INTRODUCTION
In The Netherlands, the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in humans is very low, mainly due to restrictive antibiotic use and an effective search-and-destroy policy [Reference Wertheim1]. Since 2003, a new type of MRSA has emerged in Dutch hospitals, which has been isolated specifically from people frequently in contact with livestock, in particular pigs and veal calves [Reference Huijsdens2–Reference Voss4]. This type of MRSA belongs to clonal complex (CC) 398 [Reference Armand-Lefevre, Ruimy and Andremont5] and is referred to as livestock-associated MRSA (LA-MRSA). Since the first reports, this type has been increasingly isolated from various farm animal species [Reference Broens6]. LA-MRSA was found on 88% of Dutch veal calf farms [Reference Graveland7] and on about 70% of Dutch pig farms [Reference Broens8]. On LA-MRSA-positive pig farms, 49% of personnel working intensively with pigs carried LA-MRSA [Reference Van den Broek9]. Moreover, a high prevalence of MRSA-positive farmers on veal farms was found [Reference Graveland7]. In contrast, prevalence of MRSA in the general Dutch community is <0·1% [Reference Bode10]. As a consequence of the high prevalences in these specific populations, the search-and-destroy policy in Dutch hospitals was adapted and all people in close contact with live pigs or veal calves are now screened for MRSA at hospital admission [11].
The first report on MRSA in poultry originated from South Korea, where MRSA was isolated from the joints of two chickens with arthritis and one retail meat sample between 2001 and 2003 [Reference Lee12]. Three Belgian small-scale studies detected LA-MRSA in broilers: two studies investigating 39 and 14 broiler farms, respectively, found a prevalence of 13–14% [Reference Nemati13, Reference Persoons14], and in one study examining farms, also with pigs present, 2/3 farms were MRSA positive [Reference Pletinckx15].
In 2006, LA-MRSA was first isolated from chicken droppings in The Netherlands [Reference Leenders16]. In 2010, 35% of broiler flocks and 7% of broilers were MRSA positive in 405 animals sampled at the slaughterhouse originating from 40 Dutch broiler flocks [Reference Mulders17]. Most isolates belonged to CC398; however, 28% were ST9 (spa-type t1430). This study also showed an increased risk for MRSA carriage in personnel of broiler slaughterhouses (overall prevalence 5·6%, n=466) compared to the general population, in particular in people hanging live broilers on the slaughter line (carriage prevalence 20%). Although prevalence of MRSA on pig and veal calf farms has been extensively studied, accurate estimates on the prevalence of MRSA on Dutch broiler farms are currently lacking. In particular, the prevalence and risk factors for MRSA in people working and/or living on these farms (which has great importance regarding public health), have not yet been studied.
The objectives of our study were to estimate the prevalence of MRSA-positive broiler farms and prevalence of MRSA carriage in broiler farmers, their family members and employees, and to identify and quantify risk factors.
METHODS
Study population
From July 2010 to May 2011, a cross-sectional MRSA-prevalence survey in broiler farming was conducted. Based on Mulders et al. [Reference Mulders17] and Broens et al. [Reference Broens18] sample size calculations were performed, resulting in 56 broiler farms, assuming a prevalence of positive farms of 20% and using a 5% type I error and 10% absolute precision (WinEpiscope 2.0 [Reference Thrusfield19]). One hundred broiler farms with >10 000 broilers were randomly selected from the registered broiler farms (∼670) in the Poultry Flock Information System (KIP system) and were approached for participation in the study.
The human study population was defined as the broiler farmers and their family members working and/or living on the farm and employees working on the farm. To accurately estimate human prevalence, at least 100 persons needed to be sampled, assuming a human prevalence of 20% for people in contact with live broilers [Reference Mulders17], a 5% type I error and absolute precision of 7·5% (WinEpiscope 2.0). Based on the average Dutch family size of 2·2 (CBS database [20]), it was expected this number of persons would be obtained.
