INTRODUCTION
Bovine brucellosis caused by Brucella abortus biovars is mainly characterized by abortions in pregnant cows, birth of weak calves and retained placenta [Reference Bishop, Bosman, Herr, Coetzer, Thomson and Tustin1]. Occasionally, carpal hygromata, orchitis in bulls and reduced milk yield in lactating cows have been reported [2]. Brucellosis causes both public health problems and reduced cattle productivity in many countries in Sub-Saharan Africa, including Zimbabwe [Reference McDermott and Arimi3].
In Zimbabwe cattle farming is broadly divided into commercial and smallholder sectors, with most of the cattle (60–80%) found in the latter [4]. Cattle are kept mainly for prestige, for transport, driving power for land cultivation, food (meat and milk), income and manure but they can be used in numerous social and cultural activities. In some areas of the country, smallholder household dairy herds have been established by the Dairy Development Programme (DDP) which purchased mainly Bos taurus cattle from commercial farms and the required equipment [Reference Madsen5]. These cattle were mixed and later cross-bred with the indigenous Sanga (Mashona and Tuli) cattle and kept as small semi-independent herds (median herd size 14 cattle) on individual household units (median size 10 ha) that are demarcated from neighbouring units by perimeter fencing [Reference Matope6]. The establishment of these smallholder household dairy herds together with the introduction of the land reform programme in 2000 created a unique cattle management system with the potential of changing the epidemiology of many infectious diseases that include brucellosis, but their current status is not known. Therefore, this study was conducted in order to estimate and/or identify; (a) seroprevalence and risk factors for Brucella spp. infection, (b) the association between Brucella-seropositive status and abortion, and (c) the impact of Brucella seropositivity on abortions in cattle from smallholder household dairy herds in Zimbabwe.
MATERIALS AND METHODS
The study areas
A cross-sectional study was conducted in smallholder household dairy herds in Gokwe, Marirangwe, Mushagashe, Nharira, Rusitu and Wedza areas of Zimbabwe from September 2004 to November 2005. These areas were selected for the study because they represented the different geographical regions of Zimbabwe and none of the smallholder household dairy herds in these areas were using B. abortus S19, B. abortus S45/20 and B. melitensis Rev1 vaccines.
Sampling of animals
The study design, sampling of herds and individual animals have been described in detail by Matope et al. [Reference Matope6]. In order to reduce the confounding effect of maternal antibodies in calves and young animals, we decided to sample cattle aged ⩾2 years and only herds with a minimum of 10 cattle were included in the study. The sample sizes of herds from each area were pre-calculated [Reference Dohoo, Martin and Stryhn7], by assuming that brucellosis existed at 25% inter-herd and 15% intra-herd seroprevalence [Reference Madsen5]. The eligible herds from each area were identified by numbers (written on small cards) and study herds were then randomly chosen from a bowl without replacement. The sample sizes of individual cattle per herd were estimated [Reference Jordan8] using the diagnostic sensitivity (Se) and specificity (Sp) of the Rose Bengal test (RBT) and a competitive enzyme-linked immunosorbent assay (c-ELISA) (serial interpretation) calculated as previously described [Reference Dohoo, Martin and Stryhn7], and based on previous validation data obtained using similar assay reagents and biologicals for these tests [Reference Nielsen9, Reference Nielsen10]. To balance the resources available in the project, we decided to sample at least eight cattle from each herd and a 25% sampling fraction from herds with >40 cattle. This resulted in herd Se and Sp of about 86·6% and 98·4%, respectively, when classifying herds as Brucella seropositive if at least a single seropositive animal was detected [Reference Jordan8]. Cattle were selected by systematic random sampling taking every fourth animal based on its position in the pen and blood samples were taken. In households where random sampling was not possible, eight animals were selected from those present in the herd.
