INTRODUCTION
Tularaemia is a zoonotic disease caused by the facultative intracellular bacterium Francisella tularensis, a highly virulent Gram-negative bacterium belonging to the γ-subclass of Proteobacteria [Reference Ellis1, Reference Oyston2]. Four subspecies of F. tularensis with different geographical distribution and virulence have been identified [Reference Sjostedt3, Reference Keim, Johansson and Wagner4]. Clinical tularaemia is caused by two of the subspecies: F. tularensis ssp. tularensis (type A), which occurs mainly in North America and ssp. holarctica (type B), which is endemic in many countries of the Northern Hemisphere, including Finland [Reference Sjostedt3]. The ecology of tularaemia is complex and it is still not clear where the bacterium persists in the environment and what factors induce disease outbreaks [Reference Keim, Johansson and Wagner4, Reference Oyston, Sjösted and Titball5]. Clinical manifestations depend mainly on the route of infection and disease severity on the infecting subspecies. After an incubation period of about 3–5 (range 1–14) days, disease onset is acute with non-specific flu-like general symptoms, especially fever, chills and headache [Reference Oyston, Sjösted and Titball5, Reference Tarnvik and Chu6]. If the bacteria enter by skin or mucous membranes, ulceroglandular, glandular, oculoglandular or oropharyngeal tularaemia may result [Reference Tarnvik and Chu6]. In Fennoscandia, the ulceroglandular form is most common and mosquito bites are thought to be an important transmission mechanism [Reference Uhari, Syrjala and Salminen7]. However, few controlled studies are available to demonstrate this association. Inhalation of aerosolized F. tularensis causes respiratory or pneumonic tularaemia, the most severe form of the disease [Reference Tarnvik and Berglund8, Reference Thomas and Schaffner9]. Laboratory diagnosis of tularaemia relies mainly on serology, although antibodies only develop 2–3 weeks after illness onset [Reference Tarnvik and Chu6].
To determine specific risk factors associated with ulceroglandular and pneumonic tularaemia and their public health impact in Finland, we conducted a population-based case-control study during the largest epidemic on record. Information was also collected on clinical features of the disease and patients' characteristics.
METHODS
Laboratory-based surveillance
The Finnish National Healthcare system is organized into 20 geographically and administratively distinct healthcare districts. Laboratory-confirmed tularaemia has been a notifiable disease by the diagnosing laboratory since 1995 and clinical microbiology laboratories report cases directly to the National Infectious Disease Register (NIDR) which is maintained by the National Institute for Health and Welfare (THL). Diagnostic criteria for reporting include isolation of F. tularensis in a clinical specimen or a ⩾fourfold rise in serum antibody titre or a single IgG titre of ⩾160.
Population-based case-control study
Case definition and identification
A case patient was defined as a person with laboratory-confirmed tularaemia whose first specimen (specimen date) was obtained either from 1 July to 11 August (south-western Finland cases) or from 1 September to 6 October 2000 (northern Finland cases) and tested at one of two laboratories performing tularaemia diagnostics in Finland (Etelä-Pohjanmaa Central Hospital in south-western Finland or Oulu University Central Hospital in northern Finland), and who were residents of the endemic health districts (Fig. 1) served by these laboratories. For the case-control study, we identified case patients directly through active surveillance of these two laboratories to accelerate the case identification process.

Fig. 1 [colour online]. Incidence of laboratory-confirmed Francisella tularensis infections by healthcare district, Finland, 2000. 1, North Bothnia (Pohjois-Pohjanmaa healthcare district); 2, Central Bothnia (Keski-Pohjanmaa healthcare district); 3, South Bothnia (Etelä-Pohjanmaa healthcare district); 4, Central Finland (Keski-Suomi healthcare district). (Data are from the National Infectious Disease Registry.)
Case classifications
Patients with laboratory-confirmed tularaemia and physician-diagnosed pneumonia were classified as pneumonia cases. Patients with laboratory-confirmed tularaemia who reported enlarged lymph nodes and/or skin ulcers were classified as ulceroglandular cases.
