INTRODUCTION
Hepatitis B virus (HBV) infects the liver and can cause chronic infection, resulting in a broad spectrum of disease outcomes including liver cirrhosis, liver carcinoma and death. It is estimated that about 25% of persons who become chronically infected in childhood and 15% of those who become infected later in life die from cirrhosis or liver cancer [Reference Mast and Ward1]. Globally, an estimated 360 million people are chronically infected with HBV [Reference Shepard2]. The prevalence of HBV in adults varies markedly by country: over 90% of the population in some countries in the Far East have serological markers indicating past or active infection compared to less than 5% in some Western European countries [Reference Shepard2, Reference Medley3].
Even in low-endemic countries such as The Netherlands, HBV prevention and control is a public health priority, particularly since safe and effective vaccines are available. In The Netherlands, HBV vaccination has been recommended since 1983 for high-risk occupations and certain patient groups. In 1989, universal antenatal HBV screening was implemented with passive and active immunization of infants born to mothers with chronic HBV infection [Reference Grosheide, Klokman-Houweling and Conyn-van Spaendonck4]. In 2002, a national programme was introduced for vaccination of behavioural high-risk groups [including men who have sex with men (MSM), drug users (DUs), prostitutes and heterosexuals with a high rate of partner change]. Since 2003, children of migrant(s) from countries with a moderate or high HBV prevalence have been offered HBV vaccination within the national immunization programme. In autumn 2011, universal infant HBV vaccination was implemented [5, 6].
In addition to primary prevention, recent advancements in the treatment of chronic HBV infection now allow secondary prevention. Currently, hepatitis B screening programmes in The Netherlands target individuals who are most at risk of transmitting HBV, such as blood donors and pregnant women, rather than groups with the highest prevalence of infection [Reference Hahné, Wormann Nee and Kretzschmar7].
Since new and chronic HBV infections are often asymptomatic, seroepidemiology, which studies serological markers of HBV infection in a population sample, is needed to identify population subgroups with an increased prevalence of infection. In The Netherlands, a nationally representative serological survey was conducted in 1995/1996 and 2006/2007, primarily for the evaluation of the national immunization programme (the ‘Pienter’ studies) [Reference de Melker and Conyn-van Spaendonck8, Reference van der Klis9]. With the aim of evaluating and informing national policy on primary and secondary HBV prevention, we used these surveys to assess whether the prevalence of HBV infection in The Netherlands changed between 1996 and 2007, and to identify risk factors for HBV infection in 2007.
MATERIALS AND METHODS
Study population and sample design
In the Pienter studies, cross-sectional samples of the Dutch population aged 0–79 years were taken from municipal registers in 1995/1996 and 2006/2007. For ease of reference, in this article the two surveys will be referred to as having taken place in 1996 and 2007. In each of five geographical regions, eight municipalities were randomly drawn with probability proportional to size. In each of these municipalities, individuals were randomly selected within 17 age groups. Further details of the sampling design can be found elsewhere [Reference van der Klis9]. In 1996 and 2007, 15 189 and 19 781 individuals were invited, respectively [Reference van der Klis9], including in 2007 oversampling of the largest migrant groups in The Netherlands. Participants completed a questionnaire and an informed consent form, and visited a clinic for venepuncture. A separate questionnaire was used for participants aged 0–14 years, to be completed by their parents. The questionnaire included questions on demographic characteristics, vaccination history, activities possibly related to infectious diseases and information related to sexually transmissible diseases for 15- to 79-year-olds. Residents born in a foreign non-Western country received a letter of invitation in their own language (Turkish) or a partly translated letter in English, French and Arabic along with a Dutch version. Participants received a gift voucher of €10. People who declined participation were asked to complete a short questionnaire including questions on demographic characteristics, reasons for non-participation, educational degree and general health status. The study proposal was approved by the Medical Ethics Testing Committee of the Foundation of Therapeutic Evaluation of Medicines (METC-STEG) in Almere, The Netherlands (ISRCTN 20164309).
Laboratory methods
Serum was tested for antibodies to HBV core antigen (anti-HBc) using the AxSYM Core assay (Abbott Laboratories, USA). In both surveys the same test was used with identical specifications. Samples positive for anti-HBc were tested for HBV surface antigen (HBsAg) using the AxSYM HBsAg (V2) assay (Abbott Laboratories). Anti-HBc-positive samples taken in the 2007 survey were also tested for presence of HBV-DNA using a S-region-based PCR method with a lower limit of detection of 50 genomic equivalents/ml serum [Reference Boot10]. PCR-positive samples were genotyped on the basis of the S-region sequence. Anti-HBs tests were not performed.
