INTRODUCTION
Extraintestinal pathogenic Escherichia coli (E. coli) or ExPEC are the major cause of extraintestinal infections (e.g., urinary tract and bloodstream infections) in humans [Reference Foxman1, Reference Russo and Johnson2]. Half of all women will experience at least one urinary tract infection (UTI) during their lifetime [Reference Foxman1]. ExPEC frequently exhibit resistance to one or more antimicrobial agents, making even uncomplicated UTIs challenging to manage. There is emerging evidence that the E. coli that cause extraintestinal infections in humans may have a food or food animal reservoir [Reference Nordstrom, Liu and Price3]. Outbreaks associated with ExPEC have been identified suggesting that a food or environmental source for ExPEC may exist [Reference George and Manges4]. In previous studies, chicken and pork have been identified as potential sources of ExPEC causing human UTIs [Reference Nordstrom, Liu and Price3, Reference Manges5–Reference Leverstein-van Hall7] and women who reported higher consumption of chicken and pork were more likely to develop multidrug-resistant infections [Reference Manges5]. Closely related groups of human ExPEC have been recovered from retail meats or directly from food animals at slaughter [Reference Vincent8, Reference Bergeron9]. Although food is one possible source, ExPEC have also been identified in multiple non-human reservoirs, including companion animals, wastewater and other environmental sources [Reference Johnson and Manges10].
Previous epidemiological studies have identified risk factors for the intestinal acquisition of ExPEC and development of extraintestinal infections. In one study of 290 subjects in Norway, development of a UTI caused by an extended spectrum β-lactamase-producing (ESBL) E. coli or Klebsiella pneumoniae was associated with travel to Asia, to the Middle East or Africa, exposure to fluroquinolones or β-lactam antibiotics, diabetes mellitus and freshwater swimming in the past year [Reference Soraas11]. A second epidemiological study conducted in Pennsylvania, USA, including 165 cases of UTI caused by fluoroquinolone-resistant E. coli and 1836 controls who experienced a UTI caused by a fluoroquinolone-susceptible E. coli, found that Asian race, renal disease and previous exposure to nitrofurantoin were risk factors for fluoroquinolone-resistant UTI [Reference Rattanaumpawan12]. Other studies based on laboratory and clinical data have identified exposure to health care facilities and prior antimicrobial use as risk factors for antimicrobial-resistant, community-acquired UTIs [Reference Steinke13, Reference Ikram14].
The immediate source of the E. coli that cause extraintestinal infections is typically the individual's own intestinal tract. While intestinal colonisation does not lead to any immediate ill effects, E. coli are available to cause disease when risk factors for an extraintestinal infection occur. For example, the leading risk factor for UTI in young women is sexual intercourse. The mechanics of sexual intercourse facilitate the transfer of intestinal E. coli from the anus, across the perineum to the urethra and bladder leading to infection. The most significant challenge in determining the source of ExPEC intestinal acquisition and infection is the fact that extraintestinal infections may occur weeks or months after an ExPEC isolate has colonised the gut. Therefore, trace-back investigations, which are useful for identifying the source of diarrheal disease outbreaks (where disease develops within a few days), are of limited use in epidemiological investigations of food or environmental exposures to ExPEC due to the long lag between intestinal acquisition and infection.
To investigate the possible sources of drug-resistant ExPEC, we performed a case-control study. The primary hypothesis of this study is that more frequent consumption of poultry or poultry products is associated with an increased risk of UTI caused by an antimicrobial resistant (resistance to ⩾1 antimicrobial class) or multidrug-resistant (resistance to ⩾3 antimicrobial classes) E. coli. The study also examines other potential risk factors and exposures associated with the intestinal acquisition of ExPEC and development of antimicrobial resistant UTI.
