INTRODUCTION
Tuberculosis (TB) is a major cause of morbidity and mortality globally with an estimated 9·4 million incident cases, 14 million prevalent cases, 1·3 million deaths from TB in HIV-negative and 0·38 million deaths in HIV-positive persons in 2009 [1]. Currently, Bangladesh ranks sixth in the 22 high-burden countries, with an estimated annual incidence of all forms of TB of 360 000 cases (225/100 000) and 83 000 deaths (51/100 000) annually [1]. There is limited systematically collected recent epidemiological data from Bangladesh. Two previous national TB prevalence surveys were conducted in 1964–1966 and in 1987–1988. The 1964–1966 survey estimated a prevalence of sputum smear-positive pulmonary TB of 318/100 000 population in symptomatic individuals aged ⩾15 years, while the 1987–1988 survey reported a much higher prevalence of 870/100 000 population in the same age group [2, 3]. Directly observed treatment, short course (DOTS) had been implemented in Bangladesh in 1993 and these surveys were conducted before implementation of DOTS. Several other smaller local surveys were conducted both in and outside the DOTS programme areas which reported estimates of prevalence of smear-positive TB ranging from 24 to 95/100 000 population [Reference Chowdhury4–Reference Zaman6]. The results of these surveys should be interpreted cautiously since they all used different methods and populations.
Bangladesh has achieved commendable success in TB control activities with nationwide coverage of DOTS: >70% case detection rate and >90% treatment success rate [7]. The case detection rate is based on the estimated incidence of TB which can not be assessed directly. Instead, the incidence is approximated by extrapolating data from the 1964–1966 and 1987–1988 prevalence surveys and infection rates [2, 3]. This leads to considerable uncertainties, which together with the rapid increase in DOTS coverage, population growth and involvement of non-governmental organizations (NGOs) and private sectors in TB control activities necessitates a reassessment of the TB situation in Bangladesh. The current study was undertaken with the objective of determining the prevalence of new smear-positive TB in Bangladesh and its distribution in different subgroups of the population.
MATERIALS AND METHODS
At the request of the National Tuberculosis Control Programme (NTP), Bangladesh, the World Health Organization (WHO) commissioned the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B) to implement the national tuberculosis prevalence survey. The survey was conducted during October 2007–March 2009. This was a cross-sectional community-based cluster survey and included all individuals aged ⩾15 years who slept the previous night in a selected household within 40 selected clusters.
Sample size calculation and outcome variable(s)
The sample estimation was based on the total population of Bangladesh excluding the Chittagong Hill Tracts (1·2% the total population) which could not be included due to security issues. In 2001, the population of Bangladesh was 123 851 120, divided over six divisions which are in turn divided into 64 districts [8]. The smallest administrative unit is the mauza in rural areas and the mahallah in urban areas. In total, there were 59 990 mauzas/mahallahs Footnote † [8].
According to the results of previous prevalence surveys and case notification rates, we assumed the prevalence of smear-positive TB to lie between 100 and 200/100 000 population. The sample size calculation for the survey was based on an estimated prevalence of 145/100 000 population, a desired precision of 25%, and a participation rate of 90%, leading to a required sample size of 47 000. With a cluster size of 1250 and a between-cluster coefficient of 0·25, the design effect was estimated to be 1·11, leading to an overall sample size of 50 300 [Reference Hayes and Bennet9]. To accommodate the 10% non-participation, we targeted 1400 adult individuals in each of the 40 clusters. Figure 1 shows the distribution of study sites.
Sampling method
A separate sampling frame was constructed for urban and rural strata. Sampling was performed proportionally to the population size with an upazila (subdistrict) as the primary sampling unit (PSU). Twenty PSUs were selected from each stratum. In each PSU one mauza/mahallah was randomly selected as a study cluster. The starting point for inclusion of households was randomly chosen after which households were added in a consecutive manner. Addition of households was stopped when the required sample size of eligible adults (1400 per cluster) was reached. All adult members (⩾15 years) of these households were eligible for inclusion if they slept in that household the night before the visit of the census team.
Study procedures
Census taking
Two field teams collected data from two different clusters at the same time. They visited each household within the cluster to enumerate the study population, and to obtain information on sex, age, education level, presence of cough, and previous TB treatment of all individuals aged ⩾15 years of the household.