Sample collection and questionnaires
Samples were collected by one employee of the Animal Health Service. Flocks were sampled from an age of 21 days onward. All flocks present on the farm (i.e. all broiler houses) were sampled by taking dust samples using Sodibox wipes with Ringer solution (SB 4124 E, Raisio Diagnostics B.V., The Netherlands) on five predetermined locations: (1) drinking system at the front of the barn, (2) drinking system at the back of the barn, (3) feeding system at the front of the barn, (4) feeding system at the back of the barn, and (5) ventilation opening. On each farm, one randomly chosen flock was intensively sampled by taking throat swabs (Dryswab, MW102, Medical Wire and Equipment Co. Ltd, England) from 60 broilers, a sample size which enables detection of a positive flock with a within-flock prevalence of at least 5% at the 95% confidence level (WinEpiscope 2.0). The study was performed according to Dutch law on studies with animals. Informed consent was obtained from each participating farmer.
Inside the farm residence, dust samples from the favourite armchair, TV remote control, inside and outside door handles, and favourite pet, if present, were collected using Sodibox wipes with Ringer solution (Raisio Diagnostics B.V.).
People who voluntarily participated in the study had a nose swab sample taken using gel swabs (Transwab, MW170, Medical Wire and Equipment Co. Ltd). All participants had to complete an informed consent form before participating; for children aged <18 years parental consent was requested.
Farmers completed a questionnaire on farm and farm management characteristics, e.g. farm size, hygiene measures and antimicrobial use as well as a questionnaire on their own lifestyle and health characteristics, addressing factors like age, intensity of contact with broilers, medical history relevant to MRSA infection and foreign travel. This latter questionnaire was also used for family members and employees.
Microbiological examination
Samples were analysed at the National Institute for Public Health and the Environment (RIVM). From each farm, the 60 throat swabs from broilers were pooled into five pools of 12 swabs; all other samples were examined individually.
Broiler and dust samples were analysed by transferring the pooled swabs to 20 ml Mueller–Hinton enrichment broth (BBL, 211443) with 6·5% NaCl (MHB+), while the wipes were transferred to 100 ml of MHB+ and incubated for 18 h at 37 °C. For selective enrichment 1 ml broth was transferred to 9 ml Phenol Red mannitol broth with 5 mg/l ceftizoxim and 75 mg/l aztreonam (bioMérieux, France, NL020) incubated for 18 h at 37 °C and subsequently plated onto Columbia agar with 5% sheep blood (Oxoid, UK, PB5008A) and Brilliance MRSA agar (Oxoid, UK, PO5196A), and incubated for 18 h at 37 °C. Human samples were incubated in 10 ml MHB+ for 18 h at 37 °C. Next, 10 μl of the broth was plated onto ChromID MRSA plates (bioMérieux, France, 43451), Columbia agar with 5% sheep blood (Oxoid, UK) and Brilliance MRSA agar (Oxoid, UK) and incubated for 18 h at 37 °C. Suspected colonies were tested by PCR for the S. aureus-specific DNA fragment [Reference Martineau21], the mecA gene [Reference De Neeling22] and the Panton–Valentine leucocidin (PVL) toxin genes [Reference Lina23]. All MRSA isolates were typed by spa-typing. The spa-typing method is based on sequencing of the polymorphic X region of the protein A gene (spa), present in all strains of Staphylococcus aureus [Reference Harmsen24].
Statistical analysis
Data were analysed using the statistical software package SAS version 9.2 [25]. Prevalences and their exact 95% confidence intervals (CI) were calculated based on the binomial probability function. Geographical distribution of the sampled broiler farms was compared to the distribution of all other broiler farms in The Netherlands (CBS database [20]). A probable difference between regions was tested using the χ2 test.
Broiler and farm data
A farm was classified as MRSA positive if at least one sample, either animal or dust samples from broiler houses, tested positive for MRSA. Continuous variables were classified based on median values. Analyses were performed at both farm and sample level. First, univariable logistic regression analyses were performed (Table 1). Variables with P<0·25 were entered into multivariable regression in which a backwards deletion procedure was performed until variables had P<0·05 or were confounders according to the method of Hosmer & Lemeshow [Reference Hosmer and Lemeshow26]. Confounding was considered present if deletion changed estimates of other variables by >25%, or >0·1 when the estimates were between −0·4 and 0·4, and the deleted variable was included again in the model. Interactions could not be tested due to paucity of data. At the sample level, a random farm effect using an exchangeable covariance structure was included in the model since multiple observations per farm cannot be regarded as independent.
