INTRODUCTION
Salmonella Enteritidis (SE) is one of the most common serotypes of Salmonella isolated from humans worldwide. In the United States this serotype accounts for about 25% of human salmonellosis cases [Reference Saeed1]. In the United States, in addition to farm animals, SE commonly colonizes rodents, reptiles, and amphibians [Reference Garber2, Reference Chambers and Hulse3]. Most SE outbreaks in the United States are linked to undercooked eggs [Reference Patrick4]. In the United States SE infections associated with eggs have been due to certain phage types (PT) (e.g. PT 4, 8, 13a, 14b) [Reference Saeed1].
From 2000 to 2002, seven outbreaks of SE infections associated with mung bean sprouts were identified in the United States, Canada, and The Netherlands. All were caused by SE with very uncommon phage types. In this paper we describe the first investigation in detail, review the subsequent six outbreaks and experiments to determine the adherence of the outbreak strain to mung bean sprouts. We also examine compliance by sprout growers to key elements of the US Food and Drug Administration (FDA) guidelines to prevent sprout-associated outbreaks [5].
METHODS
Outbreak 1
Background and epidemiological investigations
Routine surveillance reports revealed an increase in the number of SE infections in two counties in California in March and April 2000 (the outbreak period). Eleven patients ate at one or more outlets of a chain of Vietnamese restaurants (Chain A restaurants). To find related cases outside California, health departments in Oregon and Nevada submitted isolates from patients with SE infections in the outbreak period for molecular typing. Because the outbreak was due to a rare phage type of SE, we sought potentially associated cases by reviewing the phage typing results of all SE strains submitted to the Centers for Disease Control and Prevention (CDC) during the outbreak period.
We conducted two case-control studies for this outbreak in California. For the first study, we defined ‘case-patients’ as persons with culture-confirmed SE infections or diarrhoea (3 or more loose stools in a 24-h period) during the outbreak period and onset within 1 week after eating at a Chain A restaurant. We identified non-culture-confirmed case-patients and controls by interviewing dining companions of culture-confirmed case-patients. We queried case-patients and controls about consumption of the 85 dishes listed on Chain A's menu and about condiments and sauces that were present but not listed on the menu using a standardized questionnaire.
In the second study, we defined ‘case-patients’ as residents of the two California counties with culture-confirmed SE infection with onset in the outbreak period who had not eaten at Chain A restaurants. Through random digit dialling we sought community-based controls (two per case) who were adults aged 18–50 years (same age range as case-patients), matched to case-patients by telephone exchange (area code and first three digits of the phone number), who did not have diarrhoea in the outbreak period. Using a standardized questionnaire, we queried the case-patients about eating, during the week before onset, the three raw produce items in the appetizer implicated in Study 1 and about eating at any Vietnamese restaurant. We asked the controls about eating these items during the exposure period of the case-patients. We calculated matched odds ratios (mOR), 95% confidence intervals (CI), and P values using Epi-Info 6.04b software [6]. For analyses with small expected cell sizes we calculated exact confidence limits using EXACT software [Reference Martin and Austin7].
Patients from states other than California with SE infections matching the outbreak strain in California were asked about consumption of sprouts in the week before onset and the location of purchase. Nevada and Oregon submitted isolates for phage typing because they are adjacent to California and had SE clusters coincident with the increase in SE cases noted in the two California counties.
Environmental and laboratory investigations
For the implicated appetizers from Chain A restaurants, we collected samples of the raw produce ingredients and reviewed the preparation procedures. We traced the raw ingredients to the distributors; the sprouts were then traced to the sprout grower. We conducted similar tracebacks for sprouts consumed by patients from Nevada and Oregon.
On 17 April we reviewed seed disinfection and sprout production practices at Sprout Grower A and collected six irrigation water samples and ten environmental specimens (e.g. swabs of drains and growing bins). We collected stools for Salmonella culture from all 24 employees who handled sprouts. We collected SE isolates from a commercial laboratory that had cultured SE from spent irrigation water (water collected after washing germinated sprouts) collected on 6 March and 9 April 2000 (just before and during the outbreak period) at Sprout Grower A. Three lots of seeds were used in the outbreak period; we collected 60 one-pound samples of mung bean seeds from each of the two available seed lots.