Selection of control subjects
Control subjects were identified in the general population of the tularaemia endemic health districts. For each enrolled case patient, four controls who matched the patient by year of birth, sex and postal code of residency were randomly selected from the national population information system. Potential controls were excluded from the study if they reported having had febrile illness during the 2-week exposure period or if they were away from their permanent place of residence for more than 1 day during that time.
Data collection
A standard questionnaire was mailed to tularaemia case patients and to four selected control subjects. For children aged <15 years, the parents were requested to complete the questionnaire. The first part of the questionnaire included questions on clinical symptoms, history of febrile illness, medications, referrals to hospital, possible symptoms in household members and pet ownership. The second part of the questionnaire included questions about exposures to presumed risk factors for tularaemia. For both cases and controls, the questions referred to the 2 weeks before the illness onset date in the case patient (the exposure period). The following exposures were recorded: time spent outdoors (logging, hiking, hunting, picking berries or mushrooms), participating in farming activities (including harvesting and handling hay, cleaning barns), handling dead animals, swimming in natural waters, drinking water from lakes or wells, and insect bites (mosquito, tick, deer fly, horse fly, or other insect). Study subjects were also asked about use of insect repellents or other protective measures.
Statistical analyses
Matched odds ratios (mORs) and their 95% confidence intervals (CIs) were calculated using conditional logistic regression for three main outcomes in univariable analysis: (1) all tularaemia cases, (2) ulceroglandular cases and (3) pneumonia cases. We also constructed composite variables such as ‘exposure to hay’ which included activities such as harvesting hay, cleaning hay, barns or harvester. In univariable analysis, we assumed that missing data were missing approximately at random [Reference Schafer and Graham10, Reference Sterne11]. We used a P value of ⩽0·2 in the univariable analyses as a screening criterion for selection of variables for multivariable analyses [Reference Hosmer and Lemeshow12]. Since self-reported exposures had varying proportions of missing responses, which may introduce bias and reduce statistical power, two different multivariable models were developed. Model 1 was a frequentist model where the variable selection strategy was the backward elimination method with Akaike's Information Criterion (AIC) corresponding to binary variables with a P value <0·157 for inclusion in the model [Reference Steyerberg13]. In model 1, only subjects with complete information on variables in the final model were included. Model 2 was a Bayesian full-likelihood analysis where missing data were taken into account and became a multidimensional additional parameter [Reference Congdon14]. In Bayesian model 2 we performed a Gibbs’ variable selection. To include a variable in the final model, the required posterior probability had to be >50% [Reference Congdon14]. We also used the Kolmogorov–Smirnov test to compare distributions and the χ 2 test to assess associations between variables in case patients (independent observations). Adjusted population-attributable risks (PARs) and their 95% CIs for each independent risk factor were calculated by using the formula: PAR = pd((RR – 1)/RR) and adjusted odds ratios (aORs) from model 2 (pd = proportion of cases exposed to a risk factor). This formula produces an internally valid estimate when confounding exists and when adjusted relative risks (i.e. aORs) are used [Reference Bruzzi15]. Analyses were performed using Stata version 9.2 (Stata Corporation, USA) and Winbugs 1.4.3 (Imperial College and MRC, UK).
RESULTS
National laboratory-based surveillance
In 2000, a total of 926 cases of laboratory-confirmed tularaemia were reported nationally to the NIDR; 897 (97%) cases had specimen dates during the epidemic period from 1 July to 31 October 2000. Figure 2 shows the reported cases by date of first specimen. The overall annual incidence was 18 cases/100 000 population. Rates were highest in the health districts included in the case-control study (North Bothnia, South Bothnia, Central Finland) (Fig. 1). Tularaemia diagnosis was based on serology in 97% (of which ~13% were single titres) and on bacterial culture in 3% of cases. Median age was 48 years (range 0–85 years); 60% of the cases were males.

Fig. 2 [colour online]. Laboratory-confirmed cases of Francisella tularensis infections reported to national surveillance by date of first specimen, Finland, 1 July to 31 October 2000.