MATERIALS AND METHODS
Epidemiologic study design
Women with UTI were recruited at five university student health services (SHSs) across Canada (Halifax, NS; Montreal, QC; Guelph, ON; Toronto, ON; Vancouver, BC). Research ethics boards at each SHS site approved the study; all participants provided their informed consent (UBC REB H11-03439). UTI was empirically defined as the presence of two or more symptoms, including dysuria, increased urinary frequency or urgency, pyuria and hematuria. If a woman had recurrent UTIs, only the isolate associated with the first infection during the study period was included. Women who reported being pregnant, being recently hospitalised, having any urogenital abnormality or diabetes were excluded from the study. Aliquots of urine from all consecutive urine specimens from women with suspected UTI at each SHS site were collected from October 2012 to May 2015 and shipped to the study laboratory. Urine was cultured on a biplate containing selective media (MacConkey/blood agar) (Orion Diagnostica Uricult, Finland). E. coli was re-plated on MacConkey and identification was confirmed using CHROMagar™ MH Orientation (France) and by indole testing. A single colony was grown overnight in LB broth and then stored at −80 °C in 15% glycerol.
For the purposes of this case-control study, two sets of cases and controls were defined. In the first set, a case was defined as a woman with a UTI caused by an E. coli isolate resistant to ⩾3 or more classes of antimicrobial agents (termed multidrug-resistant or MDR) and controls were defined as a woman with a UTI caused by E. coli resistant to ⩽2 antimicrobial classes. In the second set, a case was defined as a woman with a UTI caused by E. coli resistant to ⩾1 antimicrobial classes (termed antimicrobial resistant) and controls were defined as women with UTI caused by an E. coli isolate susceptible to all antimicrobial classes.
Risk factor questionnaire
Women with suspected UTI recruited to the study completed a secure, online risk factor questionnaire. The questionnaire was devised partly based on the US Centres for Diseases Control, Foodborne Outbreak Response and Surveillance Unit Enteric Disease Hypothesis Generating Questionnaire from 2011 [15] and partly based on instruments used for nutritional assessments [Reference Boucher16]. The questionnaire focussed on exposures that may be associated with the intestinal acquisition of E. coli or that have been linked to E. coli associated with foodborne outbreaks, including diet, travel history, water source and contact with animals or animal products. The questionnaire also included questions about health status (particularly history of UTI or other infections), recent use of antimicrobial agents, age and other factors. Women were asked to recall these exposures over a period of 30 days prior to the index UTI episode (the date for the UTI health care visit). Information on regular food consumption patterns and major dietary changes over the previous 6-months was also elicited. Women were asked to estimate their average consumption of each food item, food type or exposure according to the following scale: never, a few times a month, 1–3 days per week, 4–6 days per week, or every day. We linked the recovered E. coli isolate transferred from the SHS to questionnaire data for each study participant by using unique student identification numbers in combination with date of birth.
Antimicrobial susceptibility testing
All E. coli recovered from the SHSs were sent to the Public Health Agency of Canada, National Microbiology Laboratory, Saint-Hyacinthe, Québec for antimicrobial susceptibility testing. The minimum inhibitory concentration values for E. coli were determined by broth microdilution method (Clinical and Laboratory Standards Institute (CLSI) M7-A8) using an automated system (Sensititre™, Automated Microbiology System, Trek™ Diagnostic Systems Ltd, West Sussex, England) [17]. The CMV2AGNF plate (Sensititre™, Trek™ Diagnostic Systems Ltd, West Sussex, England) was designed by the US National Antimicrobial Resistance Monitoring System and contains a panel of 15 antimicrobials agents for susceptibility testing. Resistance to an antimicrobial class was defined as resistance to any of the following agents: aminoglycosides (gentamicin, streptomycin, kanamycin); cephalosporins (cefoxitin, ceftiofur, ceftriaxone); chloramphenicol; macrolides (azithromycin); penicillins (ampicillin, amoxicillin/clavulanic acid); quinolones (ciprofloxacin, nalidixic acid); sulfonamides (sulfisoxazole, trimethoprim/sulfamethoxazole); and tetracyclines (tetracycline) [17]. Intermediate susceptibility to any antimicrobial agent was classified as susceptible.