Using standard procedures we serotyped Salmonella isolates. To further characterize SE isolates (including those from the commercial laboratory), we used pulsed-field gel electrophoresis (PFGE). Of 14 SE isolates from patients from California, 12 matched identically by PFGE. We defined the outbreak strain as this predominant strain and further characterized the strain as PT 33 (CDC Laboratory). SE isolates from outside California were considered to be ‘outbreak related’ if they matched the outbreak strain by PFGE or by phage typing. Using enrichment media, we attempted to culture Salmonella from food (including sprouts), 60 samples from each of the irrigation water, environmental specimens, and two seed lots using previously described techniques [Reference Inami and Moler8].
To examine the growth of Salmonella on mung beans during sprouting, we inoculated mung bean seeds separately with the outbreak strain of SE and S. Newport and measured the populations daily for 4 days [Reference Barak, Whitehand and Charkowski9, Reference Charkowski10]. We selected S. Newport strains to compare with SE because the attachment and colonization of alfalfa sprouts had already been studied on S. Newport which had been isolated from alfalfa seeds associated with a prior outbreak. Bacteria were grown in, or plated on, Luria–Bertani (LB) agar. Kanamycin was incorporated into the medium at 40 mg/l. Plasmid pKT-kan, in which a 131-bp nptII promoter fragment from Tn5 was fused to the GFP gene of plasmid pPROBE-KT is a stable, broad-host range vector that confers kanamycin resistance and green fluorescent protein (gfp) expression. Plasmid pKT-kan was transformed into all strains of S. Newport and S. Enteritidis PT 33. Mung bean sprout colonization assays were then performed [Reference Barak, Whitehand and Charkowski9, Reference Charkowski10]. Each experiment was performed at least three times. Mung bean seeds were sprouted in Petri plates and irrigation water was exchanged daily. Overnight bacterial cultures grown on LB agar were suspended in sterile water at a concentration of ~104 c.f.u./ml for attachment and colonization assays and at ~106 c.f.u./ml for microscopy studies. For colonization and microscopy assays, seeds were incubated in the bacterial suspension for 1 h, and then the suspension was replaced with sterile water and the seeds were sprouted for up to 4 days at room temperature. Individual seeds/sprouts were rinsed in 1 ml sterile water for 30 s then homogenized. The homogenate was dilution plated and the cultures were incubated overnight at 37°C, then the colonies were enumerated. We used stereo-fluorescence microscopy to determine the locations where salmonellae adhered to the sprouts [Reference Barak, Whitehand and Charkowski9, Reference Charkowski10].
Additional SE outbreaks associated with mung bean sprouts
Over the next 2 years, several public health agencies investigated six other SE outbreaks associated with mung bean sprouts. The methods for the epidemiological, laboratory, statistical, environmental, and traceback investigations were similar to those described for the first outbreak; there were five case-control studies and one cohort study. Details of two of these outbreaks were published [Reference Honish and Nguyen11, Reference Van Duynhoven, Widdowson and de Jager12]. Each outbreak had a distinct outbreak strain characterized by a specific SE phage type (Table 1). The public health laboratory in Florida also characterized isolates using PFGE. The types of controls (e.g. meal companion, telephone exchange match) or cohort (school children) selected for these investigations are listed in Table 1. For all outbreaks we assessed the seed disinfection and post-production screening techniques (e.g. testing of spent irrigation water) used by growers. For sprout growers who tested spent irrigation water, we collected the results of any testing during the outbreak periods and the sprout growers' responses to the positive result report, as well as the results of environmental testing of the growing facility or product conducted after the outbreak was identified.
Table 1. Outbreaks of Salmonella Enteritidis (SE) associated with mung bean sprouts, 2000–2002

OR, Odds ratio; CI, confidence interval.
* In Hawaii a cohort study of school children was conducted.