Population-based case-control study
Questionnaires were mailed to 261 laboratory-confirmed case patients and 780 control subjects. Completed questionnaires were returned by 227 (87%) case patients and 480 (62%) control subjects. The median time from onset of symptoms to laboratory confirmation of diagnosis of tularaemia in cases was 22 days (range 1–53 days) and the median time from illness onset to completing the questionnaire was 84 days (range 8–148 days) for all study subjects. Fourteen control subjects were excluded because of febrile illness compatible with tularaemia during the exposure period and five because of absence from their permanent place of residence for >1 day during the period. Forty-four control subjects were excluded because their respective case patients did not return the study questionnaire. An additional two control subjects were excluded because they were not residents of the three endemic districts. After exclusions, 227 case patients and 415 control subjects were included in the analysis.
Clinical characteristics of case patients
Signs and symptoms reported by 167 case patients (74%) met the definition of ulceroglandular tularaemia and in 20 case patients (9%) the definition of pneumonic tularaemia. The remaining 40 laboratory-confirmed tularaemia case patients did not report symptoms fulfilling definitions for either form of tularaemia; these cases were included in the study under the category ‘other’.
Ulceroglandular cases
Seventy-six per cent of ulceroglandular cases resided in North Bothnia, 7% in South Bothnia, 5% in Central Finland and the rest in other healthcare districts (Table 1, Fig. 1). In case patients with ulceroglandular disease, both genders were represented equally; age ranged from 4 months to 84 years (mean 42 years). Illness onsets were from 22 May to 1 October 2000. The most common symptoms of ulceroglandular tularaemia were fever, lymphadenopathy, cutaneous ulcer and myalgia. The median duration of illness was 18 days (range 4–81 days); 37% of cases with ulceroglandular tularaemia were hospitalized.
Table 1. Characteristics and clinical symptoms of case patients with laboratory-confirmed tularaemia infection, Finland 2000
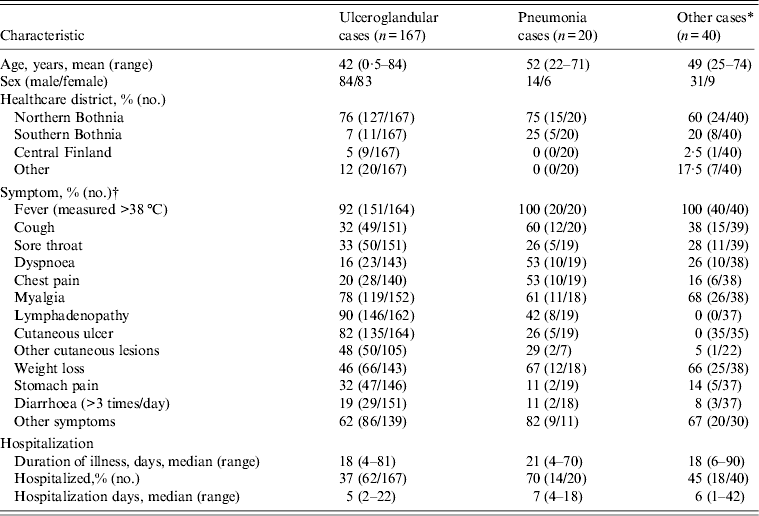
* Reported symptoms did not fulfil the case definition of ulceroglandular or pneumonic tularaemia.
† Denominators represent persons for whom data were available for a given variable.
Pneumonia cases
Seventy-five per cent of the pneumonia case patients resided in North Bothnia, the remaining 25% in South Bothnia (Table 1, Fig. 1). All pneumonia case patients were adults with a mean age of 52 years (range 22–71 years) and 70% were males. Onset of illness was from 23 June to 2 September 2000. All of the patients with pneumonic tularaemia reported fever. The majority of patients with pneumonic tularaemia also reported weight loss, myalgia, cough, dyspnoea and chest pain. Other commonly reported symptoms were headache and exhaustion. Median duration of symptoms was 21 days (range 7–40 days); 70% of the pneumonia patients were hospitalized.
