INTRODUCTION
Gastroenteritis is a common cause of morbidity and mortality worldwide. Symptoms range from mild to severe forms of vomiting, diarrhoea, and abdominal discomfort. In many instances those affected seek medical attention only when the illness is severe and/or prolonged. As a result most cases are not reported and no causative organism is identified.
In Australia, estimates show that on average every person experiences around one case of gastroenteritis per year [Reference Hall1]. Gastroenteritis is commonly transmitted via food, either by consumption of contaminated food such as raw food and meat products, or via handling and preparation of food, particularly poultry products. Additional risk factors include contact with persons having gastroenteritis, contact with animals, and consumption of contaminated water.
Domestic rainwater use, worldwide and in Australia, has been increasing in recent years as a result of several factors, including drought. Stored rainwater has been shown to have variable water quality, with faecal coliform levels at times higher than levels recommended for drinking-water supplies. With the increase in domestic tank installation and use, the potential exists for inadvertent or deliberate consumption of rainwater by a significant number of people.
Rainwater consumption has been linked to gastroenteritis in previous case-control and retrospective cohort studies [Reference Simmons2–Reference Franklin4]. These study designs use exposure data that are collected retrospectively and are therefore subject to recall bias. Additionally, in the case-control design, selection of controls can be difficult and may lead to confounding of results.
In the current study, a nested case-control design was used. Cases of gastroenteritis were identified from a defined cohort, namely rainwater consumers. Unlike the conventional case-control design, the nested case-control study starts with a cohort of persons based on their exposure status and identifies cases as they occur. Controls are then selected from those in the defined cohort who have not experienced the disease. This design also differs from the conventional case-control design in that absolute risk may be estimated reliably since participants were disease free on entering the cohort [Reference Langholz, Armitage and Colton5].
Study objectives
The objective of this nested case-control study was to investigate the risk factors for highly credible gastroenteritis (HCG) in persons using rainwater as the primary source of domestic drinking water.
METHODOLOGY
Study design
This community based, case-control study was nested within a larger randomized controlled trial conducted in South Australia between June 2007 and August 2008. Recruitment methods [Reference Rodrigo6], details of the study area, and the methodology used for this randomized study design are described elsewhere [Reference Rodrigo7]. Briefly, 300 households having no less than four members with at least two aged 1–15 years and using untreated rainwater as their usual drinking-water source were recruited. The majority of these households (n=211) met the requirement of two children aged 1–15 years; 69 had three children; 17 had four children; and three had five or more children living at home.
Participating households received either an active or a sham water-treatment unit (WTU) for filtering of all water intended for drinking or cooking. The active WTU was capable of removing microorganisms from the water, so it supplied water of a higher quality to participants. In contrast, households using the sham WTU were at their normal level of risk, utilizing untreated rainwater for their potable needs.
Written informed consent was obtained at enrolment from all adult household members and from parents/guardians on behalf of children. One adult member, designated as the reporting participant, ensured that a health diary, which recorded symptoms of diarrhoea, vomiting, nausea, abdominal pains and fever, was completed weekly for each participant [Reference Rodrigo7]. Ethical approval was obtained from Monash University Standing Committee on Ethics in Research on Humans (SCERH; 2006/555EA) and the South Australia Department of Health Human Research Ethics Committee.
Outcome criteria and case definition
The outcome, HCG, was defined as any of the following in a 24-h period: two or more loose stools; two or more episodes of vomiting; one loose stool together with abdominal pain or nausea or vomiting; or one episode of vomiting with abdominal pain or nausea [Reference Hellard8]. A case was defined as a person reporting symptoms of HCG and people could be a case on more than one occasion during the study period.
Control definition
Controls were matched with cases according to the study week in which illness was experienced by the case. All people in the cohort without symptoms of gastroenteritis in the week of, or the week prior to, onset of illness of a case were eligible to be selected as the control for that case. Using Stata version 10 (StataCorp LP, USA), one control was selected randomly to match each case.
Data collection and exposures investigated
The reporting participant was asked to telephone the study centre whenever a family member experienced an HCG event. In addition, health diaries were returned by participants every 4 weeks and were checked for HCG events on receipt, and participants were contacted and interviewed if the episode had not been previously reported.
Several attempts were made to contact cases and controls. Telephone calls were made in the evenings in order to maximize contact. For cases, telephone messages were left asking the reporting participant to contact the study centre. For controls, if contact was not made after three attempts, another random list of controls was generated.