Table 1. Broiler farm variables (in categories) derived from the questionnaire, with number of farms with farm MRSA prevalence based on 50 farms, and number of samples with sample MRSA prevalence based on 1005 samples (250 pooled throat swabs and 755 poultry-house dust samples)

a Several questionnaires were not complete, resulting in variables with missing values.
b Percentages in bold: P<0·25 in univariable analysis.
Human data
A database with data of farm characteristics and individual human data was created. First, univariable analyses were performed (Table 2). As the variable ‘MRSA-positive farm’ was highly significant (P<0·0001) in univariable analysis, this variable was used in bivariable analyses with all other variables that had P<0·25 in univariable analysis. Variables having P<0·05 in bivariable analysis were then included in a multivariable model. A backwards deletion procedure as described above was performed. As observations of humans on the same farm may not be independent, a random effect of farm was included using an exchangeable covariance structure to account for within-farm variation.
Table 2. MRSA prevalence of people living and/or working on broiler farms in relation to farm-related and individual characteristics on 47 broiler farms in The Netherlands

a A number of questionnaires were not complete, resulting in variables with missing values.
b Percentages in bold: P<0·25 in univariable logistic regression.
c 2 h was median number of hours for persons not having 0 h.
d 1 h was median number of hours for persons not having 0 h.
e Not travelled abroad or travelled to to Sweden.
f Austria, Belgium, Germany, Hungary, Switzerland, Czech Republic, France, Poland.
g Spain, Italy, Greece, Portugal, United Kingdom, Turkey, Canada, Egypt, Sumatra, China, Curacao.
Risk countries were divided into three classes based on low, moderate and high MRSA prevalence of countries, derived from data of the EARSS Annual Report 2008 [30].
RESULTS
Response and descriptive statistics
Fifty-four farms out of the 100 selected farms agreed to cooperate. When appointments for sampling were made, three farms no longer had broilers and one farmer was unavailable, resulting in a study population of 50 broiler farms (50% response rate). Geographical distribution (region, Table 1) of broiler farms sampled did not differ from the other Dutch broiler farms (P=0·89). Farms participating had on average three broiler houses (range 1–6). The median number of broilers per farm was 78 000 (range 14 400–200 000). Age of the broilers at the time of sampling ranged from 21 to 49 days, with an average of 31 days. On 85% of the farms the Ross breed was present, while on the other farms Cobb and Hubbard broilers resided.
In total, 228 household members and employees reported working and/or living on the sampled farms. Of these, 160 persons agreed to participate; however, only 145 nasal swabs with a completed questionnaire originating from 47 farms were returned, resulting in a 64% response rate (145/228). These included 47 farmers, 89 family members, and nine employees. An average of three persons per farm were included (range 1–9 persons) with an average age of 36 years (range 1–80 years).
MRSA in broilers and broiler houses
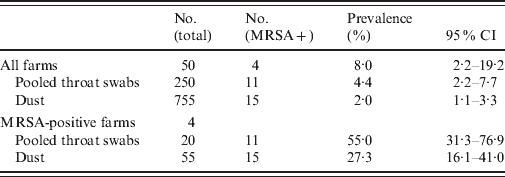
MRSA prevalence in broilers and broiler houses is shown in Table 3. MRSA was detected in 11/250 pooled throat swabs (4·4%, 95% CI 2·2–7·7) and in 15/755 dust samples from broiler houses (2·0%, 95% CI 1·1–3·3). On four out of 50 farms (8·0%, 95% CI 2·2–19·2) MRSA-positive samples were present. Two farms were positive based on both animal and dust samples, one farm on animal samples only, and one farm on dust samples only. On two positive farms, MRSA was also identified in the other flocks based on dust samples. On MRSA-positive farms, the sample prevalence was 55% (95% CI 31·3–76·9) for pooled throat samples and 27% (95% CI 16·1–41·0) for dust samples. The agreement of classification of farms based on either samples of broilers or dust samples was at least sufficient given the 95% CI of kappa (Cohen's kappa=0·85, 95% CI 0·55–1·00).