Factors associated with tularaemia
All cases
In univariable analysis, arthropod bites (any arthropod) and specifically, mosquito and horse-fly bites, outdoor activities, handling dead animals (rabbits, voles) and farming activities (land or field preparation, cleaning hay or grain barns, harvesting hay, cleaning harvester, producing silage) were associated with tularaemia (Table 2). Table 3 shows the results of multivariable models and PARs for independent risk factors. In model 1 (traditional), farming activities outdoor and handling dead animals and mosquito and horse-fly bites were associated with tularaemia. However, due to missing values, only 53% of the statistical units were included in model 1. In model 2 (Bayesian), farming activities (aOR 5·5, 95% CI 2·7–7·6), handling dead animals (aOR 5·8, 95% CI 2·3–15·9) and mosquito bites (aOR 5·4, 95% CI 2·8–10·9) were independently associated with tularaemia with PARs of 73% for mosquito bites, 40% for farming activities and 8% for handling dead animals.
Table 2. Univariable logistc regression analyses of factors associated with tularaemia, Finland 2000
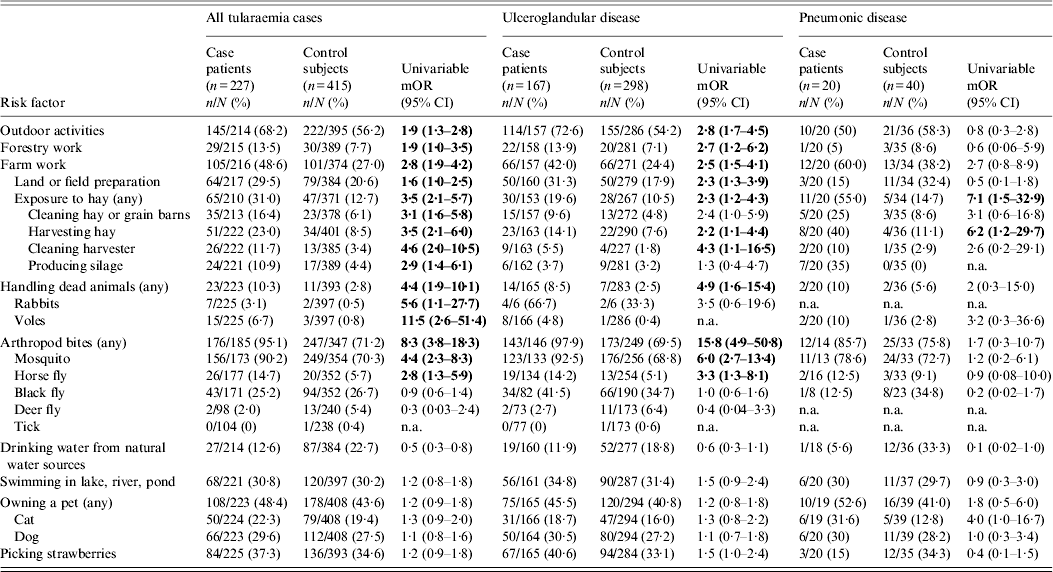
mOR, Matched odds ratio; CI, confidence interval; n.a., not available.
Bold values indicate statistically significant (P < 0·05 when 95% CI lower limit >1) mORs.
Denominator indicates persons for whom data were available for a given variable.
Table 3. Multivariable models and population-attributable risks for factors associated with tularaemia, Finland 2000
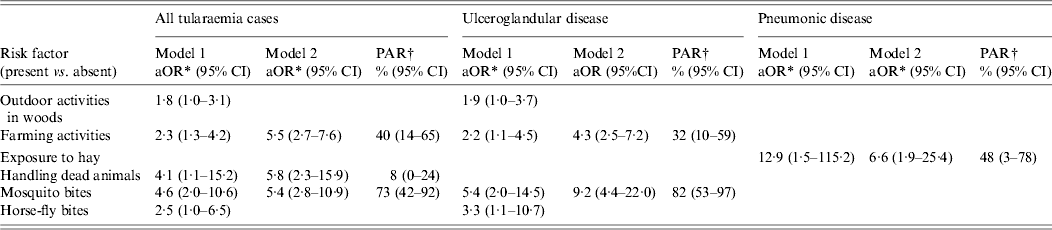
aOR, Adjusted odds ratio; CI, confidence interval; PAR, population attributable risk.