Statistical analyses
Risk factors were evaluated in univariable analyses with UTI caused by MDR E. coli (resistance to ⩾3 antimicrobial classes) or antimicrobial resistant E. coli (resistance to ⩾1 antimicrobial classes) as the outcome; odds ratios and 95% confidence intervals were estimated. We performed multiple logistic regression, for each variable significant by univariable analysis, including age, SHS and either number of lifetime UTIs or use of antimicrobials in the past 6 months. This was done to examine the independent effect of each exposure on drug-resistant UTI by adjusting for any influence of UTI history and prior antimicrobial exposure on increased drug-resistant E. coli carriage. As these two confounders are linked, separate multivariable models were run. We also examined risk factors associated with developing a UTI caused by E. coli resistant to any cephalosporin or any quinolone to compare our results with results from studies of extraintestinal infections caused by ESBL-positive and quinolone-resistant E. coli, although ESBL-production was not directly assessed. Exposure variables were grouped into (i) never (reference group), (ii) a few times a month or 1–3 days per week and (iii) 4–6 days per week or every day, based on distribution. In a few instances, responses were collapsed (at the high end of exposure) to ensure there was a minimum of 10 subjects total included in each comparison for the analyses. All analyses were conducted using the R statistical program (version 3.3.1).
RESULTS
Study subjects
From October 2012 to May 2015, 1677 Uricults were received from the SHSs and 1238 were positive for E. coli. A total of 733 unique women with suspected UTI from the five SHS were enrolled into the study and completed the online study questionnaire. Of these, 399 women completed the online questionnaire and had an E. coli isolate submitted to the study. SHSs were not able to submit urine specimens for each potential UTI case; furthermore, some women completed the survey, but bacterial concentrations in their urine were below detection and therefore these could not be included in the analyses.
In total 164 women (41·1%) had UTI caused by E. coli resistant to at least one antimicrobial class (antimicrobial resistant). Of these 164 women, 98 (24·6%) experienced a UTI caused by E. coli resistant to ⩾3 antimicrobial classes (MDR). Characteristics of the women included in the final study sample are presented in Table 1. No significant differences in demographics (e.g., age, educational attainment or income) were observed between women with UTI caused by susceptible vs. multidrug resistant E. coli, except for women with MDR UTI who reported a greater history of antimicrobial use in the past 6 months (OR = 1·64; 95% confidence interval (CI) 1·02–2·55). These women also tended to have had a greater number of lifetime UTIs (Table 1). Detailed antimicrobial resistance information is provided in Table 2.
Table 1. Characteristics of the study subjects

* Odds ratio for any antimicrobial use in the past 6 months among those with UTI caused by MDR E. coli was 1·64 (95%CI 1·02–2·55). The 98 women with MDR UTI are a subset of the 164 women with UTI caused by E. coli resistant to at least one class of antimicrobials.
Table 2. Antimicrobial resistance phenotype distribution
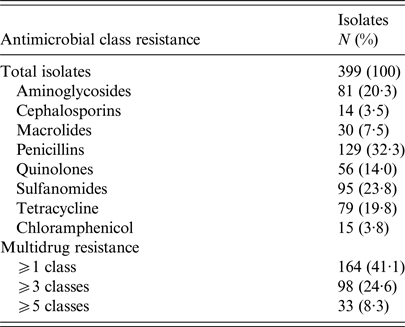
Classes were defined as: aminoglycosides (gentamicin, streptomycin, kanamycin); cephalosporins (cefoxitin, ceftiofur, ceftriaxone); chloramphenicol; macrolides (azithromycin); penicillins (ampicillin, amoxicillin/clavulanic acid); quinolones (ciprofloxacin, nalidixic acid); sulfonamides (sulfizoxazole, trimethoprim/sulfamethoxazole); and tetracyclines (tetracycline).
