Introduction
In 2015 the World Health Organization announced the ambitious goal to reach zero human deaths from dog-mediated rabies by 2030 [1]. While mass dog vaccination is anticipated to be an important part of any effective strategy, pre- and post-exposure vaccination of people living in countries endemic for rabies and of international travellers will remain a cornerstone of human rabies prevention [Reference Zinsstag2].
Rabies is a viral zoonotic disease that is widely spread across the globe. It is caused by lyssaviruses of the family Rhabdoviridae. Rabies can affect all mammals including humans and is responsible for more than 59 000 human deaths each year, most cases occurring in Asia and Africa and around 40% of cases occurring in children <15 years [Reference Hampson3]. In rabies-endemic regions more than 95% of human rabies cases are acquired by bites of infected dogs, a small proportion is due to transmission through bats, foxes, jackals, mongoose, racoons, skunks and wolves [4]. Except extremely rarely after transplantation of rabies infected tissue and organs human-to-human transmission has not been confirmed. As soon as symptoms occur, rabies is inevitably fatal leading to progressive encephalitis [4, 5].
In the European Union/European Economic Area, most member states have not seen autochthonous cases for decades and only a few cases of rabies in humans are reported each year. From 2014 to 2018 only six traveller-related cases of rabies were reported in Europe [6]. However, high awareness levels in Europe are still important as the re-emergence of rabies in northern Italy in 2008–2011 and Greece in 2012–2013 shows [Reference Tsiodras7]. In Germany rabies was eliminated after oral vaccination of foxes, whereby the last case of terrestrial rabies was detected in a fox in 2006. Today Germany is officially declared free from terrestrial rabies, only bats being a reservoir for European bat lyssaviruses (EBLV-1 and EBLV-2) [8].
Rabies is entirely preventable by active immunisation with rabies vaccine as pre-exposure and booster post-exposure prophylaxis. According to the WHO guidelines the German federal government agency and research institute responsible for disease control and prevention (RKI) recommends a rabies post-exposure prophylaxis (R-PEP) with wound washing and immediate vaccination as active immunisation after category II (nibbling of uncovered skin, minor scratches or abrasions without bleeding exposure). After category III exposure (transdermal bites or scratches, contamination of mucous membrane or broken skin with saliva, exposures due to direct contact with bats) wound washing, immediate vaccination and administration of rabies immunoglobulin (RIG) as passive immunisation is recommended. In non-immunised persons immediate intramuscular vaccination should be administered either according to the 5-dose ‘Essen’ regimen on days 0-3-7-14-28 or the 4-dose ‘Zagreb’ regimen on days 0-0-7-21 based on the current RKI guidelines [4, 8]. The 2018 updated WHO guidelines recommend a 4-dose ‘Essen’ regimen on days 0-3-7 and between 14 and 28 days instead of the 5-dose regimen [8]. However, the updated WHO guidelines have not been incorporated in the RKI guidelines until now. The RKI guidelines remain standard for clinical practice in German hospitals. Furthermore, previously immunised people should receive intramuscular vaccine on days 0 and 3 after grade III exposure.
The aim of our study was to determine the demographic characteristics of patients receiving R-PEP, the chosen regimen, the adherence to this regimen and the documentation of wound care and tetanus prophylaxis at a German University Hospital.
Methods
We retrospectively analysed all patients, who received R-PEP (category II and III exposure) at the University Hospital Bonn between 1st January 2013 and 30th June 2019. Patients > = 18 years were seen in the A&E Department either by an infectious disease specialist or by a surgeon, patients <18 years were seen in the Paediatric Department. In Germany, patients at risk for rabies infection are mostly referred to emergency departments of large hospitals as RIG needs to be available.
From an electronic database, we recorded the patients' demographic and clinical information, focusing on animal exposed to, classification according to RKI exposure category and country of exposure. Furthermore, clinical finding, time between exposure and R-PEP, R-PEP regimen and documentation of tetanus vaccination were analysed. Missing documentation of tetanus vaccination and wound washing was retrospectively analysed but was not counted as deviation from R-PEP guidelines.
This study was performed in accordance with local institutional review board (IRB) guidelines of the University of Bonn (Nr. 200/20).
Results
From 1st January 2013 to 30th June 2019, a total of 90 patients received R-PEP in the A&E Department of the University Hospital Bonn translating to 12.86 patients per year receiving R-PEP. The majority were women (53%, n = 48) and 47% (n = 42) were men. The median age was 34 years (IQR: 24 years), 9% (n = 8) were younger than 18 years old. Patients presented median 1.5 days (IQR: 6 days) after the animal bite. In 10 cases (11%) simultaneous vaccine and RIG was administered without indication for R-PEP. All these patients presented after animal bites in Germany from foxes (n = 3), squirrels (n = 2), martens (n = 2), a bat (n = 1; exposure category grade I), an edible dormouse (n = 1) and a domestic cat (n = 1). In the following analysis these cases were not included.