† During the outbreak, the commercial laboratory for the sprout grower in Hawaii reported finding Salmonella group B from spent irrigation water. The serogroup could not be confirmed by a public health laboratory because the isolate had been discarded by the commercial laboratory.
None of the sprout growers followed the FDA guidelines for disinfection of seeds and for discarding of seed lots of sprouts with spent irrigation water samples that tested positive for Salmonella.
RESULTS
Epidemiological investigation of the first outbreak
We identified 67 patients from the two California counties with culture-confirmed SE infections in the outbreak period (note that only a sample of these isolates were further characterized by PFGE and phage typing, n=6). The age range was 10–83 years (mean age 42 years); 63% of patients were female. Most patients had gastroenteritis; two patients had sepsis, and one had osteomyelitis. Seventeen patients were treated in emergency rooms and three patients were hospitalized; there were no deaths.
We found an association between illness and eating an uncooked spring roll from a Chain A restaurant (appetizer); 15/16 case-patients vs. 4/16 controls ate an uncooked spring roll (OR 45, 95% CI 3·8–2046, P<0·001). The spring rolls were made on the premises from rice crepes, rice noodles, raw cilantro, raw mung bean sprouts, and raw red leaf lettuce. In the second study (where we examined the produce ingredients among those who had not eaten at Chain A), only mung bean sprouts were associated with culture-confirmed SE infections (Table 2). Nine of ten case-patients compared with only 1/20 community controls ate raw mung bean sprouts (mOR ∞, 95% CI 3·8–∞, P<0·001).
Table 2. Results of a case-control study of 10 patients with sporadic Salmonella Enteritidis infection (who had not eaten at a Chain A restaurant) and their 20 matched community controls, California, March and April 2000
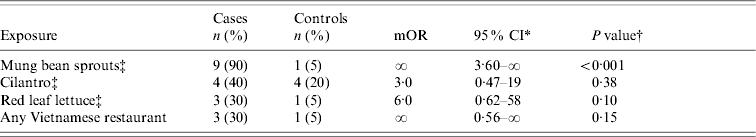
mOR, Matched odds ratio; CI, confidence interval.
* Exact 95% CI based on Fisher limits.
† Fisher's exact two-tailed test P value.
‡ We enquired about raw (uncooked) mung bean sprouts, cilantro, and red leaf lettuce.
Four Nevada residents had infections with the outbreak strain. In the week before their illness onset, all of these patients had eaten Vietnamese spring rolls with mung bean sprouts at restaurants in Nevada. Oregon public health officials independently investigated a cluster of three SE cases among high-school students who had attended a conference; all had eaten a Chinese chicken salad with raw mung bean sprouts.
Only one other SE PT 33 isolate was identified among those submitted to CDC in 2000 from other states. The patient, a 55-year-old woman resident of Massachusetts, had an onset date of 15 March 2000 (in the outbreak period) and had not travelled during her incubation period. She could not recall if she had eaten raw mung bean sprouts in the week before her onset, but she reported eating them frequently.
Environmental and laboratory investigations for the first outbreak
We did not identify major violations of proper food-handling practices or food-handler illnesses in Chain A restaurants. A traceback investigation from Chain A restaurants and other restaurants or stores where case-patients in California, Nevada, and Oregon consumed or purchased raw mung bean sprouts revealed a common sprout grower, Sprout Grower A. The distribution of sprouts from this sprout grower matched the distribution of the residences of cases in the outbreak. In contrast, the distribution of cilantro and red leaf lettuce did not.
At Sprout Grower A, there were no major violations of Good Manufacturing Practices and no reports of employees with diarrhoeal illnesses. The owner of Sprout Grower A reported decontaminating mung bean sprout seeds with 2000 ppm calcium hypochlorite, 10% of the concentration recommended by the FDA. The owner reported a preference for the lower hypochlorite concentration because of concerns about worker safety and a decreased viability (shelf-life) of the mung bean sprouts at the higher concentration. However, in compliance with FDA guidelines, Sprout Grower A did routinely test spent irrigation water for Salmonella starting in January 2000. A commercial laboratory, using methods other than those approved by the FDA, had reported positive Salmonella group D test results to Sprout Grower A from samples submitted on 6 days in March and April. Grower A had discarded sprouts from bins that had tested positive, but, sprouts produced on the same day from the same seed lot in bins that did not test positive were distributed for purchase. The grower continued using the same lots of mung bean seeds that produced the contaminated spent irrigation water for at least 6 weeks. Any of three lots could have accounted for all the cases.