Definitions
The Dutch population was defined as individuals registered in municipal registers. Countries of origin were divided in two groups: those with a low prevalence of HBV (HBsAg prevalence <2%) and HBV-endemic countries [those with a moderate to high HBV prevalence (HBsAg ⩾2%)] [Reference Mast and Ward1]. Indigenous Dutch participants were born in The Netherlands to parents born in The Netherlands. A first-generation migrant (FGM) was a person born in a HBV-endemic country, of whom at least one parent was born outside The Netherlands. A second-generation migrant (SGM) was a person born in The Netherlands, of whom at least one parent was born in an HBV-endemic country. Past or present HBV infection was defined by the presence of anti-HBc. Chronic HBV infection was defined by the presence of anti-HBc and HBsAg and/or HBV-DNA.
Statistical analyses
All analyses took account of the survey design. We estimated the hepatitis B prevalence weighted to the Dutch population, taking into account sex, age group and migrant status. We used 1997 and 2007 population estimates from the National Statistics Office (CBS). For all analyses of anti-HBc results, including prevalence estimates, we excluded children aged <18 months as it can not be excluded that their anti-HBc reflects maternal infection [Reference Boot11].
We assessed differences in the hepatitis B prevalence between 1996 and 2007 by calculating prevalence ratios (PRs). We tested whether these PRs differed significantly from 1 using the Delta method [Reference Oehlert12]. We assessed determinants for HBV infection only for the 2007 survey data, as in 1996 potentially important determinants such as injecting drug use, having received blood products and partner's country of birth were not ascertained. We used univariable and multivariable Poisson regression to estimate PRs for anti-HBc positivity and HBsAg and/or HBV-DNA positivity. Risk factor analyses were performed separately for children (aged <15 years) and older participants, for the overall dataset, as well as stratified by migrant status. Variables with a P value <0·1 in univariable analyses were included in a multivariable model. The effect of migrant status and country of birth was estimated adjusting for age and sex. The effect of other determinants was assessed by a multivariable model, which included age, sex, migrant status and all determinants with a univariable P value >0·1. The final model was selected by removing determinants with a P value >0·05, unless they changed the effect parameter of one or more of the remaining variables by >10%. Only determinants included in the final model are included in the tables. We estimated population attributable fractions (PAFs), which represent the estimated proportion of HBV infections that is attributable to a determinant, for determinants that were significantly associated with HBV infection on multivariable analysis. These were derived from the multivariable model by changing the actual individual value for the determinant to that of the reference category [Reference Deubner13]. Bootstrapping was used to obtain 95% confidence intervals (CIs) using SAS version 9.2 (SAS Institute Inc., USA) [Reference Rao, Wu and Yue14]. All other analyses were performed in Stata 10.0 (StataCorp LP, USA).
RESULTS
Information from the questionnaire and an anti-HBc test result were available for 7249 individuals in 1996 and for 6246 individuals in 2007, representing a response of 47·7% and 31·6%, respectively. In both surveys, men, certain age groups and FGMs were less likely to participate. Educational degree was not an independent determinant for non-participation. Those who perceived themselves as less healthy were less likely to participate.
The proportion of Dutch citizens born in an HBV-endemic country increased from 7·2% in 1996 to 8·7% in 2007 (P<0·0001) (source data: CBS).
The overall population
In 1996, of 7249 sera tested, 150 anti-HBc positives were found. For 142 of these an HBsAg result was available, of which six were HBsAg positive. The estimated population prevalence of anti-HBc and HBsAg was 2·9% and 0·1%, respectively.
In 2007, of 6246 sera tested, 211 anti-HBc positives were found. For 203 of these, an HBsAg result was available, of which 14 were HBsAg positive and 11 were HBV-DNA positive. Nine of the 14 HBsAg positives and two of the 189 HBsAg negatives were HBV-DNA positive. For 10 of the 11 HBV-DNA-positive samples, the genotype could be determined [A (n=4), B (n=1), D (n=4) and E (n=1)]. The estimated population prevalence of anti-HBc and HBsAg was 3·5% and 0·2%, respectively. The prevalence of HBsAg and/or HBV-DNA was also 0·2%. This corresponds to 39 469 persons with chronic HBV infection (HBsAg and/or HBV-DNA positive) in the Dutch population in 2007 (95% CI 18 572–83 721).