Sputum sample collection
In each field site the field team visited all listed households. They first explained the study and the procedures, and thereafter obtained written informed consent from all adult individuals. All eligible participants were asked to provide a spot (at that moment) and a morning sputum specimen, which were examined by fluorescence microscopy (FM) at the field site. Samples positive by FM, were re-examined by Ziehl–Neelsen (ZN) microscopy. When only one of these specimens showed acid-fast bacilli, a third specimen was collected for examination. If this was negative for acid-fast bacilli, a chest X-ray was performed. All sputum specimens were labelled with a unique identifier of the respondent, and stored under cool conditions until their arrival at the field site laboratory. The team made a return visit to the households on the following morning to collect sputum samples if a participant had been absent.

Fig. 1. Map of Bangladesh showing distribution of study sites.
Case definition
The case definition of a smear-positive TB patient was any person with either two positive samples by ZN microscopy or one positive sample by ZN microscopy and a chest X-ray suggestive for TB (Fig. 2). This definition is similar to the one used in the TB control programme [10]. It was assumed that individuals who were unable to produce a sputum sample did not have smear-positive TB. Additional information on sociodemographic, economic and care-seeking practices were also collected. The participants were asked if they had any cough on the day of the visit. If they answered yes, the interviewer enquired about the duration of the cough.

Fig. 2. Case definition diagnostic algorithm (schematic).

Fig. 3. Summary of the tuberculosis prevalence survey in Bangladesh, 2007–2009.
Laboratory procedure
Immediately after arrival at the field laboratory, the sputum specimens were assessed for macroscopic characteristics (colour, amount, nature). This information was included in the laboratory register. The samples were processed for FM, stained with auramine (0·1%, stained for 15 min) and examined by FM microscope. Slides positive were re-stained on the same day and re-examined with ZN for confirmation. All results were registered in the laboratory register. Specimens with evidence of acid-fast bacilli were stored in a cool place until transport to ICDDR,B for culture and drug susceptibility testing. Culture was performed using Löwenstein–Jensen solid media. Drug susceptibility testing was performed using the proportion method. The concentrations of anti-TB drugs tested were as follows: 0·2 mg/l isoniazid, 40 mg/l rifampicin, 2 mg/l ethambutol and 4 mg/l streptomycin [Reference Zaman11]. The specimens were kept at 4°C after collection and were transported to the laboratory in a cool box within 24 h. No transport medium was used.
Quality assurance
The completeness and quality of the collected data was ensured by regular and routine supervision, checking and monitoring. All field and field laboratory data forms were routinely rechecked by one immediate supervisor and further checked by the team leader. Data transfer was performed in the evening in presence of all team members. Each team leader prepared an update of status which was displayed each day on a noticeboard showing the progress of the field work. A strong supervision structure was established for the survey consisting of field and central level supervisors. The study investigators and the senior field research officer monitored the activities on a day-to-day basis and undertook routine field visits and repeated census taking on sample basis. Any discordance was discussed and rectified. The national steering committee provided necessary inputs on design, implementation, logistic support and supervision of the study activities on an ongoing basis.
Finally, an independent international expert made supervisory visits under the auspices of WHO technical support twice during the survey; once at the beginning and once when the survey moved into the Dhaka City field data collection period. One month after completion of the survey another monitoring visit was made to five randomly selected clusters and interviews were conducted in the selected areas about the survey in order to check the validity of the information and survey procedure.
All slides positive with ZN microscopy at the field laboratory were sent to ICDDR,B where they were re-stained with ZN and examined. Discordant results were checked by another microscopist blinded to the initial result. All positives and 2·5% of the negative slides by FM were sent to the Damien Foundation (the leading TB research organization in Bangladesh) for re-examination with FM. All identified cases were referred to a nearby DOTS centre for initiation of treatment and follow-up.
Statistical approach
Data analysis was performed using the statistical package Stata v. 10.0 (StataCorp., USA). The crude prevalence was estimated as number of cases detected among the participating population and expressed as per 100 000 population. The corresponding 95% confidence interval (CI) was calculated by Poisson regression. Adjusted analyses were based on a weighted estimation of the prevalence. The participation weight assigned to an adult respondent was the product of a stratification weight and an attrition weight. The stratification weight was the inverse of the probability of being included from the rural or urban sampling frame. The participation weight was the inverse of the probability of actually participating in the study when being eligible, which was assessed by logit estimation based on the variables age and sex. With these weights the study population was comparable to the target population of all individuals aged ⩾15 years. The overall weight (the product of the stratification weight and the participation weight) was scaled to the size of the target population to ensure that statistical tests were conducted on the appropriate number of degrees of freedom. Further analyses were performed using the complex cluster survey analysis option in Stata.