Meat consumption or exposure associated with UTI caused by antimicrobial resistant E. coli
Women who reported consumption of processed or ground chicken ⩾1–3 days per week were more likely to have a UTI caused by antimicrobial resistant E. coli (ORadj 1·58; 95% CI 1·00–2·49), after adjusting for age, a SHS and antibiotic use (Table 3), but the odds ratio after adjusting for age, SHS and UTI history was elevated but not statistically significant (ORadj 1·61, 95% CI 0·93–2·79) (Table 4). Shellfish consumption exhibited a similar risk for antimicrobial resistant and MDR UTI (ORadj = 1·95; 95% CI 1·20–3·16 and ORadj = 2·07; 95% CI 1·22–3·50), after adjustment for prior antibiotic use (Table 3) and for UTI history (Table 4), respectively. There was an increased risk of MDR UTI in women who reported consumption of raw meat (OR = 2·15; 95% CI 1·02–4·50) ⩾1–3 days per week vs. women who never ate raw meat in the univariable analyses; however, raw meat was not significant after adjustment for age and prior antibiotic use (ORadj = 1·76; 95% CI 0·81–3·83) (Table 3) or after adjustment for age, SHS and lifetime UTIs (ORadj = 2·38; 95% CI 0·98–5·78) (Table 4). Consumption of cooked ground beef ⩾1–3 days per week was associated with a lower risk of developing a UTI caused by E. coli resistant to at least one antimicrobial class (ORadj = 0·61; 95% CI 0·39–0·98 and ORadj = 0·56; 95% CI 0·33–0·95) (Tables 3 and 4), after adjustment for antimicrobial use and UTI history, respectively. Consumption of pork or other meats was not associated with increased risk of antimicrobial resistant or MDR UTI, nor was there an association between the cooking method for whole beef or hamburger/ground beef (i.e. rare vs. medium or well cooked).