Laboratory tests
The RBT, performed as previously described [Reference Alton11], was used to screen sera for Brucella antibodies. The buffered antigens and control sera were obtained from VLA (UK). Since we used a serial interpretation (which increases specificity) in which sera positive in both tests were regarded as Brucella seropositive, then only sera positive on RBT were further tested by the SvanovirTMBrucella-Ab c-ELISA test kits (Svanova Biotech, Sweden) which was performed as previously described [Reference Matope6].
Epidemiological data collection
A pre-tested structured questionnaire with close-ended questions was used to collect information on herd-level risk factors we believed to be associated with Brucella seroprevalence and abortion. Pre-testing of the questionnaire was carried out in one of the study areas by interviewing a few farmers and any lack of clarity of questions was noted and later revised. The revised questionnaire was then administered immediately after taking blood samples. The question on abortion was carefully interpreted to mean visual detection of an expelled foetus within 3 years of the study and the responses (1=yes, 0=no) for each cow were cross-checked with records where available. Similarly, a household herd was regarded as positive for abortion if at least one cow was reported to have aborted within 3 years of the study.
Statistical analysis
The epidemiological and animal bio-data were stored in a computer database and statistical analysis was performed using Stata version SE 10.0 (StataCorp., USA). The individual- and herd-level Brucella-seropositive and abortion percentages were calculated by considering the proportions positive against the total sampled. The individual animal-level data were weighted according to the inverse of sampling fraction in order to improve the estimations of the parameters [Reference Dohoo, Martin and Stryhn7]. A sampling weight was obtained as a product of the proportion of herds sampled against the total number of herds in each study area and the proportion of cows sampled in a herd. Herd-level data were adjusted for area but no weighting was done. The calculated apparent seroprevalence was adjusted for test Se and Sp to obtain the true seroprevalence [Reference Dohoo, Martin and Stryhn7]:
where p(D+)=true prevalence, AP=apparent prevalence. In individual animals, Se=0·882 and Sp=0·998 [Reference Jordan8], and in herds, Se=0·866 and Sp=0·984.
Logistic regression analyses
A two-sided Fisher's exact test was used to test the significance of categorical variables and the individual animal or herd's abortion status, while a Kruskal–Wallis test was used for age. Since age was skewed to the right, we categorized it into quartiles in order to correct for the linearity problem. Collinearity between variables was assessed through cross-tabulation using Fisher's exact test. Only variables with a P value <0·25 in univariable analysis and having counts ⩾5 in each cell were tested in the logistic regression models. Two logistic regression models were separately built to test the significance of association between individual-animal and herd-level variables as predictors and the abortion status (0=no, 1=yes) as the outcome. Since intra-household herd correlation may result in artificially low standard errors and P values, the logistic model for individual animals was compared to the generalized estimating equation 1 (GEE1) model which adjusts for clustering at herd level. The models were constructed by forward selection applying the iterative maximum-likelihood estimation procedure. The statistical significance of individual predictors to the models was assessed using Wald's test and the likelihood ratio test. The assessment for interactinon between variables and evidence of confounding were checked using the procedure of Dohoo et al. [Reference Dohoo, Martin and Stryhn7]. The models were evaluated for goodness-of-fit using the Hosmer–Lemeshow test.
RESULTS
Descriptive statistics
Of the 1291 cows investigated, 6·5% were seropositive on RBT and 83·3% of those detected on RBT were also seropositive on c-ELISA (data not shown). After adjusting for clustering by area, sampling weights and test Se and Sp, the estimated proportion of Brucella-seropositive cows was 5·5% [95% confidence interval (CI) 3·6–7·3] (Table 1). The individual-animal Brucella seroprevalence differed in the age quartiles (P=0·03) and study areas (P<0·001). The relationship between age and Brucella seropositivity is shown in Fig. 1. At herd-level, 22·9% (95% CI 15·3–30·6) of the 203 herds were estimated to be Brucella seropositive, after adjusting for clustering by area and test Se and Sp (Table 1). Brucella seroprevalence differed significantly in study areas (P=0·06) (Table 1).