Overall, 26 patients (33%) were exposed to animals in Germany, the majority being exposed to bats (46%) but also 38% to dogs and 15% to cats of unknown origin (Fig. 1). Patients exposed to animals in Germany presented median 1 day (IQR: 1 day) after the animal bite. According to the exposure category, one patient was a grade II exposure and correctly received vaccine according to the Essen regimen. After grade III exposures 84% of patients received simultaneous vaccine and RIG, while 12% of patients received vaccine only and one patient received vaccine and RIG despite previous rabies vaccination. The majority (76%, n = 19) of grade III exposure patients with domestic bites received the Essen regimen, only one (4%) patient was vaccinated according to the Zagreb regimen (Fig. 2). The regimen of one patient (4%) could not be assigned to either regimen, one patient (4%) was immunised before and received two doses of vaccine on days 0 and 3 plus RIG which was superfluous (information missing for n = 3).

Fig. 1. Animal exposure in patients receiving R-PEP.
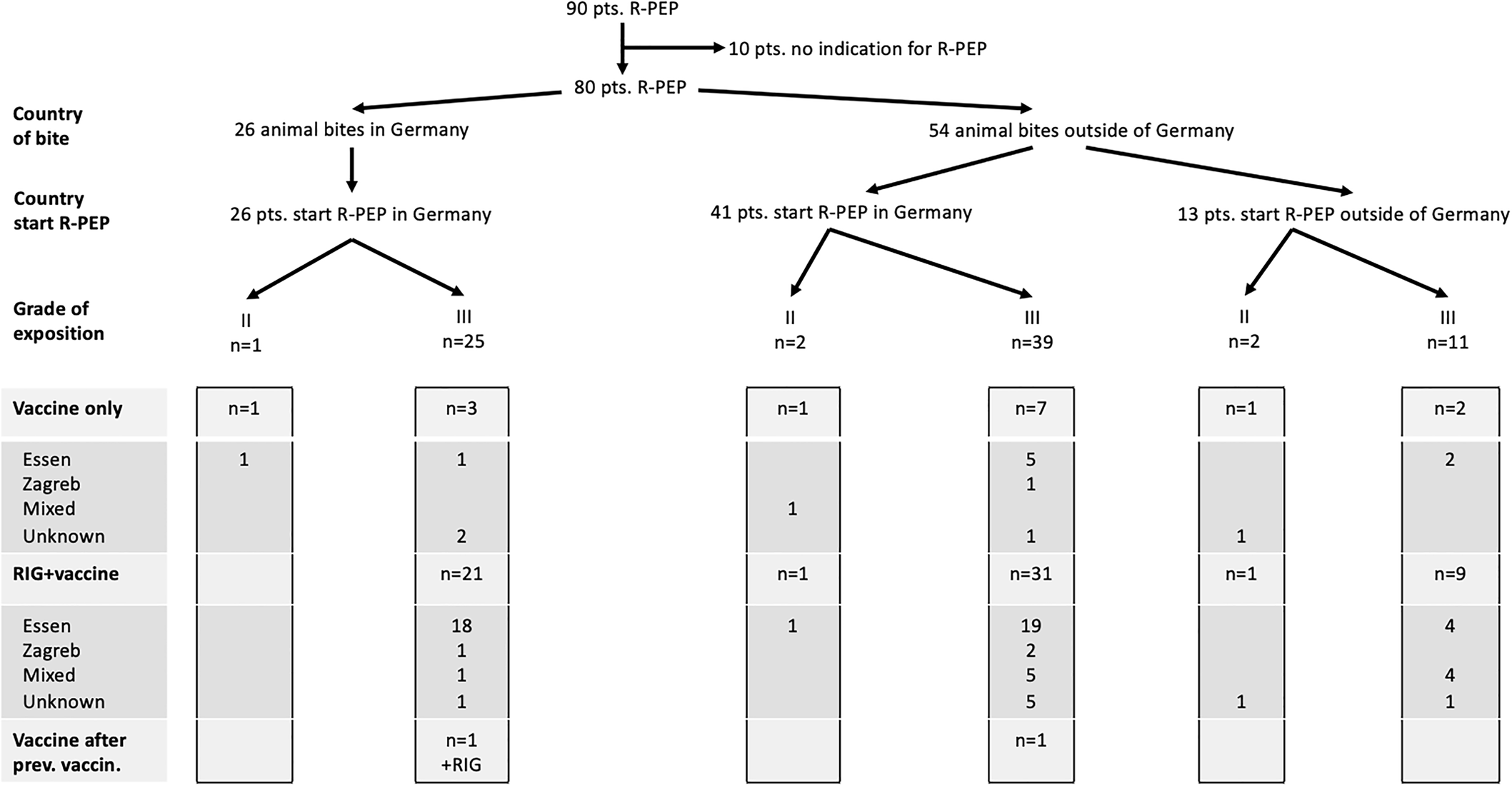
Fig. 2. R-PEP after animal bites in and outside of Germany.