Table 3. MRSA prevalence of broiler and dust samples with 95% confidence intervals (CI) at 50 Dutch broiler farms
MRSA carriage of people living and/or working on the farms
In total, eight persons from six different farms tested positive for MRSA (5·5%, 95% CI 2·4–10·6). This concerned 4/47 broiler farmers (8·5%, 95% CI 2·4–20·3), 3/89 family members (3·4%, 95% CI 0·7–9·5) and 1/9 employees (11·1%, 95% CI 0·3–48·2) (Table 2). Two of the eight MRSA-positive persons (one farmer, one family member) were detected on two different MRSA-negative farms. The farmer had recently worked on pig farms. He stated that he had tested MRSA positive previously. The family member had no known risk factors for MRSA carriage.
MRSA in the farm residence
In total, 5/233 (2·1%, 95% CI 0·7–4·9) farm-residence samples tested MRSA positive (Table 4). On three of the four MRSA-positive farms, dust samples taken from the farm residence tested MRSA positive. These samples originated from the favourite armchair and the TV remote control (Table 5). On the four MRSA-positive farms, MRSA prevalence of the farm residence was 27·8% (5/18, 95% CI 9·7–53·5).
Table 4. MRSA prevalence with 95% confidence interval (CI) in the farm residence
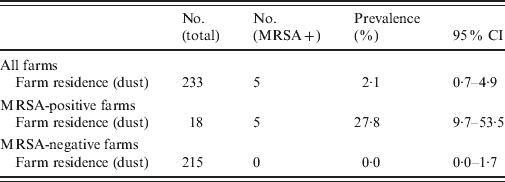
Table 5. Spa types of isolates of human, broilers and broiler houses, and residence samplesa
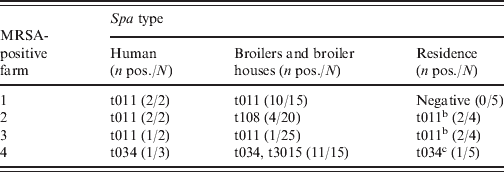
a Two additional MRSA were isolated from two different MRSA-negative farms: spa-type t011 was found in a farmer, whereas spa-type t899 was found in a family member.
b Armchair and TV remote control.
c Armchair.
Spa types
All MRSA isolates were PVL negative. Five different spa types were found: t011, t034, t108, t899 and t3015, all belonging to CC398 (Table 5). On three of the four positive farms, spa types of the human isolates were identical to those of the isolates from the broilers/broiler houses. The spa types of the farm-residence isolates were similar to the spa types of the human isolates. The isolates from the MRSA-positive persons living at two different MRSA-negative farms concerned spa-types t011 and t899.
Risk factors
Farm data
After univariable analyses at the farm level, the following variables had P values <0·25 (Table 1): region, sampling period, outside temperature, neighbours with farm animals, neighbours with veal calves, stocking density (broilers/m2), barn unloaded, presence of pigs, gassing of the barn, visitors washing their hands, control of rats and mice, flock vaccinated against infectious bronchitis (IB) and flock vaccinated against coccidiosis.
Analysis at sample level showed that 11 variables had P values <0·25 (Table 1): neighbours with veal calves, number of flocks, presence of pigs, presence of dogs, floor of barn, manure storage, use of changing room, flock vaccinated against IB, flock vaccinated against Gumboro, flock vaccinated against coccidiosis, and mortality of broilers until sampling. In an empty model (without explanatory variables), 37·4% of all non-explained variation is caused by a farm effect. This implies that there should be factors explaining farm positivity. However, given the low number of positive farms (n=4), in addition to a strong similarity in farm management, it was not possible to perform multivariable analysis and to draw statistically valid conclusions on risk factors for a farm to be MRSA positive.
Human data
Prevalence of MRSA-positive persons related to several possible risk factors is shown in Table 2. Farm effect explains 17·4% of all variation in the probability of being positive between individuals. Addition of the explanatory variable ‘living and/or working on a MRSA-positive farm’ reduces this percentage to 1·1%, indicating that this is the most important factor that increases the probability of a person being MRSA positive. Based on univariable analysis, this variable is the most significant risk factor for a persons being MRSA positive (P<0·0001), i.e. 66·7% of persons (3/3 farmers, 2/4 family members, 1/2 employees) on a positive farm were MRSA positive corresponding to 1·5% of persons (1/44 farmers, 1/85 family members) on negative farms.