The prevalence of HBsAg and anti-HBc did not statistically differ between 1996 and 2007 (Table 1). In both 1996 and 2007, the prevalence of anti-HBc increased with age until age 45 years (Fig. 1 a).
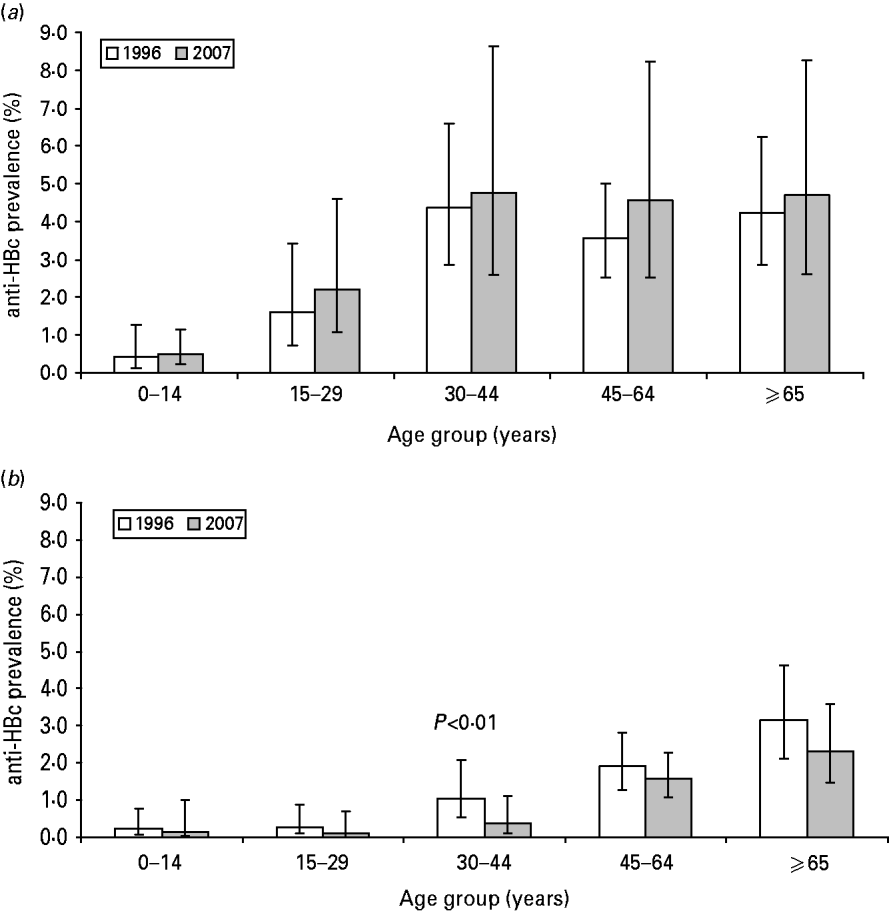
Fig. 1. Prevalence of anti-HBc in the Dutch population by age group, The Netherlands, 1996 and 2007. (a) All participants aged >18 months (1996: N=7015; 2007: N=5930). (b) Indigenous Dutch participants aged >18 months (1996: N=6209; 2007: N=4414).