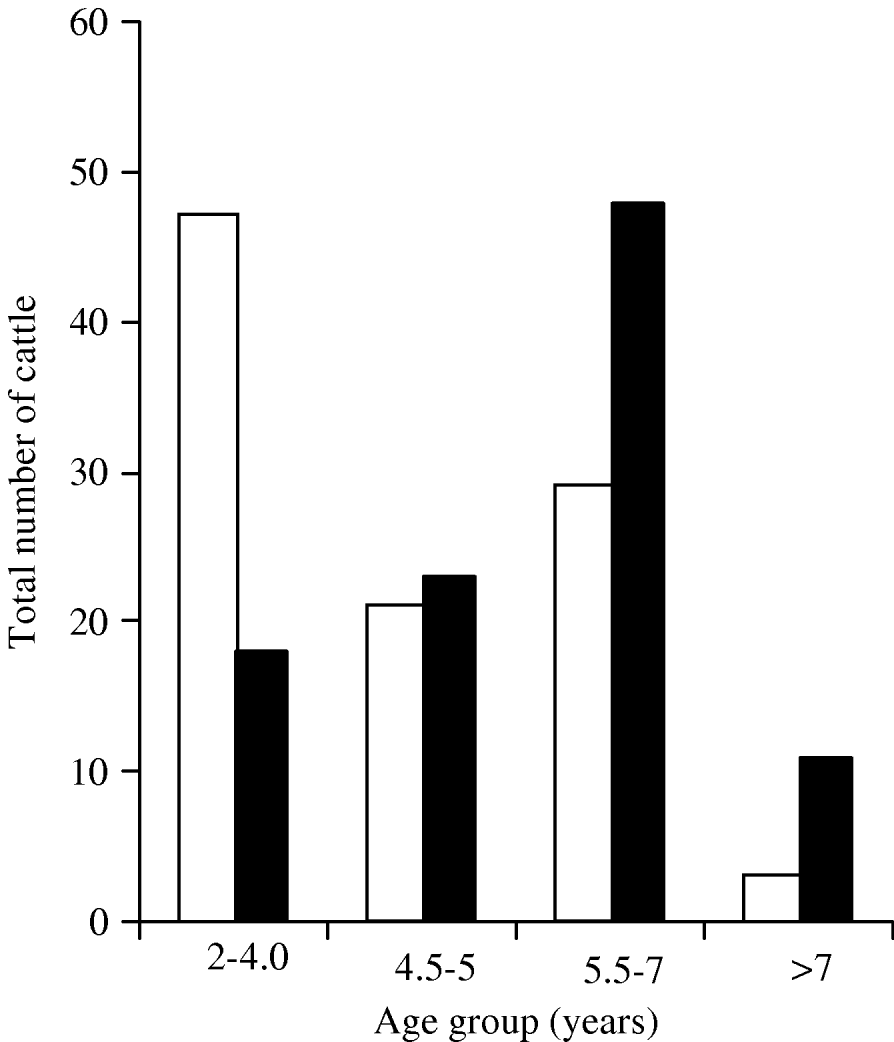
Fig. 1. The relative distribution by age groups of Brucella-seropositive (□) and aborting (▪) cows from smallholder household dairy herds in Zimbabwe (2004–2005).
Table 1. Number of cattle herds (n=203), median herd sizes (raw estimates), estimated individual animal-level (n=1291) and herd-level Brucella seroprevalence and abortions (adjusted) by study area in the smallholder households of Zimbabwe (2004–2005)
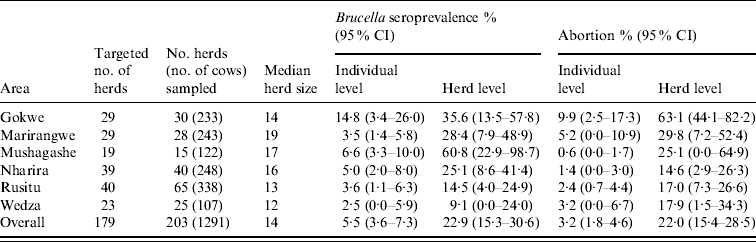
CI, Confidence interval.