RESULTS
The field data collection was conducted from October 2007 to March 2009. Figure 3 summarizes the number of people surveyed, number of people eligible to participate, number of people that actually participated and the number of smear-positive cases for rural and urban clusters. In total 63 715 adults aged ⩾15 years were enumerated in the study population, of which 52 098 (81·8%) persons participated (Table 1). The mean (±s.d.) age of the participants was 35·5±15·7 years.
Table 1. General characteristics of the survey population

S.D., Standard deviation.
The eligible population in the selected clusters varied between 1403 and 2313 adults. The mean number of actual survey participants was 1302, with the participation rate varying between 57% and 92% with an average of 82% (data not shown). This relatively low participation rate is mainly due to field activities in the first six clusters. In these clusters the households were visited only once. When eligible persons were absent, no further efforts were made in recruiting them at a later stage. Instead, more households were included to reach the anticipated cluster sample of 1400 individuals. This approach resulted in an oversampling of the cluster and a subsequent low participation rate of about 60–70%. When this was observed, the procedure was changed and the field teams were instructed to visit the households several times before assuming the absence of an eligible individual. This reduced the number of individuals on the census list and increased the participation rate in the rest of the clusters with an average participation rate of 90%. This difference between the cluster participation rates was incorporated into the design of the individual analysis weights for the respondents.
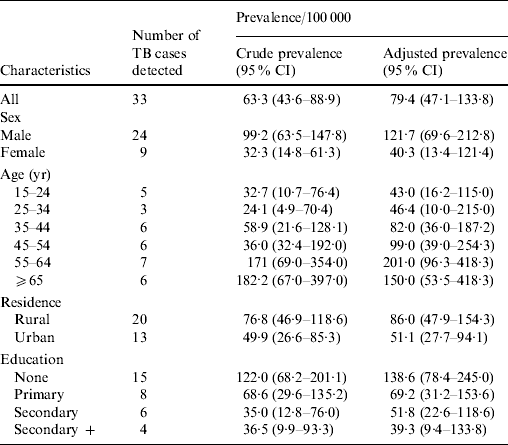
In the survey, 33 sputum smear-positive pulmonary TB cases (SS+TB) were detected. The overall crude prevalence of smear-positive TB was therefore 63·3/100 000 population (95% CI 43·6–88·9) and the adjusted prevalence was 79·4/100 000 (95% CI 47·1–133·8) in persons aged ⩾15 years. Of the 33 SS+TB cases detected, 20 (61%) were positive in two initial samples of sputum collected, 13 (39%) initially had one sample positive and had either an additional sample positive or chest X-ray positive (data not shown). A quality control process was adopted and practised throughout the survey period. All FM positive slides were restained and examined with ZN stain on a routine basis at the field site and all positive ZN slides were re-examined at the central laboratories. No discrepancies were reported. Of the 33 smear-positive cases, 29 were also culture-positive for Mycobacterium tuberculosis. Of the four culture-negative cases three were diagnosed as TB cases by X-ray with one sputum positive (scanty) on both FM and ZN microscopy and one had contamination. The majority of cases (23, 79%) were found to be sensitive to all drugs and only one (3·4%) multidrug resistant (MDR) case was detected. At the time of detection, three (9·0%) of the 33 detected cases were under DOTS treatment at a local centre and 15 (45·5%) had a history of cough of varying duration.
The crude and adjusted prevalence of SS+TB was higher in males than females and in rural rather than urban areas. The crude prevalence in males was 99·2 (95% CI 63·5–147·8) and the adjusted rate was 121·7/100 000 (95% CI 69·6–212·8), while in females the rates were 32·3 (95% CI 14·8–61·3) and 40·3 (95% CI 13·4–121·4), respectively, with a female:male ratio of 0·33. The crude and adjusted prevalence rates for the rural areas were 76·8 (95% CI 46·9–118·6) and 86·0 (95% CI 47·9–154·3), respectively, and for the urban areas the rates were 49·9 (95% CI 26·6–85·3) and 51·1 (95% CI 27·7–94·1), respectively (Table 2). The prevalence rates increased with increasing age except in the ⩾65 years age group. The prevalence decreased with advancement in education in different stages with the highest prevalence in the uneducated group (138·6/100 000, 95% CI 78·4–245·0).
