INTRODUCTION
Migrants from hepatitis B and C endemic countries account for 65% of all hepatitis B and 50% of all hepatitis C infected patients living in The Netherlands [Reference Marschall1]. These estimations are based on estimates of prevalence of hepatitis B and hepatitis C in countries of origin [Reference Kretzschmar2] and several surveys [Reference Koedijk3, Reference Kok4]. Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are often asymptomatic and therefore diagnosed late at a stage when liver damage such as fibrosis, cirrhosis or hepatocellular carcinoma (HCC) has already occurred. The challenge, therefore, is to identify patients early. Treatment options for HBV and HCV have improved in recent years. Significant progress has been made in doubling the chance of eradication of HCV even in the most difficult-to-treat genotypes [Reference Fox and Jacobsen5, Reference Jesudian, de Jong and Jacobson6]. Management of HBV has become more effective with the introduction of very potent nucleos(t)ide analogues such as entecavir and tenofovir [Reference Asselah and Marcellin7].
The biggest challenge, however, is to identify all those patients who are not yet aware of their HBV or HCV infection. In the city of Arnhem we had previously screened Turkish inhabitants and found a HBV and HCV prevalence of 3·0% and 0·4%, respectively, in first-generation migrants (FGMs) aged >24 years [Reference Richter8]. We now focused on FGMs from Afghanistan, Iran, Iraq, the former Soviet Republics and Vietnam. Reasons for selecting these five countries were, respectively, the considerably large size of the migrant population in the Arnhem area (Afghanistan, Iran, Iraq) in combination with a lack of HBV and HCV prevalence data in The Netherlands, an expected high HBV prevalence (Vietnam) and an expected high HCV prevalence (the former Soviet Republics). Vietnam is one of the countries with the highest reported HBV prevalence rates worldwide, with a prevalence of 10–20% in the general population [Reference Nguyen9]. A review of 31 studies between 2003 and 2011 consisting of data on 132 500 individuals, revealed a prevalence of 1·9% for hepatitis B and 1·1% for hepatitis C in Afghanistan [Reference Khan and Attoullah10]. Population-based studies from Iran showed hepatitis B surface antigen (HBsAg) prevalence rates of 2·6–3·38% [Reference Merat11, Reference Salehi12]. Only sparse data are available from Iraq. Chironna et al. found a HBsAg prevalence rate, indicating chronic hepatitis B, of 2·2% in Kurdish refugees from Iraq [Reference Chironna13]. In particular, we expected a high HCV prevalence rate from countries of the former Soviet Republics since Batash et al. reported a HCV seropositivity of 28·3% in immigrants from the former Soviet Republics living in New York City [Reference Batash14].
In order to assess the prevalence of HBV and HCV in FGMs from these respective countries we designed a screening project, in which FGMs received a personal invitation for an educational meeting with free onsite serological screening. We also hoped that the results of this screening project would contribute towards the formulation of an official countrywide screening strategy for migrants.
METHODS
In January 2011 we established a project group consisting of members of our infectious disease unit, the microbiology and biochemistry laboratory and an epidemiologist from our hospital, the municipal health service, and volunteers from the various participating nations. We established a project plan that was approved by the local ethics committee. We considered the project to meet the classic Wilson & Jungner screening criteria for persons with underlying chronic disease that has become a gold standard in public health [Reference Wilson and Jungner15]. We divided our project into four phases: (1) the preparation phase; (2) the campaigning phase; (3) the information and screening phase; (4) the post-screening and clinical evaluation phase.
The preparation phase
We approached physicians, nurses, paramedical services and medical students from the respective countries and asked them to assist us with the project. In total we found 25 volunteers, including some of our patients, who were educated on HBV and HCV. Together we prepared information material such as flyers and posters in the following languages: Dutch, Farsi, Arab, Vietnamese and Russian. We created a special website with videos with general information about HBV, HCV and the project, presented by physicians in the different languages. A simple, easily understandable powerpoint presentation about hepatitis B and C was prepared in which we explained the need for early screening in order to prevent illness. The Municipal Offices of Arnhem and Rheden provided us with the total numbers of FGMs aged ⩾18 years from the selected countries.