Brucella seroprevalence was adjusted for clustering by area, and test performance parameters. Brucella seroprevalence in individual animals is further adjusted by sampling weights. Reported abortions for individual animals are adjusted by area and sampling weights while herd abortions are only adjusted for clustering by study area.
Abortions were reported in 3·2% (95% CI 1·8–4·6) of the 1291 cows and there was significant difference in the study areas (P<0·001) and age quartiles (P=0·013) (Table 1). The numbers of cows with reported abortions increased with age, peaking at 5·5–7 years and declined with increasing age (Fig. 1). At herd level, 22·0% (95% CI 15·4–28·5) of the 203 herds reported abortions (Table 1). There was significant difference (P<0·001) in the herds reporting abortions in the study areas.
Logistic regression and GEE1 models for individual animals
Without adjusting for clustering within herds, the logistic regression model identified geographical area, Brucella seropositivity and age to be associated with abortion (Table 2). Brucella-seropositive cows were more likely to be reported for abortion compared to seronegative cows [odds ratio (OR) 8·7, 95% CI 4·0–18·9]. The odds of abortion increased with increasing age. Cows in the 5·5–7 years age group were about five times (OR 4·7, 95% CI 2·0–11·1) more likely to have abortions compared to 2–4 years age group. There were no significant (P>0·05) interactions among the variables in the model. The Hosmer–Lemeshow test indicated that the model fit the data (χ2=3·4, d.f.=8, P=0·91) (Table 2). After adjusting for intra-herd clustering, the P values obtained in the GEE 1 for study area, age and serological status were slightly larger but the coefficients were similar to those obtained by the logistic regression model (Table 2).
Table 2. The multivariable logistic regression and the generalized estimating equation 1 model showing the association between individual animal variables and reported abortions in cows from smallholder dairy herds in Zimbabwe (2004–2005). Results given with coefficients (b), standard errors of b (s.e.), and P values
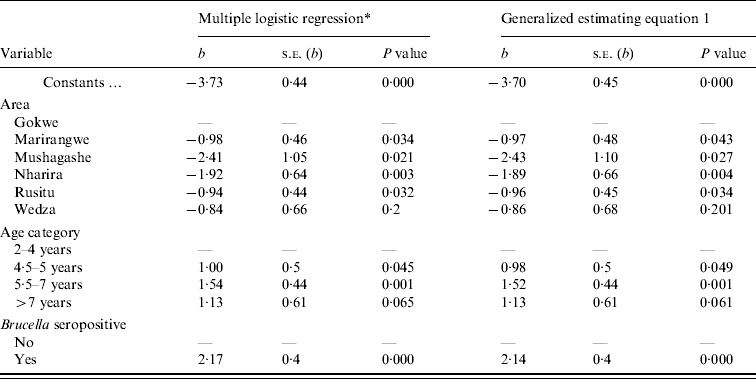
* Overall data of the model: LL=−160·8, LR χ2 (9 d.f.)=62·2, P=0·000, number of observations=1291. Hosmer–Lemeshow χ2 (8 d.f.)=3·4, P=0·91, ROC=0·78.
Logistic regression model for herds
Geographical area, Brucella seropositivity, purchase of animals and herd size were independently associated with reported abortions (Table 3). Brucella-seropositive herds had higher odds of reported abortions (OR 3·3, 95% CI 1·4–8·1) compared to seronegative herds. Herds receiving purchased cattle had higher odds of reported abortions (OR 1·9, 95% CI 1·0–4·2) compared to those that were closed herds. There were no significant interactions between the main effects and post-fit testing did not reveal major influences of outliers. The Hosmer–Lemeshow test showed that the model fit the data (χ2=3·51, d.f.=8, P=0·89) (Table 3).