Representative SE isolates from case-patients in California (n=6), Oregon (n=1), Nevada (n=1), and Massachusetts (n=1), and Sprout Grower A's spent irrigation water were submitted to the commercial laboratory shortly before and during the outbreak, and the drain swab collected at Sprout Grower A during our investigation were all phage typed as PT 33. SE was also isolated from cultures of swabs from the floor and two growing bins at Sprout Grower A, but these SE isolates were not further characterized. SE was not recovered from: any of the raw produce ingredients collected at Chain A restaurants, the 24 workers at Sprout Grower A, bean sprouts from Sprout Grower A, or the two lots of mung bean seed.
Both SE PT 33 and S. Newport increased by 2·5 logs in 48 h on mung bean sprouts and persisted throughout 4 days (the usual harvest is 3–5 days) (Fig. 1). There was no significant difference between the growth of SE PT 33 and S. Newport on mung beans. Both strains of salmonellae grew on the hilum of seeds (Fig. 1a), cracks in the seed coat, the root hairs (Fig. 1b), and cotyledon trichomes (not shown). Salmonellae also attached to a thickened region of the root. This thickened area is probably mucilage secreted from epidermal cells of the developing root (Fig. 1c).

Fig. 1. Adherence and growth of Salmonella Enteritidis phage type 33 (SE PT 33) and S. Newport on mung bean sprouts. SE PT 33 containing a GFP-expressing plasmid was added to mung bean seeds prior to sprouting. Sprouts were examined by stereo-fluorescence microscopy over 3 days. (a) SE PT 33 colonizing the hilum region of the sprout (s) and seed coat (sc). (b) Adherence to root hairs (rh). (c) Adherence to a substance secreted from the epidermal root (r) cells. (d) Growth curves of SE PT33 (- -○- -) and S. Newport (–◆–) strains over 4 days on inoculated mung bean sprouts.
Subsequent outbreaks associated with mung bean sprouts: 2000–2002
From 2000–2002, six other outbreaks of SE infections associated with mung bean sprouts were identified in Canada, The Netherlands, and other states in the United States (Table 1). These outbreaks were also caused by uncommon SE phage types: PT 11b in Alberta and Saskatchewan, Canada in April 2000 [Reference Honish and Nguyen11], PT 4b in The Netherlands in November 2000 [Reference Van Duynhoven, Widdowson and de Jager12], PT 1 in Hawaii, and SE PT 913 in Alberta, Canada in February 2001, Florida in April 2001, and Maine in February 2002.
Five of these outbreaks were linked to Vietnamese or Thai cuisine. None of the sprout growers linked to these outbreaks followed FDA seed disinfection guidelines. In outbreak 4, the sprout grower tested spent irrigation water as recommended by the FDA and received positive test results for Salmonella from a commercial laboratory. However, the sprout grower's policy was to release sprouts produced from the same seeds if those batches did not have spent irrigation water that tested positive, so sprouts from the implicated seed may have been released, similar to the action of the sprout grower for outbreak 1. In outbreak 3, two batches of sprouts harvested 3 weeks apart grew SE but the sprout grower continued using seed lots that were used to produce these sprouts; none of the other three outbreaks had laboratory evidence of SE in seeds or sprouts. In four outbreaks, the seeds were traced to China and in two outbreaks, as well as the first outbreak, the seeds were traced to either China or Australia.