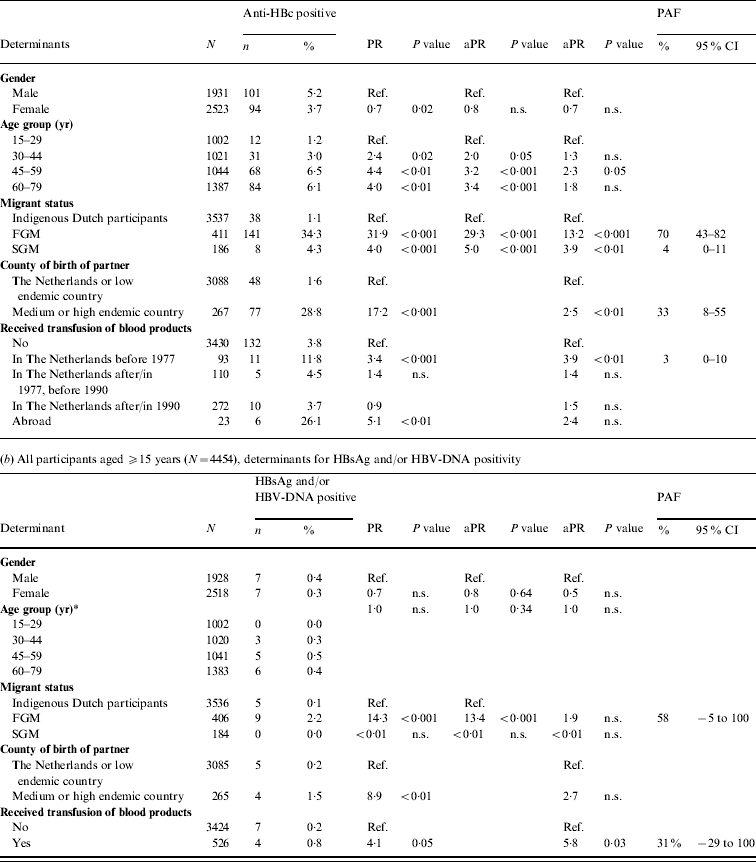
Table 1. Prevalence of hepatitis B virus (HBV) infection by age group and migrant status, The Netherlands, 1996 and 2007
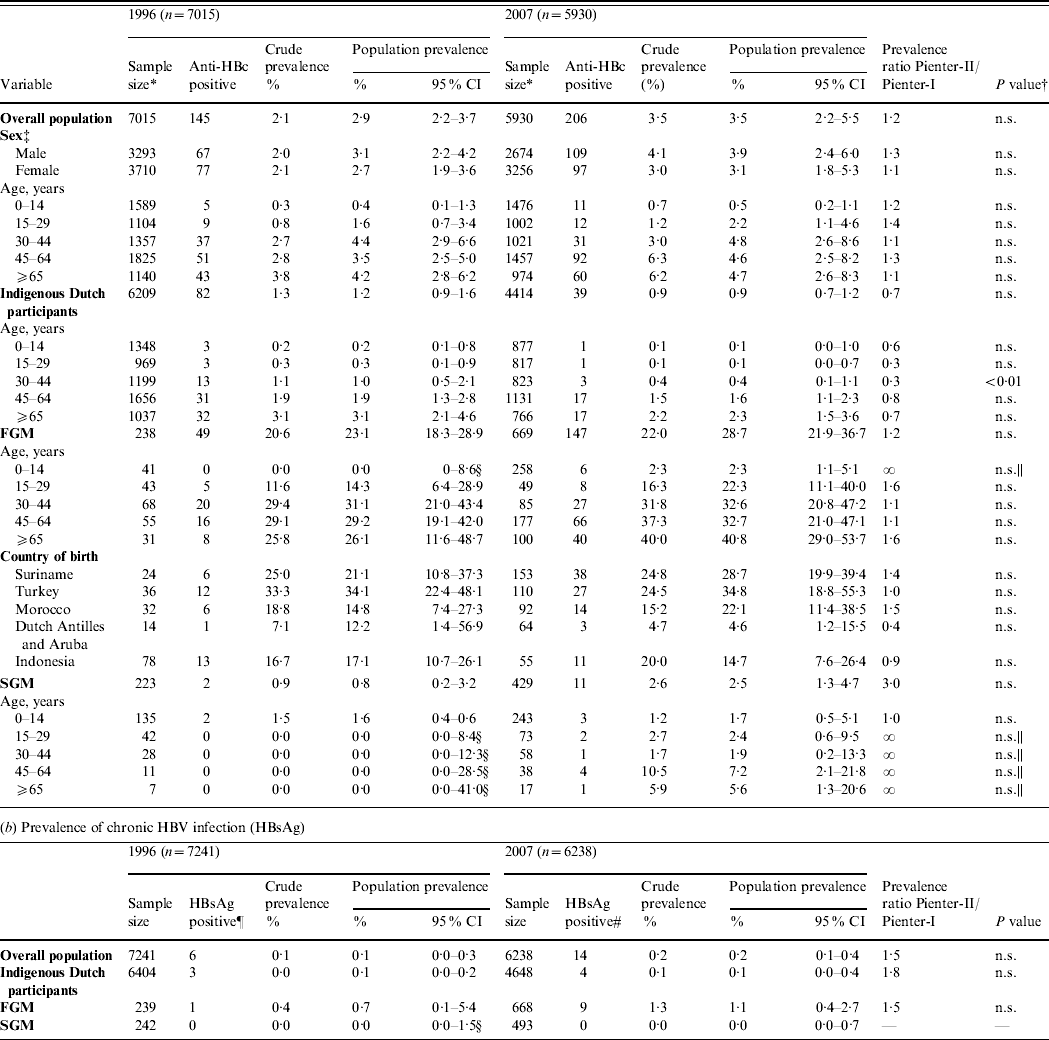
FGM, First-generation migrant; SGM, second-generation migrant; CI, Confidence interval; n.s., not significant.