Table 3. A multivariable logistic regression model showing the association between herd-level variables and reported abortions in cows from smallholder dairy herds in Zimbabwe (2004–2005). Results given with coefficients (b), standard errors of b (s.e.), and odds ratio (OR) with 95% confidence intervals (CI)
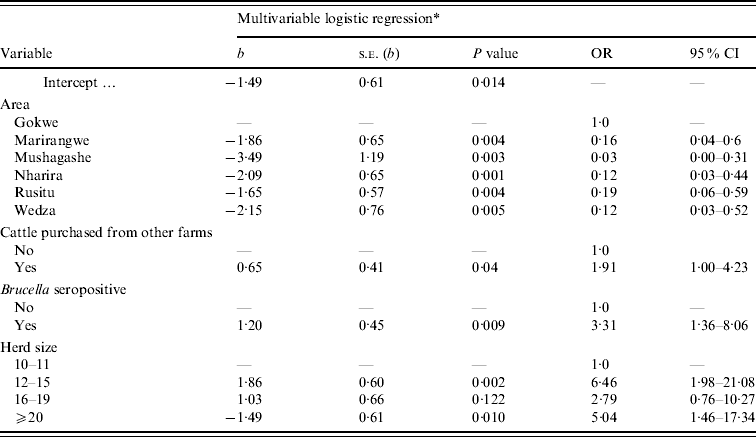
* Overall data of the model: LL=−87·1, LR χ2 (10 d.f.)=47·8, P=0·0000, number of observations=203.
The assessment of impact of Brucella seropositivity on cattle abortions
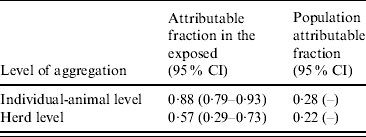
In individual animals, 88% (95% CI 79–93) and herds, 57% (95% CI 29–73) of reported abortions in Brucella-seropositive cows were attributable to them being positive (Table 4). In the total cattle population and herd-level aggregates, 28% and 22% of the abortions, respectively, were attributable to some cows being Brucella seropositive (population attributable fraction).
Table 4. The assessment of the impact of Brucella seropositivity on abortions in cattle in the smallholder household dairy herds in Zimbabwe (2004–2005)
DISCUSSION
This study investigated brucellosis, the risk factors associated with seropositivity and abortions, and the impact of Brucella seropositivity on abortions in smallholder household dairy herds in Zimbabwe. Brucella-seropositive cattle were present in all the study areas although some areas had low seroprevalence. The seropositivity was likely to be in response to field Brucella spp. infection because the c-ELISA (confirmatory test) has a high specificity in individual animals which minimizes false-positive reactions [Reference Nielsen12, Reference Samartino13]. Since we assumed that the sensitivity of RBT was 90% and given that only the RBT-positive sera were tested by c-ELISA then it is likely that 10% of the true seropositive animals were missed in the study for c-ELISA, thereby underestimating Brucella seroprevalence. One of the major weaknesses of the RBT is prozoning, where sera with high levels of antibody may yield non-visible reactions resulting in false-negative results [Reference Alton11]. However, we selected the RBT for screening sera as is conventionally done [Reference Thrusfield14] based on cost, ease of performance and also because it has a high sensitivity especially in endemic areas. Although our test regimen probably underestimated Brucella seroprevalence in individual animals, considering that we sampled at least eight animals from the median herd size of 14 (range 10–78) then false-negative classifications of herds was unlikely to be a serious problem. Although the B. abortus antigen cross-reacts with antibodies produced against other smooth Brucella spp. [Reference Nielsen12], both B. melitensis and B. suis are seldom found in the region and have not been isolated in cattle in Zimbabwe [Reference Madsen5, Reference Muma15]. The serological status in cattle has been linked to B. abortus which was isolated from aborting herds [Reference Mohan16–Reference Matope18]. We revealed that abortions were reported in all the study areas. Since questionnaire data were cross-checked with farm records for 64% of the respondents, the effect of recall bias and censoring could have been minimized. The preponderance of abortions in Brucella-seropositive cows suggested that exposure to Brucella spp. was associated with abortion. These results are consistent with the findings of brucellosis in other parts of the world [Reference Kubuafor, Awumbila and Akanmori19–Reference Silva, Dangola and Kulachelvy22]. However, since no laboratory testing was conducted to screen the cows against other infectious causes of late-term abortion such as leptospirosis [Reference Langoni23], listeriosis [Reference Malik, Barbuddhe and Chaudhari24] and campylobacteriosis [Reference Swanepoel, Blackburn and Lander17], their contribution to the reported abortions could not be determined based on the available data.