The campaigning phase
We distributed posters and flyers at shops, barbers and community centres in areas where the respective migrants lived or visited. Two weeks before the testing event, all FGMs received a personal invitation and a flyer in all languages with information on the educational meetings offering free onsite testing for HBV and HCV, forwarded by the municipal office of Arnhem and Rheden. Because of privacy restrictions we did not obtain the addresses of the FGMs ourselves. The volunteers acted as ‘ambassadors’ of the project, inviting FGMs from their own countries to attend the testing event. The local Dutch-language newspaper, de Gelderlander, and local websites publicized the project.
The information and screening phase
The information and testing events took place at three different locations of Rijnstate Hospital on three successive evenings. During these meetings all participants were registered by name, date of birth, address, and country of origin. Participants were invited to listen to the powerpoint presentation given by one of the physicians and were able to receive additional personal information in their own language from one of the volunteers. Thereafter the participants could opt for immediate onsite laboratory testing for HBsAg, antibodies to hepatitis B core antigen (anti-HBc) and antibodies against hepatitis C virus (anti-HCV). They were also invited to complete a questionnaire on risk factors they might have been exposed to in their country of origin in the past. The following risk factors were listed in the questionnaire: blood transfusion before 1992, drug use, dentist consultation, injection or surgery, circumcision and tattoo/piercing. We provided all participants with a phone number and email address. In case of any remaining questions participants could leave their phone number and one of the volunteers would call back in the requested language.
The post-screening and clinical evaluation phase
Within 2 weeks all participants received their results by letter. Those testing positive for HBsAg or anti-HCV were contacted by phone and received an appointment at our hepatitis outpatient clinic within 3 weeks. After the three onsite screening sessions we extended the opportunity for free laboratory screening for hepatitis B and C for the FGMs from the respective countries for 2 months since we received several mails and phone calls from people that had been invited but were unable to attend one of the screening sessions. We provided these individuals with a laboratory request form and a questionnaire by mail and they were able to attend the laboratory for testing without further appointment. The list of all participants who tested positive for HBsAg and/or anti-HCV was sent to the municipal health service that invited them for contact tracing and HBV vaccination of partners/family members.
RESULTS
Of the 3226 registered FGMs from the respective countries, 959 (29·7%) were tested. However, for 32 individuals the country of origin was not registered. Table 1 shows the participation rate of all FGM groups according to their country of origin, sex and three age categories (18–24, 25–50, >50 years) for the remaining 927 (28·7%) participants. The highest (36·4%) proportional uptake was seen in migrants from Vietnam and Afghanistan, the lowest (11·4%) in migrants from the former Soviet Republics. In the >50 years age group, and in women compared to men, the participation rate was higher.
Table 1. Total number of first-generation migrants from Afghanistan, Iran, Iraq, the former Soviet Republics and Vietnam* and their participation rate by age and gender
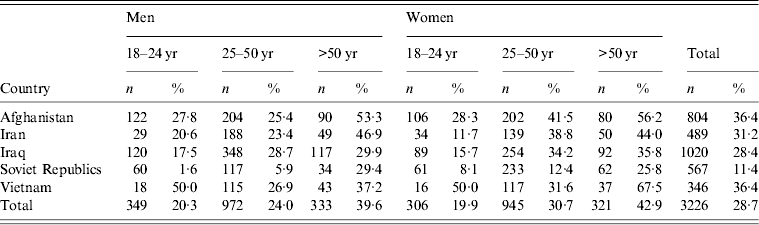
* Source: Municipal Database Arnhem/Rheden, 2011.
Table 2 shows an overview of the outcome of the HBV and HCV serological tests in the five FGM groups. In total we found 21 (2·2%) FGMs with chronic hepatitis B (HBsAg positive) and only three (0·3%) FGMs with chronic hepatitis C (HCV-RNA positive). Three of the 21 persons with chronic hepatitis B and one of the three persons with chronic hepatitis C were found by contact tracing.
