INTRODUCTION
Hantaviruses are widely emergent zoonoses that are caused by a group of rodent/insectivore-borne RNA viruses of the genus Hantavirus, family Bunyaviridae, that cause clinical diseases of two forms, i.e. haemorrhagic fever with renal syndrome (HFRS) in Eurasia and Africa and hantavirus pulmonary syndrome (HPS) in the Americas. Although there is increasing evidence that these two forms compose a single syndrome [Reference Clement1], the traditional nomenclature is used in this article. These agents are transmitted to humans through the inhalation of aerosolized excreta of rodents of the Muridae and Cricetidae families [Reference Jonsson, Figueiredo and Vapalahti2]. The geographical distributions of these reservoirs determine the viral genotypes that are involved in human infection because each virus genotype is frequently associated with a specific rodent. More recently, several genotypes of hantavirus have been identified in some species of shrews, moles and bats, but the effects of these genotypes on public health remain to be determined [Reference Suzuki3–Reference Yadav, Vincent and Nichol6].
Evidence of hantavirus infection with associated HFRS in humans was detected in the New World (Recife, Brazil) for first time in 1993 [Reference Hindrichsen7]; however, the disease became notifiable [Reference Silva8] only after the first cases of HPS in São Paulo were confirmed in 1997. Since then, HPS has been identified in 14 of the 27 Brazilian states, and great difficulties have been experienced in attempts to control this disease (Brazilian Ministry of Health, report on hantavirus cases 1993–2011, unpublished data).
HPS can be considered an emerging disease in Brazil. Several studies have reported that HPS exhibits a seasonal pattern with peaks in the winter and spring months; however, cases have been detected throughout the year [Reference Lavocat and Wada9, Reference Teixeira10]. Until now, no studies have analysed the temporal trends of the disease and possible differences in seasonality across the macroregions of Brazil. We performed a study of all reported HPS cases in Brazil over an 11-year period to ascertain the temporal trends of the disease and to determine whether seasonal patterns and regional differences in the distribution of cases of this disease exist in Brazil.
MATERIAL AND METHODS
Study design
We performed a retrospective study of all reported cases of HPS in Brazil from 2001 to 2011.
Data source
The National Disease Notification System (SINAN) is a database that contains the clinical and demographic information of patients with diseases which create concerns for public health. Cases are reported throughout the country according to national definitions and in compliance with federal laws. We extracted cases with diagnoses of HPS according to the confirmation criteria adopted by SINAN, which defines HPS cases as follows: all individuals living in or having frequently visited known transmission areas and having compatible symptoms and/or at least one positive confirmatory laboratory test (i.e. positive for IgM ELISA, rising titres of IgG, or the presence of specific ribonucleic acid sequences as determined by polymerase chain reaction (reverse transcriptase–PCR and real-time PCR) or immunohistochemistry). Additional cases are considered to be confirmed in the absence of laboratory confirmation when an epidemiological link to a laboratory-confirmed case (i.e. contact within 8 weeks) can be established. The cases included in the study were not personally identified, and no attempt to access individual medical records was made.
Analytical procedures
We investigated the evolution of the total number of cases of HPS in Brazil across the five regions designated by the Brazilian Institute of Geography and Statistics (IBGE) based on environmental, demographic and economic differences; i.e. the Southeast, Northeast, North, South and Central-West regions. The incidences of the disease were calculated based on the population estimates calculated by the IBGE for 2005 (i.e. the middle of the study period). We also constructed frequency distribution diagrams of the relative numbers of cases by month and performed spatial analyses using the Gaussian kernel method to estimate the densities of the distributions of HPS cases in Brazil and each of its regions. To ascertain the seasonal variation in the number of cases, we initially investigated the variability of the case-number time-series with the Box–Cox transformation technique. The objective of this analysis was to obtain a more symmetrical distribution that better approximated the normal distribution. After ascertaining the existence of a trend in the series, we used a more robust non-parametric test based on Spearman's rank correlation coefficient. Finally, to determine the presence of seasonality in the time-series, we used the Kruskal–Wallis test. We used R software for Windows 2.15.2 (www.r-project.org) for statistical analysis and Terraview 4.2.3 (www.dpi.inpe.br/terraview) for the kernel density estimators.
Ethical issues
This study was conducted in accordance with the ethics resolutions of Brazilian law. Identifying information was not available for this study; hence, ethical approval was not required.
RESULTS
During the period from 2001 to 2011, 1486 cases of HPS were reported in Brazil, and three of the five regions of the country (i.e. Southeast, South, Central-West) accounted for 93·7% of HPS cases. The greatest incidence of HPS was observed in the South region. The absolute number of cases and incidence rates are depicted in Table 1, and the regionally density distributions are illustrated Figure 1.