During the telephone interview, cases and controls (or reporting participants when the case/control was a minor) completed a structured questionnaire that addressed risk factors in the 7-day period before onset of symptoms. Potential risk factors investigated in the week before onset of illness were consumption of chicken, beef, organ meat (offal), fish, shellfish, raw vegetables, salad, fresh fruit, rice, milk products including cheese, eggs (runny, cooked or raw), and takeaway fast food from any source. Other factors assessed were the presence of a child in nappies in the household; changing and/or washing nappies; and contact with pets through having animals in the household or other sources (farm, zoo). All cases and controls were interviewed by trained staff using the prepared questionnaire. Demographic data collected during the enrolment for the randomized controlled trial included age, gender, education and institution attendance (childcare, kindergarten, primary, secondary), work status (of adults), and presence of pets within the household.
Statistical analysis
Logistic regression was used to study univariate associations of potential risk factors with HCG and robust standard errors were calculated to allow for familial clustering and repeated observations on individuals (as cases or controls on multiple occasions). Food exposures were analysed by logistic regression adjusting for age, gender, location (metropolitan Adelaide vs. the semi-urban Mount Barker area) and treatment group (active or sham). Age was dichotomized in these analyses (<5 years, ⩾5 years). All analyses were conducted using the Stata version 10 (StataCorp LP). A P value <0·05 was interpreted as statistically significant.
The population attributable fractions (PAF) (%) were determined using Stata software (version 10, StataCorp LP) based on ordinary logistic regression by the method of Greenland & Drescher [Reference Greenland and Drescher9] employed in the Stata program aflogit using the cc option [Reference Brady10]. PAF was estimated for risk factors having odds ratios greater than 1 in the logistic regression models.
RESULTS
A total of 769 episodes of HCG from 501 individuals were identified from the health diaries. Participants were successfully interviewed for 298 (36·5%) episodes. Cross-checking with the diary data revealed that 281 of these were valid HCG cases. Case data were not valid if the diary was not returned (n=4) or there was a discrepancy between reported data and health diary data (n=13). The 281 HCG cases occurred in 215 persons: 171 persons had one HCG episode; 31 had two episodes; and 13 had three or more episodes. Table 1 shows that cases who were not interviewed (non-contact cases) were similar to HCG cases who were contacted.
Table 1. Demographic characteristics of contact and non-contact cases, and controls

* The denominators for adults are different from the total number of participants since some data were missing (n=72 for cases, n=105 for controls).
† The denominators for adults are different from the total number of participant as some adults were unemployed (n=92 for cases, n=129 for controls).
Of the 297 control interviews that were conducted 35 individuals were interviewed twice and three individuals were interviewed three times. A total of 51 people who were a HCG case at some point in time during the study were interviewed as controls for other cases. Cases were different from controls in having a greater proportion of children aged <5 years, and a smaller proportion of adults undertaking paid work (Table 1). In line with the age difference, a greater proportion of cases were attending childcare. Overall the majority of HCG cases were in children aged ⩽15 years (63·3%).
Of the valid cases, 11·4% (32) had combined symptoms of diarrhoea, vomiting, nausea, and abdominal pains; 35·6% (100) experienced 3/4 symptoms; 26·0% (73) had two symptoms; 19·2% (54) had diarrhoea only, and 7·8% (22) had vomiting only. For 33 (11·7%) cases medical advice was obtained, with three instances requiring attendance at a hospital. Medication was prescribed in 11 (33·3%) of the cases that sought medical attention.
In the analysis of food consumption and HCG, eating beef appeared to be a risk factor [adjusted odds ratio (aOR) 2·74, 95% confidence interval (CI) 1·56–4·80] (Table 2). Protective associations were observed for consumption of salami, shellfish and raw salad prepared at home. Contrasting associations were found with handling of fresh (aOR 1·52, 95% CI 1·02–2·99, P=0·04) and raw frozen (aOR 0·46, 95% CI 0·32–0·69, P<0·001) poultry. Any animal contact was also found to be a risk factor (aOR 1·83, 95% CI 1·19–2·81, P=0·006). No statistically significant association was found when pets (cat, dog, fish, bird) were present in the household. The highest PAF was observed for the consumption of beef at 57·6%. PAF estimates for all other risk factors were relatively low (Table 3).