Table 3. Exposures associated with UTI caused by antimicrobial resistant and MDR E. coli adjusted for age, student health service* and prior antibiotic use
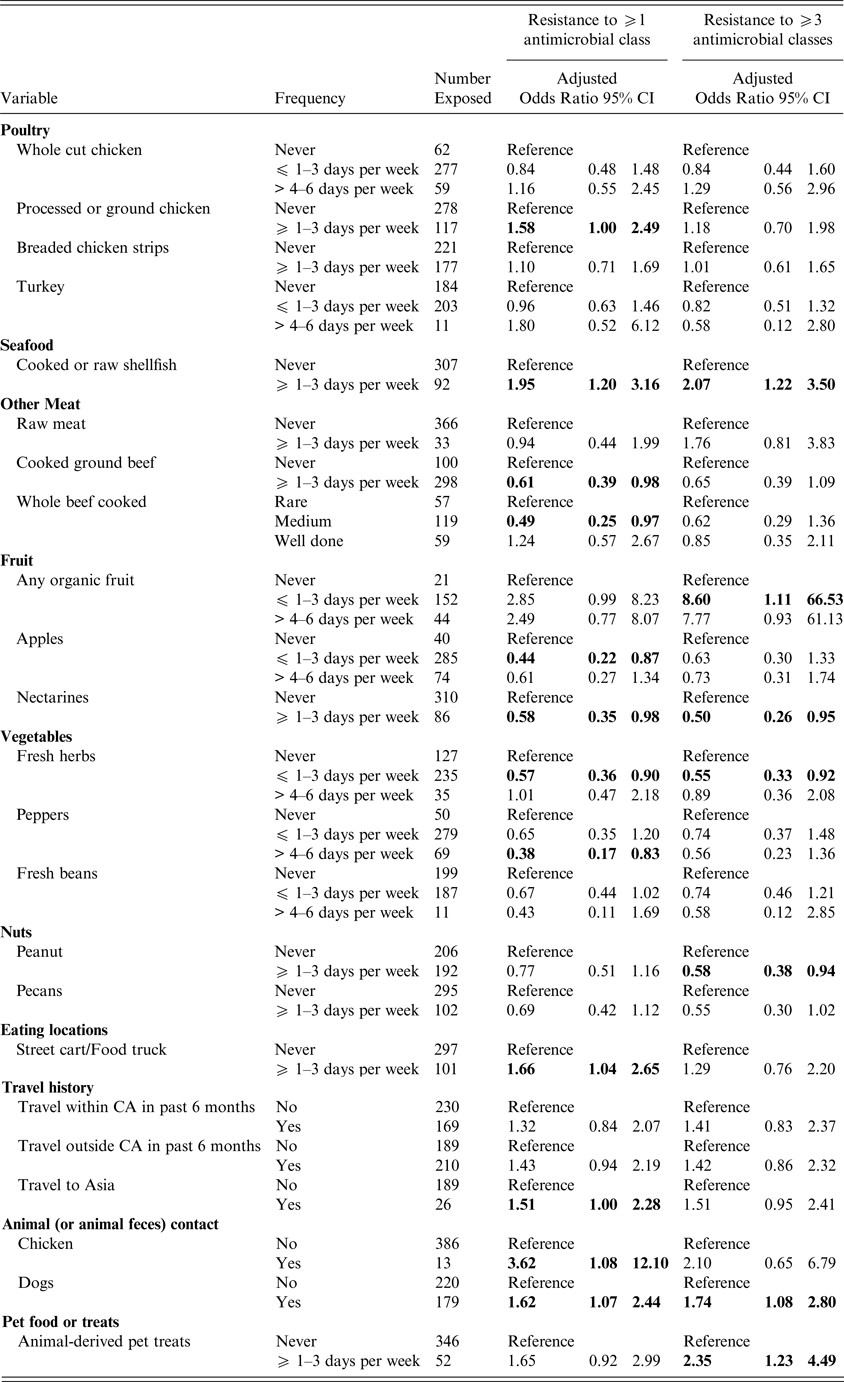
Odds ratios are adjusted; bold indicates 95% confidence limits that exclude 1.0). *The Nova Scotia site was excluded from the multivariable models, as the number of subjects (n = 5) was small. Results for all meat related exposures assessed on the questionnaire are included as meat consumption was the primary hypothesis of the study. Otherwise, only those exposures associated with an antimicrobial resistant or MDR UTI in the univariable analyses are presented.
Table 4. Exposures associated with UTI caused by antimicrobial resistant E. coli adjusted for age, student health service* and UTI history
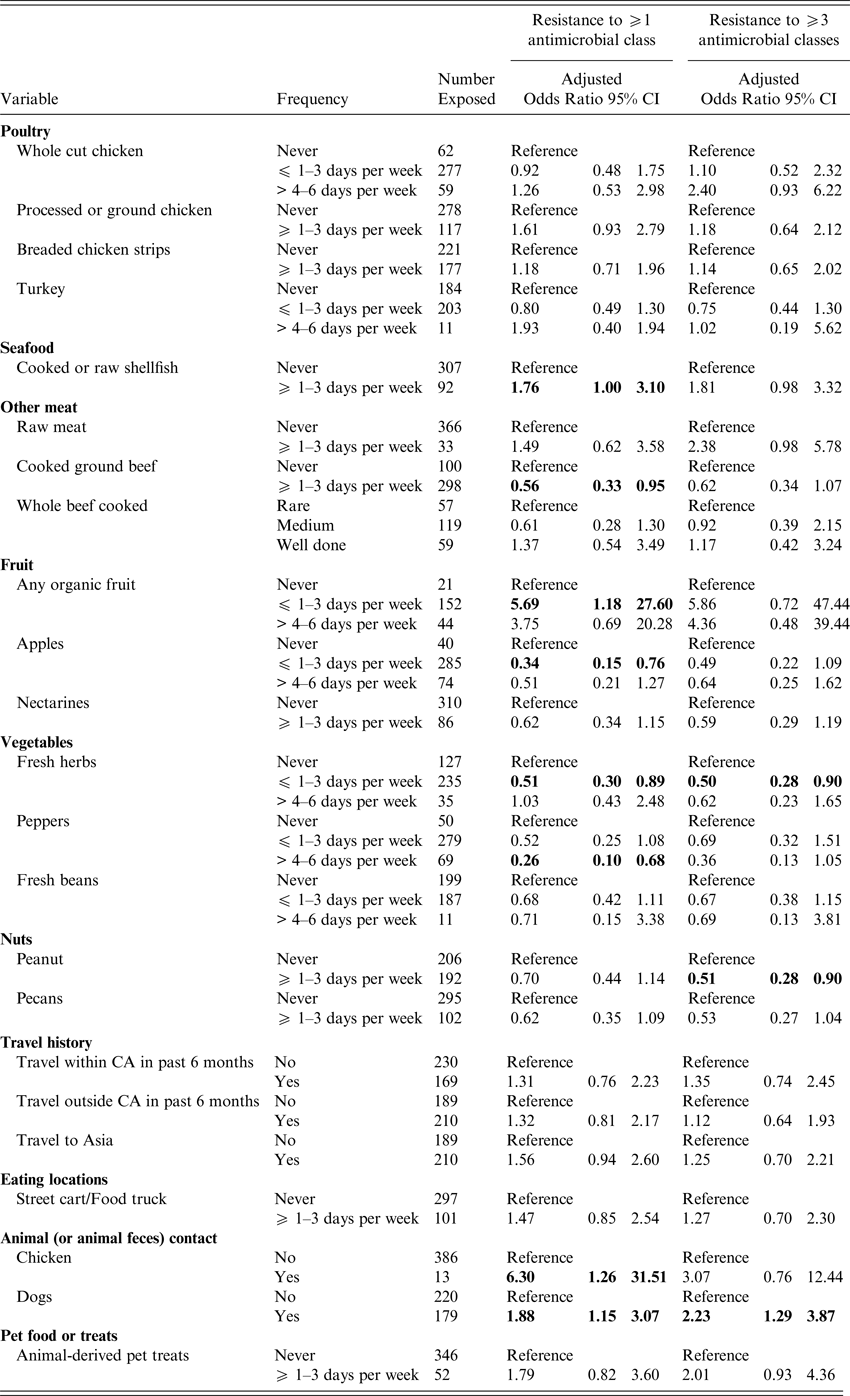
Odds ratios are adjusted; bold indicates 95% confidence limits that exclude 1.0). *The Nova Scotia site was excluded from the multivariable models, as the number of subjects (n = 5) was small. Results for all meat related exposures assessed on the questionnaire are included as meat consumption was the primary hypothesis of the study. Otherwise, only those exposures associated with an antimicrobial resistant or MDR UTI in the univariable analyses are presented.
Non-meat dietary exposures and UTI caused by antimicrobial resistant E. coli