Fig. 1. Hantavirus pulmonary syndrome case distributions based on kernel density estimations in Brazil between 2001 and 2011.
Table 1. Case distribution, incidence, time trend and seasonality of hantavirus pulmonary syndrome in Brazil and its subregions between 2001 and 2011
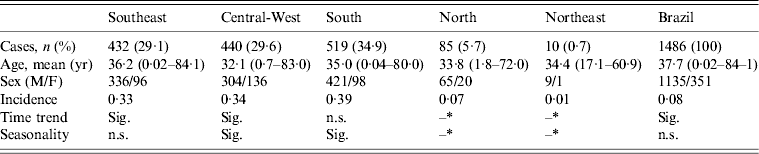
Sig., Significant; n.s., not significant.
* The North and Northeast regions were excluded from the analyses due to the low number of cases.
Analyses of the time trends of HPS across the study period revealed a change in the baseline number of cases reported to the system (P < 0·01). The Southeast and Central-West regions (P < 0·01) exhibited trends towards increases in the numbers of cases, and the South, North and Northeast regions (P > 0·05) did not. The evolution of the absolute number of cases of HPS reported in Brazil across the time period of the study is illustrated in Figure 2.

Fig. 2. Evolution of the absolute number of cases of hantavirus pulmonary syndrome in Brazil and its subregions between 2001 and 2011 (total number of cases, 1486).
The frequency distribution diagram of the relative number of cases by month for all of Brazil did not reveal a seasonal pattern, but regionally specific patterns were found when the distributions were stratified by region (Fig. 3).

Fig. 3. Relative frequency distribution of hantavirus pulmonary syndrome cases detected between 2001 and 2011 in Brazil, stratified by region.
We performed time-trend analyses to evaluate whether the findings displayed in Figure 1 were due to random chance. We found no significant seasonality for the entire country or its subregions, with the exception of the South region (P = 0·049). Table 1 also summarizes these findings.
DISCUSSION
Since 1997, when the first HPS cases were identified in Brazil, new cases have been continuously reported throughout the country and have mainly affected the Southeast, Central-West and South regions [Reference Silva8, Reference Teixeira10]. In this study, we demonstrated that the incidence of HPS increased across the duration of the study period. HPS is an emerging disease with a high mortality rate and poses a serious public health concern in Brazil and the American continents [Reference Lavocat and Wada9–Reference Elkhoury11].
The reasons for the growth in the number of cases of this disease in Brazil are not entirely clear, but epidemiological investigations have determined the effects of increased human agricultural activities in native territories and the ecological dynamics of the reservoirs [Reference Henkes and Barcellos12]. Deforestation and increased food availability near locations of intense human activity could be responsible for the displacement and increased densities of disease reservoirs [Reference Pini13]. Another possible factor is the uncontrolled growth of cities into areas that were previously native forest, which could result in the displacement of reservoirs to areas close to human dwellings [Reference Campos14, Reference Santos, Steiinke and García-Zapata15]. Indeed, the emergence of outbreaks or isolated cases of the disease in Brazil are frequently linked to specific situations and environmental risk factors that include, for example, the agricultural profile of the states in the South region and the urbanization of rural areas in the Central-West region. Despite all of this evidence, rapid improvements in the surveillance, detection and diagnosis, and the accompanying increases in physician education and public awareness may have contributed to some extent to the rise in the numbers of reported cases as occurs for all emerging diseases [Reference Hadorn and Stärk16].
In the distribution of the relative frequencies of cases, we observed clear regional differences in the occurrence of the disease. In the Southeast region, the majority of cases occurred during autumn and winter, and the majority of cases occurred in winter in the Central-West region and in spring in the South region. These patterns may be a consequence of differences in the distribution of reservoirs and viral genotypes circulating in each region. In the cerrado vegetation that is distributed throughout the Central-West region and part of the Southeast region, the main reservoir is Necromys lasiurus, and the main circulating viral genotype is Araraquara [Reference Nunes17, Reference Vieira18]. In the Atlantic Forest areas that are distributed throughout the Brazilian coastal margin, the main rodent reservoir species is Oligoryzomys nigripes, which hosts the Juquitiba variant [Reference Suzuki3, Reference Romano-Lieber, Yee and Hjelle19, Reference Donalisio20].
Regional particularities of agricultural activity also strongly influence the behaviour of reservoirs because the ideal times for planting and harvesting are unique to each region. These particularities may determine the main periods of reservoir activity, which in turn, would affect the frequencies of reservoir–human contact. In Brazil, the prevailing culture entails the planting of grains, such as soybeans, corn and coffee, and these grains serve as a source of food for the reservoir, especially during off-season periods in which the product is stocked and distributed [Reference Henkes and Barcellos12, Reference Donalisio20–22].
The limitations of this study include its retrospective design and utilization of secondary data from SINAN. The definition of hantavirus infection by SINAN includes only the most severe form of the disease or HPS [22]. The major consequence of this limitation is a lack of knowledge about the full epidemiological pattern of this disease in Brazil because mild to moderate forms are most likely the most common. The circulation of genotypes that have not yet been associated with human diseases, such as Jabora e Rio Mearim, may be involved in milder forms of the disease that cause confusion and other acute febrile illnesses [Reference Rosa23].
Despite the retrospective design and limitations of a passive surveillance system, this study indicates that HPS cases exhibit a trend towards increasing, and seasonal patterns that differ across the regions of Brazil. The determinants of these processes are not fully understood. Thus, the instruments of health surveillance should be further developed such that mild and moderate cases of the disease can be detected; specifically, a more sensitive definition of disease cases should be developed. Furthermore, control strategies that account for the epidemiological differences between regions should be planned and executed.
DECLARATION OF INTEREST
None.