DISCUSSION
Raw mung bean sprouts were implicated as the vehicle for seven outbreaks of SE infection in a 3-year period. Millions of pounds of mung beans are consumed each year in the United States. But prior to 2000, no outbreaks associated with mung beans were reported to the CDC in the United States. We found a report of only two prior outbreaks of SE due to mung bean sprouts worldwide [Reference O'Mahony13].
Our review of the literature did not uncover any prior outbreaks of SE infections associated with any type of sprout. The vast majority of outbreaks of SE infections in the United States and in California with a confirmed food vehicle, are associated with unpasteurized shell eggs [Reference Mishu14, Reference Mohle-Boetani15]. Of 371 SE outbreaks in the United States from 1985 to 1999 with confirmed food vehicles, 80% implicated eggs or items containing eggs (e.g. omelettes); none were due to raw produce [Reference Patrick4].
In all seven outbreaks, epidemiological investigations identified an association between SE infection and consumption of raw mung bean sprouts and the traceback investigations revealed a common sprout grower, supporting the epidemiological association. In two investigations, the outbreak strain of SE was also found in environmental specimens: spent irrigation water and a drain in outbreak 1 and harvested sprouts in outbreak 3. In one outbreak (outbreak 4), the commercial laboratory identified Salmonella group B from spent irrigation water but the serogroup could not be confirmed by a public health laboratory because the isolate was discarded.
Recurrent outbreaks due to alfalfa sprouts starting in 1995, led the FDA in 1999 to recommend disinfection of all sprout seeds and pre-release product screening for salmonellae [5]. A survey of sprout growers in California revealed that mung bean sprout growers were less likely to follow these recommendations than alfalfa sprout growers [Reference Thomas16].
In all of these SE outbreaks associated with mung bean sprouts, the public health response occurred only after an increase in cases was noted through routine surveillance. Neither the sprout grower nor the private laboratory reported positive Salmonella findings to public health officials. Although this is not recommended explicitly in the FDA guidelines, earlier notification to public health personnel of positive Salmonella tests would have enabled oversight of control measures in the sprout-growing facilities that could have prevented cases. In outbreaks 1 and 3 the sprout grower continued to use the implicated seed lots for several weeks after finding salmonellae in spent irrigation water or sprouts. Public health officials could have worked with the grower to ensure that implicated seed lots were returned to the distributor or discarded. In outbreak 4 the continued use of the implicated seed lot is unclear because of poor record keeping by the grower; nevertheless, the practice of this firm was to withhold only the bins of sprouts that had Salmonella-positive spent irrigation water but not all other sprouts produced with the same seed lot, despite the recommendations of the FDA.
The only prior outbreaks we identified worldwide in which mung bean sprouts were identified as the cause of Salmonella outbreaks occurred in England and Sweden in 1988 [Reference O'Mahony13]. Yet, contamination of mung bean sprouts may be fairly common: a survey in Thailand revealed that 9% of mung bean sprouts were contaminated with salmonellae [Reference Jerngklinehan and Saitanu17]. Possible reasons for few outbreaks being associated with mung bean sprouts compared with the many outbreaks associated with alfalfa sprouts may be that: (1) mung bean sprouts, unlike alfalfa sprouts, are often cooked (e.g. in Chinese cuisine), (2) consumption of mung bean sprouts may not be easily recalled by patients since these sprouts are often used as condiments in appetizers, soups, and salads, and may not be enquired about in an investigation, and (3) SE outbreaks associated with mung bean sprouts could have been difficult to detect because SE is one of the most common serotypes in the United States, Europe, and Canada [Reference Herikstad, Motarjemi and Tauxe18]. In the United States only one isolate is routinely phage typed from each outbreak. Cases in prior outbreaks could have been widely dispersed and not linked because they were due to more common PFGE types.
The association of mung bean sprouts with outbreaks of SE infections but not with outbreaks due to other serotypes of Salmonella is notable. Among 18 outbreaks in North America in 1995–2002 in which a single type of sprout was implicated, all seven outbreaks linked to mung beans were caused by SE while all 11 outbreaks linked to alfalfa or clover sprouts were caused by other S. enterica serotypes (e.g. S. Newport, S. Kottbus). Although association between mung bean sprouts and SE could be due to an affinity for SE to grow on mung beans compared with other salmonellae, our laboratory studies do not support this idea.