Consumption of organic fruits was associated with an increased risk of developing MDR UTI (ORadj = 8·60; 95% CI 1·11–66·53) after adjustment for age, SHS and antibiotic use, but not after adjustment for UTI history. However, an association with antimicrobial resistant UTI was present after adjustment for UTI history (Tables 3 and 4). More frequent consumption of street foods (e.g., food carts or food truck) was associated with antimicrobial resistant UTI (ORadj = 1·66; 95% CI 1·04–2·65) (Table 3). Foods prepared in the home, restaurants, or campus dining halls were not associated with a resistant UTI. Consumption of apples, nectarines, peanuts, fresh herbs and peppers were variably associated with a decreased risk of developing an antimicrobial resistant or MDR UTI after adjustment (Tables 3 and 4).
Contact with animals, animal waste or pet products and risk of UTI caused by antimicrobial resistant E. coli
Contact with live chickens (or chicken feces) was associated with a greater risk of developing a UTI caused by an E. coli resistant to at least one antimicrobial class (ORadj = 3·62; 95% CI 1·08–12·10 and ORadj = 6·30; 95% CI 1·26–31·51) after adjustment for age, SHS and prior antimicrobial use or UTI history (Tables 3 and 4). Contact with dogs (or dog feces) was associated with a UTI caused by a MDR E. coli isolate (ORadj = 1·74; 95% CI 1·08–2·80 and ORadj = 2·23; 95% CI 1·29–3·87) after adjustment for prior antimicrobial use or UTI history, respectively. After adjustment for prior antimicrobial use, exposure to animal-derived pet treats was also associated with MDR UTI (Table 3).