Table 2. Outcome of HBV and HCV serological tests
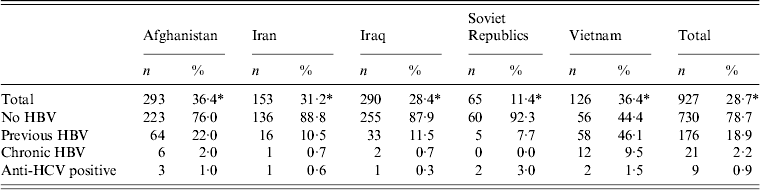
* Percentage of total first-generation migrant population in Arnhem/Rheden. Source: Municipal Database Arnhem/Rheden, 2011.
Prevalence of chronic hepatitis B was by far the highest in Vietnamese participants (9·5%), followed by participants from Afghanistan (2%), Iran and Iraq (0·7%) and the former Soviet Republics (0%). Previous HBV infection (positive for both anti-HBc and for antibodies against HBsAg) was also most common in FGMs from Vietnam (46·1%), followed by participants from Afghanistan (22%). Prevalence of anti-HCV positive individuals was highest in FGMs from the former Soviet Republics (3·0%), followed by those from Vietnam (1·5%) and Afghanistan (1%). Chronic hepatitis C (HCV-RNA positive) was only found in FGMs from the former Soviet Republics (3%) and Vietnam (0·8%). Figure 1 illustrates the data on clinical evaluation of the patients with chronic hepatitis B and hepatitis C. Three participants already had liver cirrhosis at presentation: one with hepatitis B from Afghanistan and two with hepatitis C, one from Vietnam and one from a former Soviet Republic (found by contact tracing). Of the 21 patients positive for HBsAg, seven (33%) were hepatitis B e-antigen (HBeAg) positive, 10 (48%) had a HBV-DNA score of >2000 IU/ml and seven (33%) had an alanine aminotransferase (ALT) score of >1 ULN (upper limit of normal). Four patients with chronic hepatitis B qualified for treatment according to the 2012 EASL guidelines [16] based on the combination of three criteria: serum HBV DNA levels, ALT levels >1 ULN and severity of liver disease. These four patients have since started treatment for HBV. In one patient (HBeAg negative and normal ALT value), treatment was started because he was receiving immunosupressive therapy for another disease. For one HBeAg-positive patient with HBV DNA >2000 IU/ml and