Table 2. Exposure for gastroenteritisFootnote †
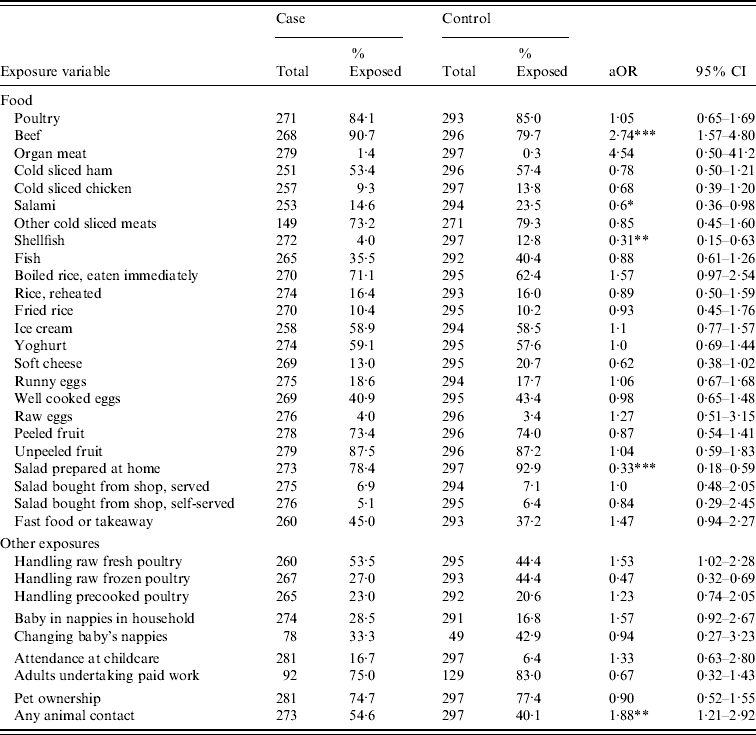
aOR, Adjusted odds ratio; CI, confidence interval.
† Adjusted for age (age <5 years and ⩾5 years), gender, location and treatment group (active or sham water-treatment unit).
* P<0·05, ** P<0·01, *** P<0·001.
Table 3. Estimated population attributable fraction (PAF)

DISCUSSION
The randomized controlled trial investigated whether consumption of untreated rainwater contributed to community gastroenteritis and involved collection of health data including the incidence of gastroenteritis within the cohort [Reference Rodrigo7]. This information was then used to identify cases for this case-control study and to explore the risk factors for gastrointestinal illness. The findings of the larger study suggest that consumption of untreated rainwater does not contribute appreciably to community gastroenteritis [Reference Rodrigo7].
This nested case-control study provides novel data regarding risk exposures associated with community-based endemic gastroenteritis. Most previous data regarding high-risk foods or high-risk behavioural factors linked with gastrointestinal illness come from community outbreaks rather than from a predefined cohort already under observation. Many water or foodborne gastroenteritis outbreaks are detected via surveillance systems that signal the onset of greater than normal occurrences of illness, so are not designed to identify endemic levels of disease. Consequently, it is unclear whether the same risk factors – or indeed any significant factors – would be expected to emerge in a longitudinal community-based non-outbreak study. However, an increased likelihood of gastroenteritis with consumption of beef, as well as following handling raw poultry or having animal contact was found. Eating beef showed the greatest association with illness having and odds ratio of ~3. Additionally, the estimated PAF implied that 57·6% of gastroenteritis was attributable to beef consumption.
The high attributable fraction of beef to endemic gastroenteritis seems surprising, but there are precedents to support this finding. First, a previous Adelaide survey also showed consumption of minced beef to be associated with an increased likelihood of illness [Reference Heyworth and Rowe11]. Second, minced beef has previously been shown to have the potential for contamination with enteric pathogens such as pathogenic strains of E. coli, particularly E. coli O157:H7 [Reference Vogt and Dippold12, Reference Sekla13]. Studies have reported pathogenic E. coli to be a common cause of community gastroenteritis [Reference Sinclair14]. However, since most strains of pathogenic E. coli are not notifiable and since, even in people providing a faecal specimen during an episode of gastroenteritis, examination for E. coli is not routine, the contribution of pathogenic strains of E. coli to community gastroenteritis is incompletely understood.
Given the known association of minced beef with E. coli, it would have been helpful if participants had been asked to specify whether the beef product they consumed was minced beef or steak, but unfortunately this question was not included. It is plausible that E. coli may be associated, not just with mince, but also with steak, particularly if the meat is undercooked. In Australia, barbeques are very common, especially during the summer (December–February) and autumn (March–May) periods, and meat (both steak and mince) is often a major part of these events and commonly consumed relatively rare.
Limited water-quality testing of rainwater samples taken from tanks of selected study participants showed that E. coli was present in 30·1% of samples, with levels ranging from 0 to 2400 c.f.u./100 ml (S. Rodrigo, unpublished observations). A priori, this suggested to us that partial immunity to E. coli would be likely to be higher in this cohort of rainwater consumers than in the general population. Previous data have shown that lower bacterial levels may be insufficient to cause illness in persons regularly exposed to bacterial indicators [Reference Strauss15] since partial immunity may be acquired [Reference Eisenberg, Bartram, Hunter, Fewtrell and Bartram16]. For most waterborne pathogens the protection conferred to a host after exposure to the agent of disease is partial and temporary with such partial protection lasting for months or years [Reference Eisenberg, Bartram, Hunter, Fewtrell and Bartram16, Reference Hunter17]. In the case of rainwater consumers, the sources of E. coli are most likely to be birds and small animals that have access to the catchment surface (usually the house roof). Consequently, it is possible that higher pathogenic E. coli doses may be required in order for symptomatic infection to occur in study participants [Reference Eisenberg, Bartram, Hunter, Fewtrell and Bartram16]. The high attributable fraction for beef in this population is therefore particularly interesting, and if anything may underestimate what may be found in the general population.