* This excludes infants aged <18 months (234 in 1996 and 316 in 2007).
† Determined by the Delta method.
‡ For 12 participants in 1996 the sex was unknown.
§ Estimated with the exact method.
|| Estimated with Fisher's exact test.
¶ For two HBsAg-positive individuals migrant status could not be classified [country of birth missing (n=1), born in India to Dutch parents (n=1)].
# For one HBsAg-positive individual migrant status could not be classified (no information on country of birth of the mother).
The 2007 anti-HBc prevalence in FGMs and SGMs was, respectively, 32·7 and 2·9 times higher than in indigenous Dutch participants (P<0·01 and P=0·06, respectively). The HBsAg prevalence in FGMs was 10·4 times higher than in indigenous Dutch participants (P=0·2). The HBsAg and/or HBV DNA prevalence was 1·4% in FGMs. This corresponds to 20 284 FGMs with chronic HBV infection in the Dutch population in 2007 (95% CI 10 399–39 301). This constitutes 51% of the estimated total number of people with chronic HBV infection while only 9% of the population were FGMs. The anti-HBc prevalence in SGMs was much lower than in FGMs (PR 0·09, P<0·01). There were no HBsAg- and/or DNA-positive SGMs in 1996 or 2007.
Combining data from both surveys, 4% of the anti-HBc positives (10/240 with information on this question) and 20% (3/15) of the HBsAg and/or HBV-DNA positives reported they had previously been diagnosed with hepatitis B.
In 2007, independent risk factors for anti-HBc positivity in adults were being a FGM (PAF 70%) or a SGM (4%), having a foreign-born partner (33%) and having received a blood product before 1977 (3%). The PR for being a FGM was more than halved when the variable ‘partner born abroad’ was added to the model. This suggests that a considerable proportion of HBV infections in FGMs are sexually acquired. For SGMs the PR changed less. Independent risk factors for HBsAg and/or HBV-DNA positivity were being a FGM (PAF 58%) and having received a blood product (31%) (Table 2 b).
Table 2. Prevalence rate ratios (PRs) and 95% confidence intervals (CIs) for determinants of hepatitis B virus infection, 2007
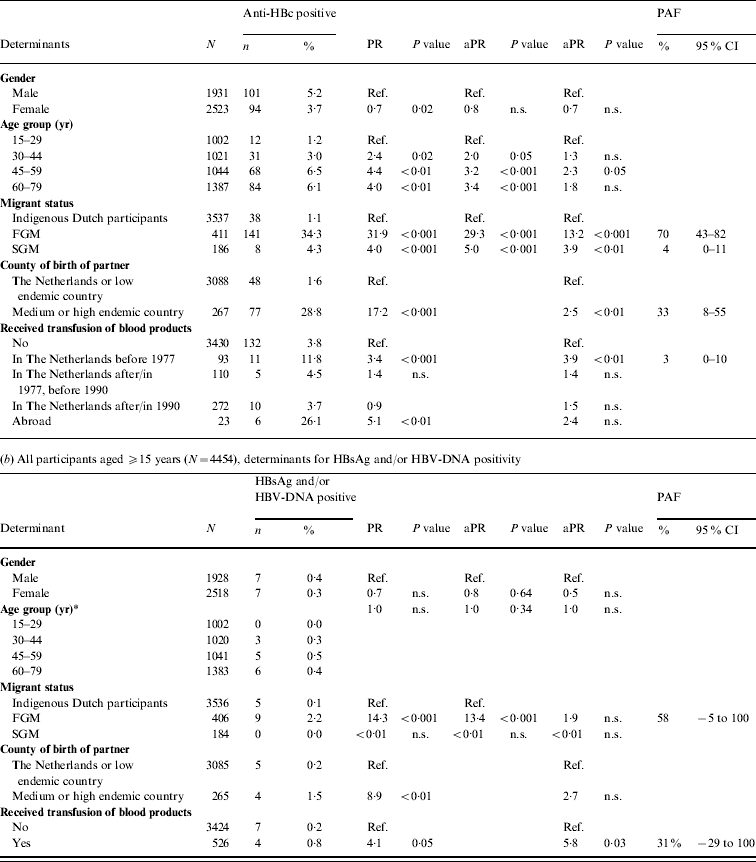
Ref., Reference category; n.s., not significant; aPR, adjusted prevalence ratio; PAF, population attributable fraction; FGM, first-generation migrant; SGM, second-generation migrant.