The majority (68%, n = 54) of animal bites occurred during touristic travel abroad. Travellers presented median 6,5 days (IQR: 8 days) after the animal bite. Most international travellers (37%) returned from Asia, 19% from Africa, 11% from Southern Europe, 15% from Eastern Europe, 11% from South America and 4% from Central Europe (travel destination missing for n = 2). Most travellers experienced rabies risk contacts with dogs (52%), other animals were cats (28%), monkeys (15%) and bats (2%) (information missing for n = 2) (Fig. 1). Four travellers experienced grade II exposures and correctly received vaccine, two of them also received RIG which was superfluous. A grade III exposure was experienced in 50 travellers, 40 patients (80%) receiving simultaneous vaccine and RIG, while nine patients (18%) received vaccine only, one patient received vaccine after previous rabies vaccination. The majority (n = 31) of patients received the Essen regimen, only three patients were vaccinated according to the Zagreb regimen (Fig. 2). The regimen of 10 patients could not be assigned to either regimen (information missing for n = 9), one patient was immunised before and received in compliance with the guidelines two doses of vaccine on days 0 and 3. Altogether, 13 patients (n = 2 grade II, n = 11 grade III) had started R-PEP in the holiday destination.
Deviations from R-PEP guidelines were found in 51% (n = 41/80) of all patients receiving R-PEP. There was no significant difference concerning deviations from guidelines comparing patients being treated by infectious disease specialists vs. surgeons and paediatricians. Twelve patients (15%) with category III exposure were wrongly only given vaccine instead of vaccine and RIG simultaneously. These patients had no history of rabies immunisation. Seven patients were bitten by dogs, five patients by cats. Countries of exposition included Turkey (n = 3), Germany (n = 3), Brazil (n = 1), Cuba (n = 1), Latvia (n = 1), Sri Lanka (n = 1), Tanzania (n = 1) and Thailand (n = 1). Administration of RIG around the bite was performed correctly in 20 cases. In 24 cases the site of RIG administration deviated from the guidelines as RIG was not infiltrated around the bite, but only given intramuscularly. The documentation of site of administration of RIG was incomplete in 20 cases. The Essen and Zagreb vaccine schemes were followed correctly in 51 and 4 patients, respectively (information missing for n = 12). Two patients were not vaccinated according to the Essen/Zagreb regimen as they had a history of rabies vaccination and correctly received vaccine on days 0 and 3. There was a deviation from the chosen vaccine scheme in 14% (n = 11/80), as five patients vaccinated according to the Essen regimen received their fifth vaccine on day 21 instead of day 28. Furthermore, incorrect intervals in the vaccination schedule occurred in one patient and in five patients vaccination cycle was incomplete.
Extensive wound washing according to R-PEP guidelines was performed and documented in 23 cases, 10 of those presenting ≥3 days after the bite. In 11 patients wound washing was not performed and in 46 patients documentation of wound washing was missing. Twenty patients had a sufficient tetanus vaccination before animal bite, five patients were vaccinated simultaneously to R-PEP in our University hospital and five patients had been vaccinated abroad after exposure. However, in 50 patients tetanus vaccination status was not documented.
Discussion
Adherence to WHO R-PEP guidelines by patient and health-care practitioner following a suspected rabies exposure is essential to end human deaths from rabies [4]. In our study we analysed epidemiologic characteristics of patients initiating R-PEP at an A&E Department of a large German University Hospital over a 6-year period. Our study found challenges associated with vaccine completion, site of RIG administration, indication of R-PEP and consequent documentation of wound washing and tetanus prophylaxis.
Vaccine completion rates vary substantially between countries (16.3–92%) and are positively impacted by free provision of R-PEP and easily accessible vaccination centres [Reference Tarantola9]. In the past several efforts have been made to simplify rabies PEP and make the regimen more convenient, including reduction of the number of doses and visits [Reference Rupprecht10]. Especially in endemic low-income regions intradermal (ID) vaccination regimens have proven to be more cost-effective than intramuscular (IM) ones. Despite easily accessible availability of vaccination at no charge in our study we still found that 14% of patients on R-PEP did not receive their vaccine regimen correctly. This was mainly due to deviations from vaccination regimen (8%) and missed vaccinations (6%). Furthermore, despite WHO category III exposure only vaccine but not simultaneous vaccine and RIG was performed in 15% of patients. This cannot be explained by lack of access to RIG, as in Germany emergency depots guarantee country-wide availability of RIG. In 24 patients RIG was not applied around the wound but only injected intramuscularly in the deltoid muscle. These defaults may be explained by the rare indication of R-PEP as in our study only 12.86 patients received R-PEP per year. Our findings suggest that further efforts are needed to educate providers and patients, as adherence to R-PEP guidelines are crucial for reducing rabies mortality. Refresher trainings for health care providers should regularly be performed in institutions distributing R-PEP. However, there may also be a missing risk perception in patients living in rabies low-burden countries or countries free from terrestrial rabies explaining deviations and low completion rates. Patients may have been misled by a false sense of security receiving an initial dose of vaccine and RIG. Given the suboptimal completion rates found in our study, we investigated whether any of the patients from our study died due to lack of adherence. Using the rabies national surveillance system from the RKI we found no documented rabies death during our observation period (assuming no patient moved to another country).