There was no significant risk of illness associated with consumption of poultry; however, there was a significantly increased risk with the preparation of raw fresh poultry in the household. Fresh poultry is often implicated as the source of illness due to microbiological cross-contamination of surfaces that occurs during handling of raw chicken. Surprisingly a protective effect was noted when frozen chicken was the source of poultry prepared. Freezing does lower bacterial levels, for example Campylobacter levels have been stated to be reduced 100-fold or more [Reference El-Shibiny, Connerton and Connerton18], but thawing may result in microbial growth. Perhaps the strong public health messages regarding the potential for chicken as a risk factor for disease have improved preparation methods over years and reduced the risk of endemic disease associated with poultry.
Other factors associated with a significantly lower likelihood of illness included consumption of salad prepared at home, and consumption of salami and shellfish. While these may have been chance findings, the protective effect of salads prepared at home has been noted previously [Reference Mitakakis19]. It is possible that persons who prepare salads are more concerned about health, making home-prepared salads a proxy for good food hygiene. Surprisingly, salami and shellfish consumption were associated with a reduced odds of illness. Previously these two food types have been implicated in foodborne illness [Reference Mitakakis19, Reference Dowell20]. Therefore the observed association found in this study may be an artefact.
Animal contact was the only non-food exposure significantly associated with gastroenteritis. Animals are known to harbour a variety of pathogens, so exposure to animals – particularly non-pet animals such as those found on farms – is not an unexpected risk factor for illness.
A notable PAF was also obtained when rice that was boiled and eaten immediately was considered (25·9%). In this case there was a difference in frequency of consumption between cases and controls where more cases consumed this food compared to controls. Boiled rice has been associated with outbreaks of illness due to poor handling and cooking practices [Reference Schmid21]. It is difficult to speculate on the reasons for the PAF obtained as participants were not asked about food preparation and handling practices.
As documented in previous studies, children experience a greater rate of gastrointestinal illness compared to adults and are considered to be a more susceptible population [Reference de Wit22–Reference Kuusi24]. Therefore, it is not surprising that the majority of cases in this study were found to be children aged ⩽15 years.
Advantages of the nested design include feasibility, convenience, cost-efficiency, high validity and statistical power [Reference Langholz, Armitage and Colton5]. Another advantage of the nested study is related to baseline collection of exposure data that enables a temporal sequence to be established, an important criteria for causation. However, not all exposure data of interest, such as food consumption and other risk factors, were collected prior to illness. Retrospective collection of such data was the main disadvantage of this study design, resulting in the potential for recall bias.
Selection of controls from the same well-defined cohort as cases (rainwater consumers) minimizes systematic differences (bias and confounding) that can occur in conventional case-control studies. While matching can be conducted in the nested case-control design, for this study an unmatched design was utilized. The unmatched design was convenient, reduced cost in time and labour, and minimized the complexity involved in selecting matched controls from a finite pool.
A limitation of this study was the relatively low capture of cases, with only one third of those reporting HCG completing an exposure questionnaire. It is therefore possible that bias may have been introduced, such that cases interviewed were systematically different from those not interviewed. However, we were able to compare baseline demographic characteristics of cases captured with those not contacted, and the similarity in demographic characteristics of the two suggests minimal if any selection bias as a result of non-contact.
CONCLUSIONS
This study identified a number of factors associated with endemic gastrointestinal illness, particularly showing that consumption of beef was associated with a high odds of illness and with a PAF of 57·6%. In an outbreak setting, exposure to a single food item as a risk factor for illness is often found, but detecting such a high PAF for beef in a non-outbreak setting was unexpected. This result should prompt further research into the contribution of beef to community-based gastroenteritis. If our finding is confirmed, stronger public health messages regarding appropriate preparation of beef will be warranted. Conversely, several foods normally considered to be risk factors for gastroenteritis were found to be associated with a lower likelihood of illness, perhaps indicating that the population involved in this study was aware of some of the previously identified potential risk factors for illness and as a result practised good hygiene for these food types in their daily lives.
In summary, this nested case-control study in a population of rainwater drinkers provides interesting data regarding potential risk factors for gastroenteritis in a non-outbreak setting, and suggests that further consideration of beef consumption as a significant contributor to community gastrointestinal illness is warranted.
ACKNOWLEDGEMENTS
The authors thank Julia Bell who contacted participants for this case-control study. This work was supported by the National Health Medical Research Council [NHMRC Project Grant No. 384226] and the Cooperative Research Centre for Water Quality and Treatment.
DECLARATION OF INTEREST
None.