Determinants that were significant only in univariable analyses were for anti-HBc: sexual preference, travel to Asia, travel to Central or South America, net monthly income and educational level; for HBsAg and/or DNA none of the determinants was only significant in univariable analyses.
* Age included as a continuous variable since effects for grouped estimates could not be calculated.
In 2007, there were 11 anti-HBc-positive and two HBsAg-positive children among the participants. Independent risk factors for anti-HBc positivity in children were being a FGM or a SGM (PAF 59% and 56%, respectively). By adding travel to the model, the PR for migrant status decreased, suggesting part of the increased risk in migrant children is explained by travel to endemic countries (Table 3).
Table 3. Prevalence rate ratios (PRs) and 95% confidence intervals (CIs) for determinants of hepatitis B virus infection (anti-HBc positivity) in children aged <15 years, 2007 (N=1476)
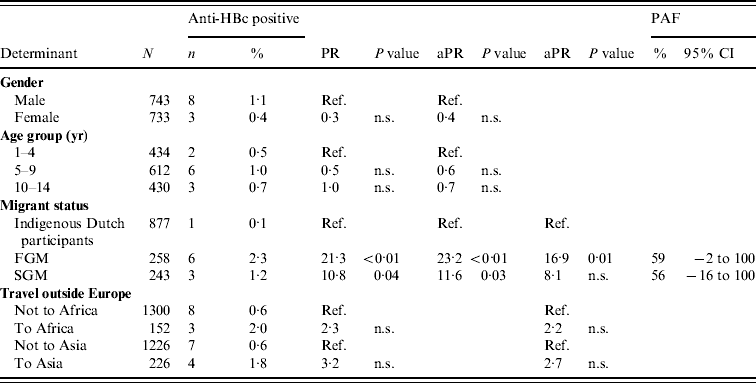
Ref., Reference category; n.s., not significant; aPR, adjusted prevalence ratio; PAF, population attributable fraction; FGM, first-generation migrant; SGM, second-generation migrant.
Indigenous Dutch participants
There were 6410 and 4649 indigenous Dutch participants in 1996 and 2007, respectively. There was a borderline significantly lower prevalence of anti-HBc in 2007 compared to 1996 (0·9% and 1·2%, respectively, P=0·06). The difference was largest in the 30–44 years age group where the prevalence decreased from 1·0% to 0·4% (P<0·01) (Table 1, Fig. 1 b). When stratified by educational level, the anti-HBc PR between 2007 and 1996 was 0·6, 0·8 and 0·5 in participants with a low, medium and high educational level, respectively (P=0·08, 0·79 and 0·04, respectively). The HBsAg prevalence in indigenous Dutch participants did not differ in 2007 from 1996 (0·1% in both years). The proportion of indigenous Dutch participants with a record of at least three doses of HBV vaccination was higher in 2007 than in 1996 (3·50% and 0·07%, P=0·03).
In 2007, older age and having received a blood product before 1977 or between 1977 and 1990 were the only significant independent risk factors for anti-HBc positivity in indigenous Dutch participants (PAF 14% and 6%, respectively) (Table 4). Having received a blood product was the only significant risk factor for being HBsAg and/or HBV DNA positive [adjusted PR 11·7, P=0·01, PAF 46% (95% CI –12 to 100)].
Table 4. Prevalence rate ratios (PRs) and 95% confidence intervals (CIs) for determinants of hepatitis B virus infection (anti-HBc) in indigenous Dutch participants ⩾15 years of age, 2007 (N=3537)
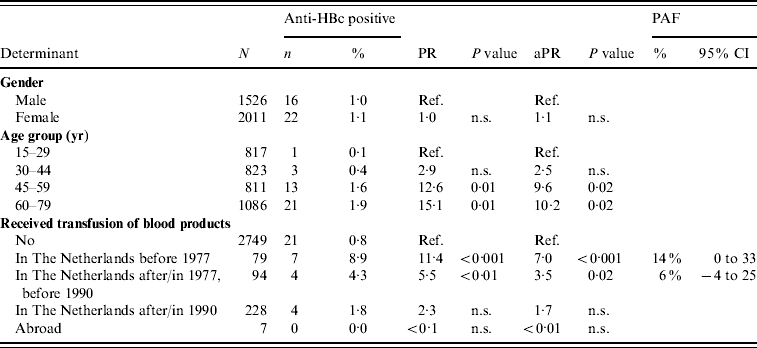
Ref., Reference category; n.s., not significant; aPR, adjusted prevalence ratio; PAF, population attributable fraction.