Travel and risk of UTI caused by antimicrobial resistant E. coli
In univariable analyses, travel within Canada was associated with an elevated risk of antimicrobial resistant UTI (OR = 1·50; 95% CI 0·99–2·24), while travel outside of Canada (OR = 1·50; 95% CI 1·00–2·24), especially travel to Asia (OR = 2·82; 95% CI 1·20–6·63) was associated with a greater risk of MDR UTI. Travel to Asia remained elevated after adjusting for age, SHS and antibiotic use (ORadj = 1·51; 95% CI 1·0–2·28), but was no longer significant following adjustment for age, SHS and lifetime UTI (Tables 3 and 4).
Factors not associated with risk of UTI caused by antimicrobial resistant E. coli
Other dietary variables not found to be associated with drug-resistant UTI included: alcohol consumption; vegetarianism; adhering to any special diet (e.g., Kosher); organic foods (except fruit); antibiotic-free foods; any significant diet changes; conventional fruits (melon, pears, peaches, apricots, plums, citrus fruits, any berries, grapes, bananas, exotic fruits, dried fruits, avocados, unpasteurised juices); nuts (almonds, cashews, hazelnuts, peanut butter, pistachios, walnuts); vegetables (tomatoes, salsa, leafy greens, sprouts, cabbage, cucumbers, zucchini, celery, carrots, radishes, peas, broccoli, cauliflower, onions, scallions, mushrooms, water chestnuts, vegetable juices); and unpasteurised cheese, raw or undercooked eggs; eating at fast food or sit-down restaurants, delis, home prepared meals and campus dining halls. The frequency of sexual intercourse was not associated with a drug-resistant infection, nor was the type of housing (e.g., dormitory, family home, apartment, communal housing) or a number of individuals sharing a kitchen. Animal-related exposures such as dry pet food, raw meat pet food, rodents or processed pet chews were not associated with antimicrobial resistant UTI; nor was exposure to cats, cattle, fish, goats, horses, pigs, reptiles, rodents, turkeys, or other birds. Travel to Mexico, Central and South America, or Africa was not associated with experiencing any drug-resistant UTI.
DISCUSSION
Consumption of poultry, in this case, processed or ground chicken and contact with chickens were associated with an increased risk of developing UTI caused by antimicrobial resistant E. coli. However, there was no indication that exposure to whole cut chicken, chicken strips or turkey was associated with the development of a UTI caused by either antimicrobial resistant or MDR E. coli. These results provide partial support for our primary study hypothesis and complement observations from other studies, which have implicated chicken as a possible reservoir for drug-resistant ExPEC [Reference Manges5, Reference Leverstein-van Hall7–Reference Bergeron9]. We did not observe any relationships between pork consumption and antimicrobial resistant or MDR UTI, nor ampicillin or cephalosporin-resistant E. coli in this study; in contradiction to our earlier observations [Reference Manges5].
Consumption of several high-risk foods was associated with antimicrobial resistant and MDR UTI, including consumption of cooked or raw shellfish, raw meat and street foods. Seafood has been reported to be an important reservoir for antimicrobial resistant opportunistic pathogens and may increase the risk of drug-resistant infections [Reference Janecko18–Reference Rubin, Ekanayake and Fernando21]. Organic fruit consumption was also associated with an increased risk of MDR UTI. This may be the result of waste products used as fertilisers in organic farming [Reference Ruimy22]. An antimicrobial effect of pesticides or non-organic fertilisers could be a possible explanation, although this is an untested hypothesis.
Increased risk of MDR UTI with exposure to dogs and pet treats (which may or may not contain meat products) is not surprising given the prior associations of drug-resistant ExPEC in companion animals and associated family members [Reference Johnson and Clabots23–Reference Platell27].
Self-reported Asian race or travel to Asia have been reported to be risk factors for antimicrobial-resistant ExPEC infections in past studies [Reference Soraas11, Reference Rattanaumpawan12, Reference Peirano28]. Travel to Asia was identified as a significant risk factor for MDR UTI development in our study. Increased prevalence and diversity of antimicrobial resistant ExPEC in Asia has been demonstrated in several community-based studies of colonisation and extraintestinal infection and in returning travellers [Reference Peirano28–Reference Jakobsen33]. In contrast to these studies, we did not observe any associations between developing a UTI caused by E. coli resistant to any cephalosporin or any quinolone and dietary intake, Asian race, swimming, recent antibiotic use, kidney infection, or travel history [Reference Soraas11, Reference Soraas12].