elevated ALT, and alcohol abuse, treatment had not yet started. One of the three patients with chronic hepatitis C started treatment, the second had a contraindication for treatment because of decompensated liver cirrhosis with concurrent alcohol abuse, while the third patient had no liver fibrosis and no urgent need for treatment.
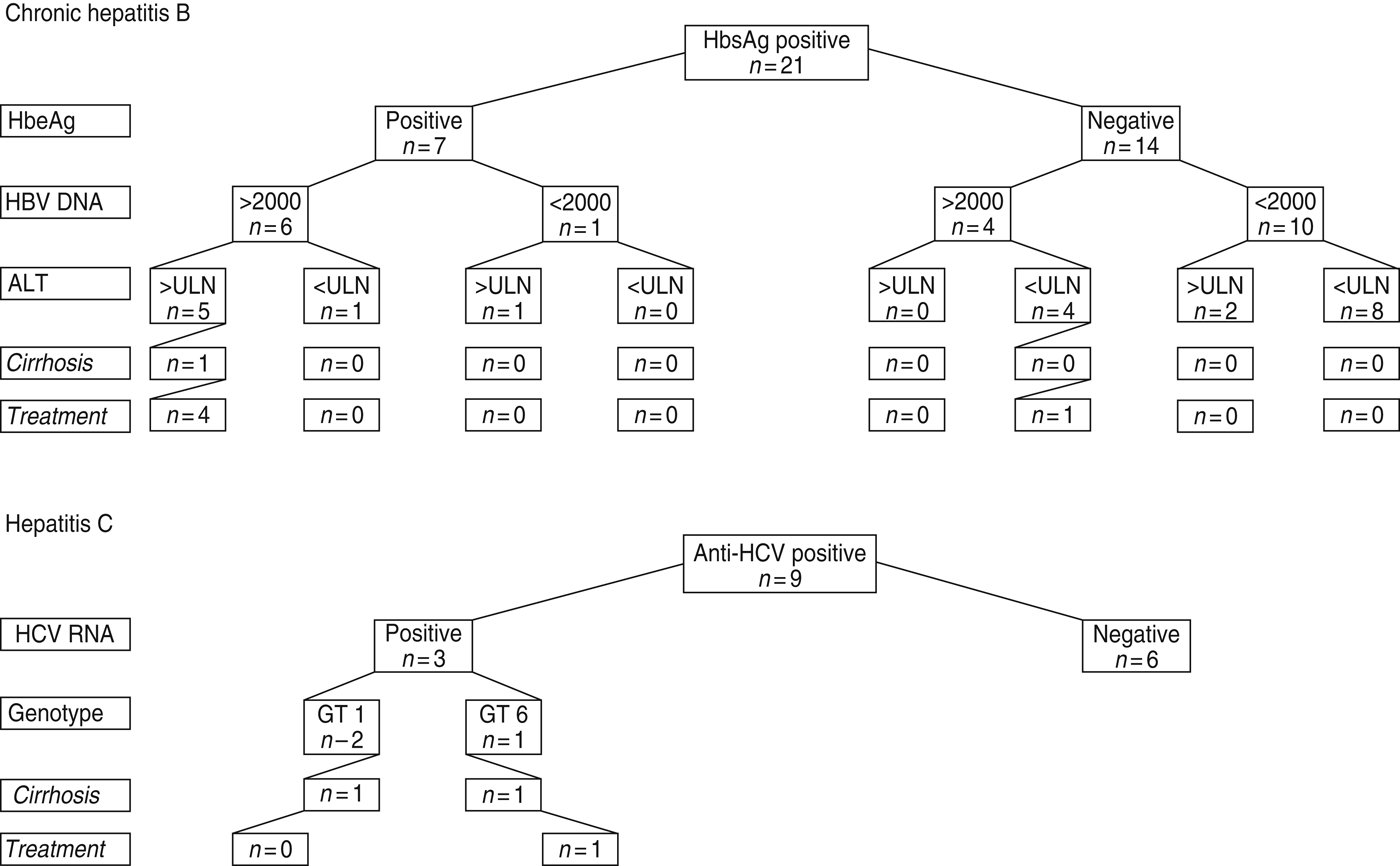
Fig. 1. Clinical evaluation. ULN, Upper limit of normal.
In total, 771 participants (83·4%) completed the questionnaire concerning risk criteria as described above. Both patients with chronic HCV infection reported having been exposed to at least one of the risk factors described. In total, 11 (61%) of the 18 HBsAg-positive patients who completed the questionnaire reported one of the listed risk criteria. Of patients with laboratory features of past hepatitis B infection (anti-HBc positive, HBsAg negative), 63% (106/168) reported at least one of these risk criteria for transmission. However, we did not find a significant difference in exposure to any individual risk factor between participants with or without infection.
DISCUSSION
In this screening project focusing on FGMs from Afghanistan, Iraq, Iran, Vietnam and the former Soviet Republics we achieved a much higher attendance rate of 27% compared to our previous screening project for migrants from Turkey [Reference Richter8], in which we achieved only a 10·2% participation rate. The major difference was that in the earlier ‘Turkish’ project we had to rely on a 100% community approach since we could not send a personal invitation to the migrants from Turkey. Interestingly, Niessen et al. achieved a comparable participation rate of 25·2% in Turkish migrants from the northern part of The Netherlands by making use of a personal invitation [Reference Niessen17], showing that personal invitation of FGMs from Turkey is more efficacious than even an extensive community-based approach.