Model 1: Conditional logistic regression model (traditional).
Model 2: Bayesian full-likelihood conditional logistic regression model.
* Each aOR is adjusted for the remaining variables shown in the table in addition to the matching factors (see Methods section for details).
† Calculated from aORs derived from multivariable logistic regression model 2.
Ulceroglandular cases
Of case patients with ulceroglandular tularaemia, 98% reported arthropod bites in the 2 weeks before illness compared to 70% of controls (Table 2). Of the specific arthropods, however, only mosquito bites and horse-fly bites were significantly associated with disease. Of the case patients, 93% reported mosquito bites compared to 69% of controls; horse-fly bites were reported by 14% of cases and 5% of controls. Other factors associated with ulceroglandular tularaemia in univariable analysis were outdoor activities, forestry work, farming activities and handling dead animals (Table 2). In multivariable model 1, mosquito bites, horse-fly bites and farming activities were significantly associated with ulceroglandular tularaemia (Table 3). However, due to missing values only 55% of the statistical units were included in model 1. In model 2 where missing data excluded from model 1 was taken into account, mosquito bites (aOR 9·2, 95% CI 4·4–22·0) and farming activities (aOR 4·3, 95% CI 2·5–7·2) remained independently associated with ulceroglandular tularaemia with PARs of 82% and 32%, respectively.
Pneumonia cases
In univariable analysis (Table 2), the composite variable ‘exposure to hay’ was significantly associated with pneumonic tularaemia. In the 2 weeks before illness onset, 55% of pneumonia patients were exposed to hay, compared to 15% of controls. Exposure to hay remained a strong risk factor for pneumonic tularaemia in both multivariable models 1 and 2 (aOR 6·6, 95% CI 1·9–25·4) with a PAR of 48% (Table 3). In the pneumonia group, 85% of the statistical units were included in the analysis in model 1.
DISCUSSION
We report results from national surveillance of tularaemia and a large, population-based case-control study during a major tularaemia epidemic in Finland. Our results indicate that mosquito bites, farming activities and handling dead animals were independently associated with tularaemia infection overall, and that some 73% of all cases could be attributed to mosquito bites. Analyses focusing on risk factors for different clinical outcomes of tularaemia showed that mosquito bites and farming activities in general were associated with ulceroglandular tularaemia, while exposure to hay dust was the only risk factor independently associated with pneumonic tularaemia.
Mosquito-borne transmission has been considered an important mode of transmission of F. tularensis holarctica in Fennoscandia [Reference Lundstrom16–Reference Eliasson18] and some of the largest epidemics have reported a link to mosquito bites. However, no previous studies have included a population-based, controlled design or quantification of the public health impact attributable to various exposures. Our results are consistent with a Swedish study conducted during the same outbreak year which also found an association between tularaemia infection and mosquito bites and farming [Reference Eliasson18]. Mosquitoes may already acquire the bacterium as larvae from their aquatic habitat [Reference Lundstrom16, Reference Ryden17, Reference Broman19], and persistence of the bacterium in natural waters of endemic areas could explain the uneven geographical distribution of tularaemia [Reference Tärnvik, Sandström and Sjöstedt20–Reference Splettstoesser23]. In Finland, the provinces of Central Finland and North and South Bothnia have the highest incidence of human disease, with fewer cases in other districts. The association of farming with all forms of disease is probably an indicator of outdoor exposure to mosquitoes.
In the case-control study, study participants were asked about being bitten by the most frequent arthropods present in Finland. Mosquito and horse-fly bites were the only specific species associated with tularaemia infection in univariable analysis; after adjustment for other variables in multivariable analysis, only mosquito bites remained statistically significant. However, few subjects were exposed to arthropods other than mosquitoes thereby reducing the statistical power to evaluate these associations. Study participants may also have had difficulties in indentifying the various arthropod species or even noticing being bitten by an arthropod, potentially resulting in misclassification of exposure. Because the link to mosquito bites is widely known in the population in the epidemic area in Finland, case patients may also have reported bites more readily than controls creating the potential for recall bias. Although ticks are an important vector for F. tularensis in the USA [Reference Petersen, Mead and Schriefer24], none of the case patients in our study reported tick bites.
