plating might have increased detection rates of MRSA from swab specimens. Culturing sites in addition to nares might have increased detection rates of MRSA colonization [Reference Suggs20]. Although we did not culture for methicillin-susceptible S. aureus, we identified 27·8% of subjects to be colonized with methicillin-resistant CNS. This is consistent with the results of a limited number of studies assessing methicillin-resistant CNS colonization rates [Reference Hisata21].
This study had certain limitations. First, confirmation of resistance to methicillin and other β-lactam antibiotics by testing for the mecA gene on isolates was not performed in the study. Approximately half of the subjects were enrolled at the sports recreation centre, thereby representing young adults who were mainly college students, a group that might not be representative of the general population. Second, we enrolled subjects from clinics; however, only 10 adults and 33 children were enrolled from clinic sites. A paediatric clinic was chosen as an enrolment site for convenience and to avoid logistic and legal hurdles associated with enrolling children at more open sites, where identification and verification of subjects legally authorized to sign consent for minors may not be entirely possible. Using strict screening minimized the possibility of enrolling subjects with hospital exposure. On the basis of previous experience, we had expected to identify a higher colonization rate in persons enrolled in the study at the clinics. Last, a larger sample size might have enabled us to narrow the confidence interval of our results; however, this was beyond the resources available for this study.
In conclusion, we identified a low prevalence of asymptomatic colonization in healthy children and adults in New Orleans prior to hurricane Katrina. The Louisiana Office of Public Health plans to continue with similar studies in specific population groups, at potentially elevated risk (e.g. prison inmates, police officers, armed forces, school personnel) to more accurately describe the prevalence of MRSA in New Orleans. Additional population-based sampling from other geographic locations is needed to elucidate the differences in regional prevalence.
ACKNOWLEDGEMENTS
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention.
DECLARATION OF INTEREST
None.