The estimated incidence of potential rabies exposures requiring R-PEP among international travellers is 0.4 per 1.000 per month of stay [Reference Gautret11]. This has increased in the past years probably due to greater diversity of travel destinations and number of international travellers [Reference Gautret12]. Our study supports previous study findings reporting rabies exposures among international travellers most frequently in Asia. However, of major concern is the finding that only a small number (24%) of international travellers received or started R-PEP in the holiday destination leading to delay of initiation of R-PEP. This supports recent studies showing that only 5–20% of travellers received RIG in the country of exposure when indicated [Reference Carroll13–Reference Gautret15]. However, this finding may be multifactorial and possibly due to the global limited low-threshold availability of R-PEP in these travel destinations [Reference Abela-Ridder16, Reference Jentes17]. This may be aggravated by the insufficient awareness of international travellers on the indication of R-PEP after animal bites. More than 90% of international travellers in our study had not received rabies vaccination before travelling. The availability of RIG and vaccine abroad is unpredictable everywhere and cannot be relied on. All travellers, especially to Asia and Africa, should be encouraged to attend travel clinics for vaccination. Since one course lasts a lifetime, it is an investment [Reference Jentes18]. WHO has updated the recommendation of vaccine to two doses on days 0 and 7, after several studies demonstrated similar immunogenicity compared to three-dose regimens [4]. Reducing number of doses and subsequently time frame for vaccine may help to reach a higher rabies vaccination coverage in travellers.
Germany has been officially declared free from terrestrial rabies and is only found in bats. Thus, the relatively high number of R-PEP after animal bites in Germany was surprising and mostly due to the fact that the origin of the animal was unknown. This may explain why RIG was not given after grade III dog bites in Germany as rabies risk may have been estimated low by the treating clinicians.
The R-PEP guidelines inform about the importance of extensive wound washing and also recommend evaluating the tetanus status and vaccinating in case there is no protection. Extensive wound washing and present tetanus status was only documented in 29% and 37%, respectively. The RKI guidelines recommend tetanus prophylaxis irrespective of type of animal bite. Thus, the low documentation rate cannot be explained by the number of bat bites in our study where the need for tetanus vaccination may be questionable [8]. Introducing standardised operating procedures for R-PEP in A&E Departments of institutions distributing R-PEP may help to completely fulfil the R-PEP guidelines.
Our study has limitations. Because this was a single centre study our findings may not be a representative for the general population in Germany. Data collection was done retrospectively, thus our analysis was dependent on the electronic documentation and e.g. questioning the patient about extensive wound washing and present tetanus protection may have been performed and not documented. Thus, the high percentage of missing information about wound washing and tetanus protection may be estimated too high and was thus excluded from the deviations of R-PEP guidelines. Unfortunately, the number of patients seeking advice for R-PEP after animal bites, but not qualifying for R-PEP, was not available in our study.
Findings from this evaluation have important implications for R-PEP practice. First, the vaccination completion rate is much lower than expected in a country of high-standard health care. This indicates the need for a national surveillance system following two variables: initiation of R-PEP and vaccine completion rates. Second, refresher trainings of health care providers distributing R-PEP should be regularly performed to keep a consistent standard of care. Third, before travelling abroad international travellers should receive rabies risk assessment, seek advice for travel vaccination and be educated by health-care practitioners about avoiding contact with animals and behaviour after animal bites. Meanwhile we should strengthen communication on rabies knowledge, vaccination schedule, R-PEP guidelines and make sure that the guidelines are followed correctly.
Despite rabies elimination in Germany patients frequently seek advice for R-PEP in A&E departments. Our data show that there is a high need for education on indication for rabies vaccination before travel, R-PEP during and after travel and for implementation of precise R-PEP guidelines in daily clinical practice. A more comprehensive study is needed to understand why high-risk individuals deviate from R-PEP vaccine regimen.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of Interest
None.
Data
Data were analysed as part of a quality control and may not be made available publicly in an individualised form. By legal restrictions we are only allowed to present aggregated data.