Exposure to hay dust was the only exposure significantly associated with pneumonic tularaemia; some 55% of these patients reported exposure to hay. Farming activities, such as harvesting hay, have the potential of aerosolizing environmental pathogens and airborne tularaemia outbreaks have been linked to farm work [Reference Dahlstrand, Ringertz and Zetterberg25–Reference Allue28]. However, this is the first study quantifying the association in a controlled study design. Interestingly, five (26%) of the patients with pneumonic form of disease also reported having had a cutaneous ulcer. It is possible that their illness may have been caused by haematogenous spread of F. tularensis to the lungs as a complication. Respiratory tularaemia is considered an occupational hazard for farmers in endemic areas. Most patients with pneumonic tularaemia were males, supporting the link with farming occupation. Some small outbreaks of airborne tularaemia in Central Europe an North America have also been associated with other outdoor activities such as lawn mowing [Reference McCarthy and Murphy29, Reference Feldman30] or hunting [Reference Siret31, Reference Hauri32].
An association with cat ownership and tularaemia was suggested in a Swedish study in which the authors hypothesized that the association could be due to rodents brought home by the cat [Reference Eliasson18]. Although cat ownership was more common in cases than controls with pneumonic tularaemia (32% vs. 13%) in our study, the number of cases was small and this association was not statistically significant. However, cats are common on farms and their potential association with pneumonic tularaemia may be confounded by the overall association with farm work.
The main limitation of all case-control studies includes potential for selection and recall bias. Because of active surveillance for cases, population-based design and relatively high participation rates in both cases and controls in the study, selection bias does not appear to be a major concern in our study. Although the control subjects who reported febrile illness were excluded from the study, it was not feasible to test control subjects serologically and some may have had subclinical infection. This potential for misclassification of subjects, however, is probably small and non-differential.
Possible recall bias in reporting various exposures, however, must be considered, since clinical diagnosis of tularaemia relies on serology and increased antibody levels are generally only present 2 weeks after illness onset. Although we identified the case-patients rapidly through active, laboratory-based surveillance, a few weeks’ delay from the date of specimen collection to completing the questionnaire was inevitable. Control subjects may obviously have had difficulty in remembering certain exposures despite the instructions to use memory aids, such as calendars. A frequent additional problem in self-reported surveys is missing information. In our study, potential bias from missing data was reduced by a Bayesian full-likelihood modelling approach which has the ability to take missing data into account [Reference Schafer and Graham10], further increasing the validity of our findings. Effects which are clearly significant in either the frequentist or Bayesian models are usually significant in both models, but the full-likelihood analysis has the ability to better discriminate the effects. Thus, the point estimates are generally larger in the Bayesian model.
In conclusion, this large population-based case-control study of F. tularensis infection contributes new information about the relative public health importance of various risk factors and provides a comprehensive description of the clinical characteristics. Tularaemia causes severe illness and places a substantial burden on the healthcare system during recurring epidemics. Few population-based studies of tularaemia are available, and our findings indicate that risk factors for ulceroglandular and pneumonic forms of tularaemia are different, enabling targeting of prevention efforts accordingly. Physicians in the endemic areas should consider tularaemia in patients with febrile illness and should be trained in how to make the diagnosis. People living in endemic areas should be educated about activities associated with increased risk of infection, preventive measures and the symptoms of tularaemia. The risk of tularaemia infection can be reduced by protecting against mosquito bites, using disposable gloves if handling dead rodents, and by minimizing exposure to hay dust potentially contaminated with bacteria by wearing masks for respiratory protection while performing farming and landscaping activities. Future studies should be designed to evaluate how to prevent transmission of mosquito-borne tularaemia infection to humans.
ACKNOWLEDGEMENTS
We thank Elisa Huovinen, Salla Toikkanen, Outi Lyytikäinen, Karin Nygård, Pekka Holmsröm, Pirjo Matero, Pirjo Kiiski, Leila Balk, Raili Ronkainen, Irmeli Behm and Marika Seger for their assistance in the investigation.
DECLARATION OF INTEREST
None.