There was no evidence that either the sprout growers or their employees were a source of contamination of the sprouts for any of the outbreaks. Since Sprout Grower A did not distribute sprouts to Massachusetts, SE infection with the same rare phage type in a Massachusetts woman who ate raw mung bean sprouts is consistent with contamination of the mung bean seeds for outbreak 1. Finding SE in spent irrigation water (outbreak 1) or sprouts harvested 3–4 weeks apart (outbreak 3) is also consistent with contaminated seed rather than contamination of the environment at the sprout grower's [Reference Van Duynhoven, Widdowson and de Jager12]. Similarly, in the outbreaks in England and Sweden, mung bean seeds were the source of contamination [Reference O'Mahony13]. Moreover, most outbreaks in the United States associated with alfalfa and clover sprouts were similarly linked to contaminated seeds [Reference Taormina, Beuchat and Slutsker19]. The negative culture results in the samples of the mung seed lots that could be tested in outbreak 1 does not rule out seed contamination. The contamination could have been in the lot of seed that was not tested or could have been at low concentration or unevenly distributed in the lots that were tested. In four outbreaks the seeds were from China and in three outbreaks the seeds were from either China or Australia (Table 1).
The rare phage types in these outbreaks may relate to the source of the contamination. The SE strains commonly found in the United States are PT 4, 8, and 13a [Reference Saeed1]. In contrast, the outbreaks associated with mung bean sprouts were due to PT 33, 11b, 4b, 1, and 913. Prior to these outbreaks, PT 33 and 913 had never been isolated in the United States (B. Holland, CDC, unpublished data). If the phage types that are common in China or Australia differ from the phage types commonly found in North America or Europe, then contamination of seed on the farm or in storage in those areas could explain why the SE phage types noted in these outbreaks were ‘unusual.’ For example, SE PT 34 is rare in North America but is common in Japan [Reference Kusunoki20]. SE PT 33 was first noted in 1988 in southern England in an outbreak associated with poultry and was subsequently found most commonly from patients from India in 1992–1996 (L. Ward, HPA, UK unpublished data). Although suggestive of contamination abroad, the finding of these rare serotypes does not rule out an origin in North America. An outbreak of a rare phage type of SE (PT 30) was due to almonds and this phage type was found on an almond farm in California [Reference Isaacs21].
Mung bean seeds could be contaminated on the farm by growing them in fields fertilized with untreated manure. One farm in California, implicated in an outbreak of S. Montevideo infections associated with alfalfa sprouts, fertilized fields with raw chicken manure [Reference Mohle-Boetani22]. Rodents carry SE and could contaminate seeds in storage [Reference Garber2]. Unfortunately, despite referral of these outbreaks to the FDA, traceback investigations to the farms or storage sites in China or Australia were not conducted and the source of seed contamination could not be determined. The common phage types of SE in China and Australia are not available.
Although the sprout growers probably did not introduce SE into the mung bean sprouts, none of the growers used the concentration of calcium hypochlorite recommended in the FDA guidelines for sprout seed decontamination [5]. Similarly, other Salmonella outbreaks associated with alfalfa sprouts have been linked to poor adherence to the FDA guidelines for disinfection [Reference Gill23]. An additional contributory factor is that only two sprout growers tested their irrigation water for Salmonella despite FDA recommendations. As noted earlier, both of these firms released sprouts produced from the same seed lot that produced Salmonella-positive test results and one continued using the seed lot associated with the contaminated irrigation water for several weeks after learning of the positive result.
Sprout growers should follow the FDA guidelines to test irrigation water for each batch of sprouts. Sprouts should not be released until the spent irrigation water testing results are reported as negative. If the irrigation water tests positive, there should be: (1) no distribution of any sprouts prepared with the same seed lot and (2) no further use of the implicated seed lot. Although not in the FDA guidelines, we strongly urge that public health officials be notified by the laboratory or the sprout grower of confirmed positive findings from irrigation water or product samples; this would enable oversight of procedures to prevent illness. Seed suppliers could also be notified of the results to prevent further distribution of the implicated seed lots.