Other possible risk factors with P<0·25 are: region, sampling period, absence of changing room, age, type of person, smoking, foreign travel during past year, and visiting risk countries. However, in bivariable analysis with ‘living and/or working on a MRSA-positive farm’ five variables remained with P<0·05, i.e. foreign travel during past year, visiting risk countries, region, period of sampling and absence of changing room. After a backwards deletion procedure, multivariable analysis resulted in two significant risk factors. These were ‘MRSA-positive farm’ (P<0·0001) and ‘absence of changing room’ (P=0·02).
Analogous to research on pig farmers and veal calf farmers [Reference Graveland7, Reference Van den Broek9], it was expected that high degree of contact with live broilers could be a risk factor, but variables related to that had P>0·25. This information was only given by about half of the respondents; i.e. 83 and 79 persons, respectively, completed the number of hours per week present in the broiler house and number of hours per week having physical contact with live broilers. Both variables, the average number of hours in the broiler house and number of hours with physical contact with broilers, were significantly different (P<0·0001) between type of persons, i.e. farmers (2·8 and 1·8 h), partners (0·7 and 0·5 h), other family members (0·4 and 0·1 h), and employees (1·8 and 0·6 h), but were not different between MRSA-positive and MRSA-negative farms (P=0·57 and P=0·82, respectively).
Similar to the farm-data analysis, due to the low number of positive persons and similarity of farm management, it is difficult to draw statistically valid conclusions on the risk factors, except that living and/or working on a MRSA-positive farm is a major risk factor for people becoming MRSA positive.
DISCUSSION
In this study, a prevalence of 8% LA-MRSA-positive Dutch broiler farms was found. This prevalence appears to be lower than the 35% found in the study performed in broiler flocks at Dutch slaughterhouses [Reference Mulders17]. A possible explanation for the higher prevalence at the slaughterhouse is transmission of LA-MRSA during transport and in the slaughterhouse, as was shown earlier for slaughter pigs [Reference Broens18]. Moreover, the prevalence at the slaughterhouse was based on samples taken from both animals and transport crates. The animal prevalence of 6·9% found at the slaughterhouse seems slightly higher than the 4·4% pooled throat swabs in the present study; however, due to pooling no conclusions can be drawn on animal prevalence. For classification of farm status with respect to MRSA, pooling is not expected to decrease the probability of detecting a MRSA-positive farm based on studies on pig farms [Reference Broens8]. However, the throat might not be the optimal sampling site for poultry.
Prevalence of LA-MRSA-positive broiler farms appears to be much lower than of MRSA-positive pig farms (68–71% positive farms) [Reference Broens8, Reference Broens27] and veal farms (88% positive farms) [Reference Graveland7]. This may be explained by the short duration of the production cycles in broiler farming (maximum 7 weeks) and the use of an all-in/all-out system, which possibly reduces the risk of LA-MRSA introduction and persistence on broiler farms compared to pig or veal farms. In addition, poultry may be less susceptible to colonization with MRSA ST398 than pigs (and other animals) as suggested by Pletinckx et al. [Reference Pletinckx15]. In their study on three farms with both broilers and pigs, the within-flock prevalence of MRSA in broilers appeared to be lower than the within-herd prevalence in the pigs.