Peanuts, fresh herbs, peppers, apples and nectarines were observed to be ‘protective’ against antimicrobial-resistant UTI. The mechanism for this effect, if true, is unclear. We explored the consumptions of these ‘protective’ foods by racial/ethnic groups but did not observe any significant differences (data not shown). These ‘protective’ factors may reflect confounding due to their relationship to other behaviours associated with a lower risk of drug-resistant UTI or greater overall health. Consumption of cooked ground beef ⩾1–3 days per week was associated with a lower risk of antimicrobial resistant UTI; these relationships may reflect certain preferences for beef over poultry or reflect food handling and preparation practices. Retail beef typically has the lowest levels of antimicrobial resistant generic E. coli contamination (compared with retail pork and poultry meats) [34–Reference Vieira, Collignon and Aarestrup36].
The strengths of this study include a large sample of women with uncomplicated, community-acquired UTI. The sample included all consecutive UTI cases willing to participate in the study without bias due to clinical laboratory screening algorithms or by selective inclusion of women with relapsing or recurrent infections. Exposure information was systematically collected on all risk factors likely to be associated with the intestinal acquisition of ExPEC. Direct intestinal acquisition of antimicrobial ExPEC was not measured in this study. We elected to study E. coli recovered from cases of UTI, rather than E. coli intestinal colonisation alone, because we wanted to be sure to capture E. coli that can cause extraintestinal infections. There is a consensus that the primary source for E. coli associated with community-acquired UTIs is the intestinal tract of infected individuals [Reference Yamamoto37]. There are several limitations to the study. Subjects whose UTI-causing E. coli were classified as susceptible or resistant to fewer than 2 classes of antimicrobials may still have been intestinally colonised by another MDR ExPEC strain. The result of this misclassification would be to underestimate the odds ratios estimated in this study. An outbreak investigation-like case-control approach was used to address our primary hypothesis and to investigate exposures that may put individuals at risk of antimicrobial resistant UTI, which, by definition, means multiple comparisons were made. Therefore, some of these observed relationships are likely to be false positives. In some instances, statistical adjustment for confounding by age and SHS, and either antimicrobial use or UTI history led to attenuation in our effect estimates. We were not able to explore in detail the role of other healthcare contact as a potential confounder, nor were we able to collect detailed information on timing or location of exposures. Women who did not complete the survey may have differed systematically from the women who did participate, contributing to a selection bias.
In response to increases in antimicrobial-resistant UTI incidence, we sought to investigate the relationship between the consumption of meat, especially chicken, as well as other environmental exposures and the development of antimicrobial resistant or MDR UTI caused by E. coli. The use of antibiotics for treatment, prophylaxis and growth promotion in food animals can select for antimicrobial resistant enteric pathogens [Reference Nordstrom, Liu and Price3]. It would be a serious concern if certain antimicrobial use practices in food animal production also contribute to the selection of antimicrobial resistant E. coli causing UTI or other extraintestinal infections (e.g., pyelonephritis or bloodstream infections) in humans. Our study suggests that specific high-risk foods, including processed poultry meat, travel and contact with chickens and dogs may be associated with the acquisition of antimicrobial-resistant ExPEC. Given the breadth of environmental exposures related to antimicrobial UTI highlighted this study, the focus on antimicrobial stewardship in human clinical medicine alone will not be sufficient to manage the growing level of antimicrobial resistance in community-acquired infections. Rather multi-sectoral stewardship approaches including veterinary medicine, food animal production, produce farming practices and global cooperation to control the ecologic proliferation and selection of antimicrobial resistant ExPEC will be necessary for public health.
ACKNOWLEDGEMENTS
This work was supported by the Canadian Institutes of Health Research (CIHR), Institute of Infection and Immunity (MOP-114879) and the Public Health Agency of Canada to A.R.M.
DECLARATION OF INTEREST
None to declare.
ETHICAL STANDARDS
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.