Disinfection of the seeds with calcium hypochlorite reduces the bacterial load and may reduce the risk of sprout-associated disease [Reference Gill23]. A greater than 1000-fold reduction of Salmonella on alfalfa seeds was achieved by soaking them in calcium hypochlorite at a concentration of 1800 μg/ml active chlorine [Reference Wiessinger and Beuchat24]. However, this procedure cannot reliably eliminate pathogens and outbreaks have occurred from seeds that were disinfected [Reference Brooks25, Reference Procter26]. Damaged or ‘wrinkled’ seeds may harbour high concentrations of microbial flora, including pathogens [Reference Charkowski, Sarreal and Mandrell27]. Sprouts are produced at incubator-like temperatures with high humidity, promoting the growth of bacteria on seeds. Salmonella increased 2·5 logs after 2 days in our investigation and 4–5 logs in another investigation [Reference Stewart28]. Salmonella can be internalized in sprouts during sprouting so that external cleaning of sprouts will not eliminate contamination [Reference Warriner29]. Our growth experiments show how diffuse the contamination can be, illustrating that elimination of pathogens by the consumer is nearly impossible.
Since the consumer cannot remove pathogens, either seed or sprout contamination must be eliminated prior to sale. While seed contamination could be prevented by growing and storing sprout seeds intended for human consumption in fields and facilities separate from those used to grow and store seeds for livestock and other agricultural purposes, these measures would increase the cost of production and would not eliminate the potential for contamination from wild birds and animals. Pathogens on seeds could be eliminated by using chemical treatments at higher concentrations than the FDA guidelines and ammonium fumigation [Reference Lee30–Reference Himathongkham32]. However, these methods can damage seeds and reduce their capacity to germinate. One solution is irradiation of sprouts after harvesting and packaging and just before distribution which could eliminate pathogens even if the contamination were internal [Reference Bari33, Reference Rajkowski and Thayer34]. If irradiation is deemed useful, public education could help to promote acceptance [Reference Young35, Reference Bruhn36].
Until there is a method to eliminate pathogens from the seeds or from the sprouts, sprout growers should follow the FDA guidelines to decontaminate seeds (regardless of the type of seed) and should test spent irrigation water. Seed lots associated with spent irrigation water that test positive for Salmonella should be withdrawn from use for human consumption. Sprouts produced from implicated seed lots should be discarded. Confirmed Salmonella-positive test results of sprouts or their spent irrigation water should be reported to local public health officials so that appropriate follow-up measures can be taken. Raw sprouts should not be consumed by, or served to, persons at high risk of developing complications from Salmonella infections, and the general public should be advised that raw mung bean sprouts are potentially hazardous.
APPENDIX
The Investigation Team includes: Paul Effler, M.D., Rebecca Kanenaka (State of Hawaii Department of Health), Geoff Beckett, M.P.H., Jennifer Gunderman-King (Division of Disease Control, Maine Department of Human Services), Dean Bodager, R.S., M.P.A., B.S.N., Roberta Hammond, Ph.D. (Bureau of Community Environmental Health, Florida Department of Health), Bill Toth, M.P.H., Donna Walsh, R.N. (Orange County Health Department, Orlando, FL), Zuber Mulla, Ph.D. (University of Texas Health Science Center at Houston, School of Public Health Epidemiology), Sandy Isaacs, B.Sc.N., M.Sc. (CIDPC-PPHB Health Canada), Lance Honish (Environmental Health Services, Edmonton, Alberta), Emily Harvey (Division of Epidemiology and Immunization, Massachusetts Department of Public Health, Epidemiology and Immunization Division, Boston, MA), Yvonne van Duynhoven, Ph.D. (Center for Infectious Disease Epidemiology, National Institute of Public Health and the Environment, Bilthoven, The Netherlands).
DECLARATION OF INTEREST
None.