The observed differences in Brucella seroprevalence in the study areas could be explained by cattle management and other agro-ecological factors that influenced contact between herds [Reference McDermott and Arimi3, Reference Matope6, Reference Reviriego, Moreno and Dominguez25]. Brucella seroprevalence was low in Wedza and Rusitu, both having small median herd sizes, and increased in other areas with larger median herd sizes. Similarly, Gokwe with a high proportion of herds that shared facilities for grazing and water showed a higher individual animal-level seroprevalence compared to other areas (data not shown). The risk of exposure to Brucella spp. tended to increase with herd size and/or mixing of herds [Reference McDermott and Arimi3, Reference Omer26, Reference Muma27]. Our results for Brucella seroprevalence are similar to those of previous studies in Zimbabwe [Reference Madsen5, Reference Mohan16, Reference Bryant and Norval28].
Although the decrease of Brucella seropositivity with age of cows differed with other reports [Reference Muma15, Reference Kadohira20, Reference Silva, Dangola and Kulachelvy22, Reference McDermott29], it concurred with the findings from another study [Reference Omer26]. However, other factors may be at play here that account for the difference observed in our study. It is likely that in endemic areas the risk of Brucella infection, and thus seroconversion, is greater in younger animals compared to older cows, some of which could be seronegative possibly due to latency [Reference Muma15, Reference Ficht30, Reference Gul and Khan31].
The high odds of abortion (OR 4·7) for the 5·5–7 years age group could be related to the naivety to Brucella infection of cows mostly during their first pregnancy. On average cows of the Sanga breeds first calve at mean age 4 years (range 3–6 years) [Reference Payne32]. The odds of abortion decrease in multiparous cows due to the gradual build-up of solid immunity [Reference Cunningham, Crawford and Hidalgo33]. At herd level, the close association between Brucella seroprevalence and abortion indicated the similar nature of the risk factors for these outcomes. Herds receiving purchased cattle from other farms have high odds of abortions due to increased risk of Brucella infection through introduction of infected cattle [Reference Omer26, Reference Muma27, Reference Crawford, Huber, Adams, Nelson and Duncan34, Reference Kabagambe35]. Smallholder households usually purchase cattle from commercial farms, in order to improve the genetics of their herds. Despite the variability of the definition of a large herd [Reference Salman and Meyer36], the odds of abortion also increased with herd size. The practice of keeping large herds was partly influenced by the availability of grazing pasture. This is likely to favour the transmission and maintenance of Brucella spp. [Reference McDermott and Arimi3, Reference Omer26].
Further, we showed that by preventing exposure to Brucella spp. in smallholder household herds, the reported abortions at cow level and herd level would be reduced by 28% and 22%, respectively. Considering this impact on abortions and given the public health importance of brucellosis, it is prudent that the disease be controlled in smallholder household herds. This could improve cattle productivity and reduce the public health risks of brucellosis in these communities.
In conclusion, the age of cows and geographical area are associated with Brucella seroprevalence. Exposure to Brucella spp. and age are important determinants of abortion. In herds, geographical area, Brucella-seropositive status, purchase of cattle and herd size were independently associated with reported abortions. Exposure to Brucella spp. had a significant impact on abortions in smallholder household herds.
ACKNOWLEDGEMENTS
The principal author was a recipient of a Ph.D. scholarship from the Norwegian Council for Higher Education and Development (NUFU), project vote: pro/2002/06. We are grateful for the contribution of all the stakeholders involved in the project. We thank all the smallholder farmers who allowed us to use their cattle for this research.
DECLARATION OF INTEREST
None.