Overall, the prevalence of LA-MRSA in persons living and/or working on broiler farms (5·5%) appears to be significantly higher than the prevalence in the general human population of The Netherlands (<0·1%) [Reference Bode10]. Prevalence of MRSA in family members other than the partner (children, parents, siblings, n=54) was 0·0%, which indicates that living on a MRSA-positive farm in itself is not the decisive factor for becoming a MRSA carrier. It is speculated that this difference in MRSA prevalence could be explained by the difference in time of exposure to MRSA in the poultry houses as shown by the fact that farmers and their partners spend significantly more time in the broiler house compared to other family members. This is supported by the fact that intensity of animal contact was found to be an important risk factor for LA-MRSA carriage in veal and pig farming [Reference Graveland7, Reference Van den Broek9]. Prevalence of MRSA-positive people on broiler farms (5·5%) appears to be lower than on veal and pig farms (16% and 14% [Reference Graveland7, Reference Van den Broek9], respectively), but is comparable with the presence of MRSA in broiler slaughterhouse workers (5·6%) [Reference Mulders17]. We speculate that the difference in prevalence with veal and pig farmers is mainly caused by the lower prevalence of MRSA-positive broiler farms, although other factors might explain the difference. However, the MRSA prevalence in people living and/or working on MRSA-positive broiler farms was high (6/9) and ‘living and/or working on MRSA-positive farms’ was found to be a major risk factor for MRSA carriage. This is in line with the findings on risk for people on pig and veal calf farms [Reference Graveland7, Reference Van den Broek9].
A marked finding of our study was the high percentage (nearly 30%) of MRSA-positive environmental samples in the farm residences on MRSA-positive farms while none of the samples on MRSA-negative farms was found positive. The spa types were the same as those found in humans on that particular farm, indicating that humans play an important role in transmission. Contamination of the farm residence could be caused either through MRSA on the hands of MRSA-positive persons, by transferring contaminated dust from broiler houses (e.g. on clothing or hair) or by spreading the organism from colonized sites from farmers, partners or employees (e.g. by sneezing). Good hygiene precautions, like changing clothes and taking a shower before (re)entering the farm residence after working in the barns could partly prevent contamination of the farm residence. Good facilities like changing rooms in broiler houses are important in order to prevent transmission of MRSA between barns, between barns and farm residence and between farms.
Spa types found in this study are commonly occurring types in livestock [Reference Huijsdens2–Reference Armand-Lefevre, Ruimy and Andremont5, Reference Graveland7–Reference Van den Broek9] and all belong to CC398. In particular, types t011 and t108 are found in 84% and 86%, respectively, of all animal and dust samples in veal calf and pig farming in The Netherlands [Reference Graveland7, Reference Broens8]. Spa types t011, t034, and t108 have been found in Dutch poultry slaughterhouses [Reference Mulders17], in contrast, t3015 was not detected in this environment [Reference Mulders17], but has been identified in poultry meat [Reference De Boer28]. In contrast, MRSA ST9 and spa-type t1430 which were found frequently in Dutch slaughterhouses [Reference Mulders17] were not found in present study. This might be explained by the fact that broilers originating from other countries are also slaughtered in Dutch slaughterhouses and MRSA ST9 spa-type t1430 isolates have been reported from chicken products originating from Germany [Reference Fessler29]. On three farms the spa types found in humans and broilers were identical, but in one case the spa types differed. This difference might be explained by exposure of the farmer to broilers from another origin in earlier production cycles or by exposure to other sources of MRSA. Further studies using optical mapping and a microarray for the detection of resistance and virulence genes are planned to gain further insight into the transmission between animals and humans.
To summarize, prevalence of LA-MRSA-positive broiler farms is low in comparison with pig and veal farms, and living and/or working on a positive farm was a major risk factor for human carriage. Moreover, the results of this study are in agreement with our previous study on poultry slaughterhouses, indicating that people in contact with broilers are at an increased risk of MRSA carriage compared to the Dutch general population. Given the full range of recent worldwide publications on LA-MRSA and the fact that broiler husbandry systems are not unique to The Netherlands, this might imply that people worldwide in contact with live broilers are at risk for MRSA carriage.
ACKNOWLEDGEMENTS
We thank all the farmers, their family members and employees for their participation in this study. We are grateful to Erik de Jonge (Product Board for Poultry and Eggs), Piet Doornenbal, Jan Workamp and Rob Nijland (Animal Health Service) for help and advice on the selection and sampling of farms, Alex Spieker (Dutch Organization of Poultry Farmers) and Jan Brok (Dutch Poultry Farmers Union) for approaching the farmers for participation in this study, and Birgit van Benthem (National Institute for Public Health and the Environment) for advice. This research received funding from The Netherlands Food and Consumer Product Safety Authority, the Dutch Product Board for Poultry and Eggs, and the Dutch Ministry of Health, Welfare and Sport.
DECLARATION OF INTEREST
None.