Table 2. Estimated number of sputum smear-positive tuberculosis cases and prevalence in Bangladesh, 2007–2009, by age, sex, and area of residence (per 100 000)
CI, Confidence interval.
DISCUSSION
This survey is the first nationwide representative survey to determine the prevalence of smear-positive TB since the introduction of the DOTS programme in Bangladesh in 1993. The survey found a significantly lower prevalence of sputum-positive TB compared to previous surveys [2, 3]. The survey estimate is in agreement with a regional survey conducted in the rural area of Matlab upazila where the prevalence was reported to be 95/100 000 adult population [Reference Zaman6]. However, the prevalence rate was much lower in a survey conducted in another area by the Damien Foundation where the overall prevalence of smear-positive TB was only 24/100 000 [Reference Salim5]. Both the surveys screened for suspected TB cases and then collected specimens from them for microscopy. The surveys varied in their sampling techniques, including population and content of the screening interview. The survey results showed a much lower prevalence than the recent WHO estimate of 142/100 000 in all age groups [12]. The WHO estimate is based on a limited tuberculin survey conducted in 1960 [2] and on routine case notification data. Given the weaknesses in routine surveillance and notification of TB in Bangladesh, the WHO estimate has marked uncertainties. Results from the population-based prevalence survey of smear-positive TB other countries revealed higher rates in Viet Nam (196·8/100 000) and Eritrea (90/100 000) than in Bangladesh [Reference Hoa13, Reference Sebhatu14].
The prevalence was found to be higher in the rural population (86·0/100 000) compared to urban (51·1/100 000). Similar findings have been observed in the Viet Nam survey [Reference Hoa13]. In Bangladesh urban areas are densely populated and about one third of the population lives in crowed slums. A lower number of cases in urban areas was also notified in the NTP in 2009 [15]. In 2009, the NTP notified a total of 151 062 of all forms of TB cases (104/100 000 population) nationwide, of these 17·7% were reported from urban areas. The urban areas of Bangladesh currently contain about 30% of the total population and are characterized by high mobility and internal migration. The DOTS coverage started later and was less organized than in the rural areas. Of note, in urban areas health service delivery is pluralistic in nature with the presence of a large number of primary, secondary to tertiary public and private health centres as well as qualified and non-qualified private practitioners [Reference Perry16, Reference Hossain17]. There has been intensified advocacy, communication and social mobilization (ACSM) activities for TB control nationwide over the last few years which might have impacted health-seeking behaviours in the urban population. Further, the urban population included in the study could have been more affluent (since few individuals from the slum population were included randomly in the survey) compared to the rural population. The interaction of all these factors might be the reasons for lower prevalence of SS+TB in urban areas compared to rural areas.
In this survey the prevalence of TB in males was threefold higher compared to females. The findings are consistent with routine case findings and with local sporadic surveys conducted by different organizations [Reference Chowdhury4–Reference Zaman6]. Since 1993 a higher male:female ratio has also been found consistently in notified cases in the NTP, except for the <14 years age group [15]. The higher prevalence in the elderly age group is probably an indication of shifting of infection pattern and transition of disease prevalence. Since 2006, the average age of suspect cases examined was >35 years in the NTP [15]. This also indicates a shift in age. Moreover, about 60% of new SS+cases notified to the NTP in 2009 were in this age group. The inverse relationship of TB prevalence which was highest in uneducated persons, as noted in our survey, is probably related distally with knowledge, stigma, poverty, and health-seeking behaviour in TB cases.
In our survey the decision to adopt the approach of collecting specimens from all individuals was made primarily for logistical reasons. Moreover, guidelines for conducting TB prevalence surveys were not available at the time the survey was planned. Considering the availability of infrastructure facilities and other support the chosen strategy was found to be a feasible and affordable approach for the survey. All eligible individuals were asked to provide two sputum samples for smear microscopy. Theoretically this would provide the best estimate for smear positivity in the population because nobody was excluded from examination. The strategy applied in Bangladesh had already been tested in Eretria [Reference Sebhatu14]. The applied approach might have limitations if the participation rate is not adequate, the quality of the collected specimens is poor, consistent and standard field and laboratory procedures are not maintained, and microscopy is conducted poorly. In this survey, even though we had lower participation in a few early clusters (60–70%), this was soon corrected to >90% in the rest of the clusters. Standard sputum collection methods were applied throughout the survey. No specimens were rejected based on macroscopic aspects and rigid quality control measures were applied.