Travel to Africa was only significant in univariable analysis.
FGMs from endemic countries
There were 240 and 673 FGM participants in 1996 and 2007, respectively. There was no difference between the anti-HBc and HBsAg prevalence in FGMs in 2007 compared to 1996 (Table 1). In 2007, being of Asian origin was a risk factor for anti-HBc positivity in FGMs (PAF 15%). Having a partner born in an endemic country was a borderline significant risk factor (P=0·06) (Table 5). None of the determinants studied was an independent risk factor for HBsAg and/or DNA positivity in FGMs.
Table 5. Prevalence rate ratios (PRs) and 95% confidence intervals (CIs) for determinants of hepatitis B virus infection (anti-HBc) in first generation migrants (FGMs) aged ⩾15 years, 2007 (N=411)
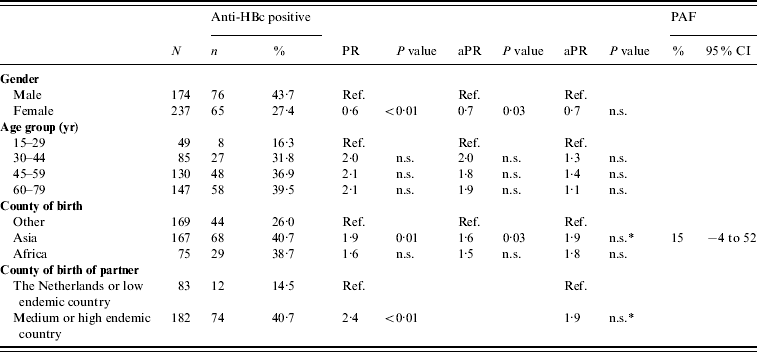
Ref., Reference category; n.s., not significant; aPR, adjusted prevalence ratio; PAF, population attributable fraction.
Determinants that were significant only in univariable analyses were: having a net income <€1750 per month, having male homosexual contact and having a low educational level.
* p=0·06.
SGMs from endemic countries
There were 243 and 495 SGM participants in 1996 and 2007, respectively. There was no difference between the anti-HBc prevalence in SGMs in 2007 compared to 1996 (Table 1). In 2007, independent risk factors for anti-HBc positivity in SGMs were having a foreign-born partner (PR 9·1, P=0·04, PAF 31%, 95% CI−17 to 100) and a history of injecting drug use (PR 32·4, P=0·01). There was only one SGM who reported injecting drug use, and this participant was anti-HBc positive. The PAF could not be estimated due to low numbers.
DISCUSSION
Our analyses of two large, population-based HBV seroprevalence studies showed that the prevalence of HBV infection in the Dutch population did not differ between 1996 and 2007, and remained in 2007 among the lowest worldwide (anti-HBc 3·5%, HBsAg 0·2%) [Reference Nardone15–Reference Merrill and Hunter17]. FGMs had a much higher HBV prevalence than the indigenous Dutch participants. Moreover, in SGMs the anti-HBc prevalence in 2007 was higher than in the indigenous population, although their prevalence was much lower than in FGMs. This is the first time the increased risk of hepatitis B in SGMs compared to indigenous Dutch people has been documented [Reference Baaten18]. Since 2003, SGMs have been targeted for HBV vaccination within the national immunization programme [19]. As our study was conducted only 4 years later, the impact of this targeted vaccination programme can not yet be assessed.
In indigenous Dutch participants, the prevalence of anti-HBc was lower in 2007 than in 1996 (P=0·06), with the largest and significant difference in 30- to 44-year-olds. It may be argued that this is a biased observation due to a lower representation of high-risk groups in indigenous Dutch participants in 2007 compared to 1996. However, the proportion of participants reporting risk behaviours that may be related to acquisition of HBV (male homosexual contact and a high rate of partner change) was not lower in 2007 compared to 1996. Given also that the laboratory tests used did not differ, the lower anti-HBc prevalence in indigenous Dutch participants in 2007 compared to 1996 probably reflects a genuine difference. The higher HBV vaccination coverage in indigenous Dutch participants in 2007 compared to 1996 is a probable explanation for the reduced anti-HBc prevalence, and may reflect the impact of targeted vaccination programmes such as for travellers and behavioural and occupational high-risk groups. However, the lower prevalence in the more recent survey could also reflect other prevention strategies such as improved screening of blood products. Surveillance of acute hepatitis B infections coupled with phylogenetic analyses and behavioural surveillance will be crucial to monitor and disentangle the impact of different prevention strategies [Reference van Ballegooijen20].