In the present study we had full support from the municipality and were therefore able to invite all registered FGMs from the respective countries personally. The educational evenings with onsite blood testing gave us the opportunity to further explain to and discuss with the participants all the issues concerning viral hepatitis. It is unsurprising that the attendance rate of FGMs from Vietnam was highest. We learned from this group that their awareness of viral hepatitis was already very high, because many participants had family members or friends with liver disease. The participants from Afghanistan, Iran and Iraq asked many questions about HBV and HCV and were very keen to be tested: they considered it self-evident to attend and get tested if they might achieve some health benefit. Moreover, some of them were already aware of the danger of viral hepatitis. It was also obvious that the majority of the participating migrants from these countries was highly educated and spoke fluent Dutch or English. We received far fewer questions from migrants from the former Soviet Republics, although some of these were worried about blood transfusions in the past. We were not surprised that we could not find a correlation of individual risk factors with infection, since we believe that the strongest risk factor is country of origin by itself.
By far the highest prevalence rate of chronic HBV infection rate was found in FGMs from Vietnam (9·5%), resembling the reported 10–20% prevalence rate in the general population of Vietnam [Reference Nguyen9]. This high prevalence rate is also comparable to the recently published high rate in the Chinese community living in Rotterdam [Reference Veldhuijzen18]. Unsurprisingly, the majority (55·6%) of the Vietnamese migrants had serological signs of past or chronic HBV infection, reflecting the fact that HBV is highly endemic in Vietnam. The FGMs from Afghanistan also had a considerably high rate of past or chronic HBV infection (>20%), in contrast to the three other countries/regions where the large majority had no serological features of past or present infection. We found that nearly half (48%, 10/21) of the newly diagnosed patients with chronic hepatitis B had a HBV DNA level of >2000 IU/ml with increased risk of developing liver cirrhosis or HCC. This re-emphasizes the importance of this kind of screening project. We were also able to demonstrate that combined testing for hepatitis B and hepatitis C is useful; although the number of detected cases with chronic hepatitis C was small, two already had liver cirrhosis. One woman from Vietnam was started on treatment immediately. Interestingly, one of the patients identified with chronic hepatitis C from a former Soviet Republic asked us to screen her ex-husband as well and indeed he appeared to already have decompensated liver cirrhosis due to chronic hepatitis C in combination with alcohol abuse, without ever having attended a hospital. In three of the patients with chronic hepatitis B, the infection had already been discovered several years before, but these patients had become lost to follow-up. In two of these patients treatment was started.
In summary, we demonstrated that screening projects for hepatitis B and C in FGMs making use of personal invitations can achieve high attendance rates. We observed that educational meetings combined with free onsite testing were highly appreciated since it gave the participants the opportunity to ask a variety of questions. The fact that participants received their results and if necessary an appointment at the hepatitis outpatient clinic within several weeks was also much appreciated. We hope that the results of our study can contribute to the formulation of a national policy for testing FGMs from HBV- and HCV-endemic countries in The Netherlands. This and other studies [Reference Richter8, Reference Veldhuijzen18–Reference Urbanus20] are important since we must be aware that the prevalence of hepatitis B and C in the country of origin does not necessarily reflect the prevalence in FGMs in a country like The Netherlands [Reference Uddin21].
Two studies have been published on the cost-effectiveness of targeted screening for hepatitis B and C in The Netherlands [Reference Veldhuijzen22, Reference Helsper23] and concluded that it might be cost-effective since serious complications leading to hospital admissions and even liver transplantation can be prevented. We believe that early detection of hepatitis B and C in high-risk population groups such as migrants from HBV- and HCV-endemic countries should be a major public health priority in Western countries, comparable to universal hepatitis B vaccination for children which was finally introduced in The Netherlands in 2011.
ACKNOWLEDGEMENTS
This study received unrestricted funding from Jansen Cilag, Merck Sharp & Dohme, Gilead and the Municipal Health Service. The authors thank all volunteers for their assistance in the campaign; Marja de Leeuw for her help in administrative issues; Tonnie Berendsen and others from the laboratory of clinical chemistry who assisted with drawing blood samples, and staff from the Department of Microbiology for their efforts in supplying the test results as quickly as possible. We also thank Dr Matthew McCall for his useful comments.
DECLARATION OF INTEREST
None.