Initial smear microscopy was performed using FM because this technique allows for a more rapid assessment of the specimen. It has at least the same sensitivity and specificity as conventional ZN microscopy – estimated to be between 52% and 97% [Reference Steingart18]. Furthermore, FM is assumed to perform better than ZN microscopy in identifying paucibacillary specimens [Reference Steingart18, Reference Ba and Rieder19]. Similarly, a sample (2·5%) of negative slides was re-examined and no false-negative results were reported. Considering the low sensitivity of the sputum microscopy the underestimation and uncertainty of our estimate might be greater. Assuming 75% sensitivity of the fluorescence and with no false-negative results reported in the field, the prevalence of smear-positive TB might be as high as 105/100 000 (95% CI 79–137) population aged ⩾15 years.
In our study >50% of sputum smear-positive cases did not have any symptoms of cough. The survey did not use cough as a screening criterion for inclusion. These cases would have been missed and the prevalence underestimated, if cough had been used as a screening criterion. Another approach for the prevalence survey was screening of individuals using chest X-ray, which may miss some cases as not all SS+TB cases show abnormalities on chest X-ray at any given time point. Similarly first screening for suspects based on chest symptoms might also result in missing mildly symptomatic and asymptomatic cases. In this survey we observed that about half of the detected cases did not report cough at the time of the survey. Further, only three cases were under DOTS at the time of survey indicating most cases were unknown to DOTS. Similar findings have also been reported from Viet Nam [Reference Hoa13]. This would have resulted in a serious underestimation of prevalence if this strategy had been adopted. Screening using a combination of chest X-rays and symptoms, the method advocated by WHO for TB prevalence surveys, is costly and needs much expertise in the field. Adopting this strategy produced an overestimation in Viet Nam [Reference Hoa13].
Some limitations of the study should be noted. The study did not cover the population aged 0–14 years which makes it difficult to calculate prevalence in the whole population. There is a paucity of TB data for Bangladeshi children and sputum samples are rarely available from children. Moreover, workplace areas, prisons and other institutionalized populations were not covered. The overall sampling strategy selected 20 urban/semi-urban and 20 rural clusters regardless of the underlying population in rural and urban areas. This disproportionate sampling in the two groups was corrected during the analysis by including a stratification factor in the overall weighing of participants. This resulted in a valid estimate of the denominators used in the calculations, but a loss of statistical power due to inefficient sampling reflected in a wide interval around the estimate. The statistical power was also negatively influenced by the small number of identified cases which was reflected in the overall design effect of 2·7. Due to logistical reasons only 2·5% of the negative slides were rechecked. This leaves the possibility for ascertainment bias in the survey leading to a possible underreporting of smear-positive TB cases. Further, there was no culture performed on smear-negative cases which precludes assessment of the prevalence of bacteriologically confirmed TB.
Despite the limitations and logistic constraints, the survey has produced a valid and reliable estimate of the prevalence of smear-positive TB in the population aged ⩾15 years. It has highlighted the higher prevalence in rural areas and aged population, and a disproportionate distribution in the male and female population. The TB control programme in Bangladesh has achieved good coverage of case detection and treatment success rates over the past few years, but the present findings warrant intensified TB control activities be continued, with increased emphasis given to older people and the rural population of the country. It is recommended that control surveys are conducted at regular intervals in order to monitor the TB situation in Bangladesh.
ACKNOWLEDGEMENTS
This research study was funded by World Health Organization (WHO) and Global Fund to Fight AIDS, Tuberculosis and Malaria (GFATM), grant number SEBAN TUB 001 XW06U. ICDDR,B gratefully acknowledges the commitment of WHO and GFATM to its research efforts. We also gratefully acknowledge the contribution of the National Tuberculosis Control Programme (NTP) of Bangladesh for their support and guidance in the performance of this study. The study team is deeply indebted to the KNCV Tuberculosis Foundation of The Netherlands for technical assistance and support. We also express our sincere thanks and appreciation to BRAC, Damien Foundation, National Institute of Diseases of Chest and Hospital (NIDCH), and all other organizations and individuals involved for their effective participation in all steps of implementation of the study. Finally we acknowledge the support of all our project staff and the community members throughout Bangladesh, without whom this study would never have materialized.
DECLARATION OF INTEREST
None.