In indigenous Dutch participants, no current independent risk factor was identified. However, blood transfusion before 1990 was a significant risk for being HBsAg- and/or anti-HBc-positive, with the highest risk when transfused before 1977. The actual question in the 2007 questionnaire asked about the most recent year a blood transfusion was received. The number of transfusions and first year of transfusion were not ascertained. It is therefore not possible to establish the exact period associated with an increased risk of hepatitis B.
In FGMs and SGMs, having a partner from an endemic country was an important risk factor, consistent with our earlier work [Reference Hahné21]. It suggests that a considerable proportion of FGMs and SGMs resident in The Netherlands acquire HBV through sexual contact. This conclusion is further supported by the observation that in FGMs and SGMs only 5% of the anti-HBc positives were HBsAg positive (10/209, Table 1), suggesting acquisition of infection took place during or after adolescence [Reference Edmunds22]. Current vaccination for FGMs and SGMs is only targeted at children. Our results suggest an assessment of the need for catch-up vaccination of older FGMs and SGMs may be required.
The Pienter studies are the only source of information on the prevalence of HBV infection in the general Dutch population. Marschall et al. estimated the general population HBsAg prevalence in The Netherlands as between 0·4% and 0·6%, based on the 1996 Pienter survey data with an adjustment for under-representation of high-risk groups including migrants [Reference Marschall23]. This estimate is considerably higher than our current weighted estimate for 1996 of 0·1%. A likely explanation is that Marschall et al. assumed an HBsAg prevalence of 3·8% in FGMs, whereas in our 1996 and 2007 data we estimated this as around 1%. In 2004, the HBsAg prevalence in FGMs in Amsterdam ranged from 0·6% to 4·8% in a survey where only adults were included [Reference Baaten18].
The main limitation of our study is the relatively low response, particularly in the 2007 survey. Under-representation of males and certain age groups and migrant groups was adjusted for by weighting prevalence estimates. However, our HBV prevalence estimates probably underestimate the true population prevalence, as high-risk groups for HBV such as undocumented migrants and injecting drug users are likely to be under-represented. Non-response analyses indicated that those who perceived their health status as relatively unfavourable were less likely to participate. However, among participants, this was not an independent risk factor for being anti-HBc positive.
Conversely, our estimate of the population anti-HBc prevalence could be somewhat overestimated due to the relatively low positive predictive value of a positive anti-HBc test due to non-specific reactivity [Reference Schmidt24]. This affects low-risk populations more than those with a high prevalence. Indeed, in our 2007 sample, indigenous Dutch participants had more quantitative anti-HBc values close to the cut-off than FGMs (data not shown). Since this bias may have led to an underestimation of the difference in anti-HBc prevalence between FGMs and indigenous Dutch participants, it is unlikely to have affected our conclusions.
Last, a limitation of our study is that despite oversampling of migrants, the power of our study to identify risk factors for chronic HBV infection was limited as only 16 chronically infected individuals were found in 2007.
In summary, our study has confirmed that The Netherlands remains a very low-prevalence country for HBV, despite increases in the proportion of the population born in endemic countries. We identified FGMs as the most important high-risk group, accounting for 70% of prevalent infections. Hepatitis B screening and treatment of Dutch FGMs was recently deemed a cost-effective intervention to prevent morbidity and mortality from sequelae of chronic hepatitis B [Reference Veldhuijzen25]. Further work is urgently needed to collate the evidence for screening programmes so that policy-making on secondary hepatitis B prevention can proceed.
ACKNOWLEDGEMENTS
We thank the participants, the Public Health Services and municipalities involved, the ‘Pienter-project’ team, and the following persons for their contribution to this study: Roel Coutinho, Hendriek Boshuizen, Jan van de Kassteele, Maarten Schipper, Yolanda van Weert, Anton van Loon. This study was funded by the Dutch Ministry of Health, Sports and Welfare.
DECLARATION OF INTEREST
None.