INTRODUCTION
The incidence of invasive meningococcal disease (IMD) has generally decreased in the USA over the past two decades [Reference Thigpen1–Reference Rosenstein4]. While the use of meningococcal vaccines has contributed to the decline, this decline in the USA began before widespread use of polyvalent polysaccharide meningococcal vaccine (MPSV4) or the introduction of polyvalent conjugate meningococcal vaccines (MCV4) [Reference Harrison5].
We examined trends in reported IMD in Massachusetts for the period 1988–2011, taking into consideration vaccine introductions and changing recommendations that occurred during that time. Massachusetts has been a ‘universal vaccine distribution’ state, with the Massachusetts Department of Public Health (MDPH) supplying all recommended childhood vaccines to all Massachusetts children (aged ⩽18 years) at no cost [including Haemophilus influenzae type b (Hib) vaccine since 1991 and pneumococcal conjugate vaccine (PCV7) from 2000]. The MDPH supplied doses of MPSV4 for children at high risk (late complement component deficiency, asplenia, etc.) over the whole period, and added MCV4 to the universal system for the recommended age group (11–12 years) and for ‘catch-up’ of older children when it became available in 2005. The MDPH also promoted national recommendations for the vaccination of college students living in dormitories in 2000 and enforced a 2004 legislative requirement for universities and colleges to offer meningococcal vaccine to students, but only provided state-supplied MCV4 for those aged ⩽18 years. The Massachusetts experience may be distinctive in the provision of essentially every dose of childhood vaccine administered and the apparent absence of outbreaks of epidemic meningococcal disease.
METHODS
Only confirmed cases of IMD were included. A confirmed case of IMD required a positive culture for Neisseria meningitidis from cerebrospinal fluid (CSF), blood or other usually sterile body site, in accordance with the Council of State and Territorial Epidemiologists (CSTE)/U.S. Centers for Disease Control and Prevention (CDC) case definitions for national notifiability in place throughout the period. Since 1989, clinical laboratories in Massachusetts voluntarily submitted isolates of N. meningitidis to the William A. Hinton State Laboratory Institute for serogroup characterization. Since 2004, submission of isolates has been mandatory.
Cases of IMD are reported by clinicians to the local health department and/or ascertained by a laboratory report of a positive culture for N. meningitidis reported to the MDPH. Follow-up of cases and completion of a case report form is performed by staff of the local health department or MDPH. Since September 2006, all case reports and supplementary information have been entered into Massachusetts Virtual Epidemiologic Network (MAVEN), the state's web-based surveillance and case-management system. The initiation of electronic laboratory reporting in 2005 enhanced the completeness and timeliness of reporting of IMD cases in the latter period. All data on all confirmed cases reported from 1988 to September 2006 were migrated to MAVEN subsequent to its adoption. A file of demographic and clinical information on all IMD cases, including an ‘event date’ (date of onset of symptoms, date of positive culture specimen collection, or date of report, whichever was earlier) was created as an Excel (Microsoft, USA) file for analysis and stripped of personal identifiers for further analysis.
For case-fatality rate (CFR) calculations, cases of reported IMD, for which mortality was not ascertained in the MAVEN extract, were matched to Massachusetts death certificate records (1990–2011) obtained from the Massachusetts Registry of Vital Records.
Estimated vaccination rates for adolescents/teens in Massachusetts for 2006–2011 were obtained from the National Immunization Survey (NIS-Teen) [6–11], a random digit dial telephone survey of U.S. households funded by the CDC.
Cases were grouped in four-year intervals to increase cell sizes for analysis, especially in the last 4 years with, on average, a total of <15 cases of IMD per year (all age groups). Groups were defined (0–4, 5–24, 25–59, ⩾60 years) to allow for the analysis of specific age groups of concern while maintaining statistical power in each age group. Poisson regression was applied to estimate the relative risk (RR) for IMD before and after certain time periods; time periods preceding the cut-off year being the reference. Cut-off years (1991 and 2000) were chosen to explore correlation between IMD and vaccine introduction in recommended vaccine target age groups, and in recognition of apparent secular trends in incidence. Population data were obtained, and age-specific rates were calculated, using interpolation and extrapolation of 1990, 2000, and 2010 U.S. Census data for Massachusetts.
Analyses were performed using Excel and ‘R’ version 2.14.0 (R Foundation for Statistical Computing, Austria). Ninety-five percent confidence intervals (CIs) for means were calculated and P values ⩽0·05 were considered statistically significant.
RESULTS
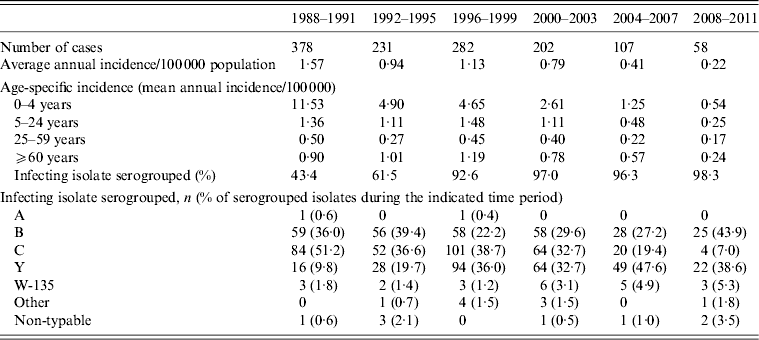
Over the time period 1988–2011, 1258 cases of IMD were reported to MDPH, of which 631 (50·3%) were males and 623 (49·7%) were females (sex was missing for four cases). Of the cases, 418 (33·2%) were aged <5 years, 388 (30·8%) 5–24 years, 243 (19·3%) 25–59 years, and 209 (16·6%) were aged ⩾60 years. Notably, the age distribution of cases changed markedly over time (Table 1), with a mean and median age, respectively, of 16·3 (95% CI 12·3–20·3) and 7·8 years in 1988; 33·3 (95% CI 25·2–41·4) and 18·8 years in 1999; and 41·1 (95% CI 27·0–55·2) and 41·3 years in 2011.
Table 1. Characteristics of reported invasive meningococcal disease cases in Massachusetts, 1988–2011
The incidence of reported IMD cases (based on event date) peaked in winter, held steady through spring, was lowest during summer, and then another peak occurred in October. The basic seasonal pattern of cases held steady despite reduced numbers of cases in later years.
Over the time period, there were three instances of pairs of cases due to the same meningococcal serogroup which were attributed as co-primary cases of IMD (in 1989, 1995, 2000), as defined by onsets within 24–48 h of each other, a common exposure setting and no subsequent contact. There were also two instances of single secondary cases (exposure and incubation period suggested transmission from IMD index case) in non-prophylaxed close contacts (in 1991, 1996), but no recognized outbreaks or geo-temporal clusters of cases involving the same serogroup.
The annual incidence of IMD decreased markedly from 1·57/100 000 population in the 4-year period 1988–1991 to 0·22 in the 4-year period 2008–2011 (Table 1, Fig. 1). After 1991, 0- to 4-year-olds had a 53% reduction in the risk for IMD (RR 0·47, 95% CI 0·38–0·59) comparing pre-1991 to post-1991. This reduction is not seen in other age groups in that time period. There was, however, a significant reduction in risk in all age groups after 2000 (0–4 years: RR 0·27, 95% CI 0·21–0·36; 5–24 years: 0·49, 95% CI 0·39–0·61; 25–59 years: 0·69, 95% CI 0·52–0·90; ⩾60 years: 0·49, 95% CI 0·36–0·66) comparing pre-2000 to post-2000.
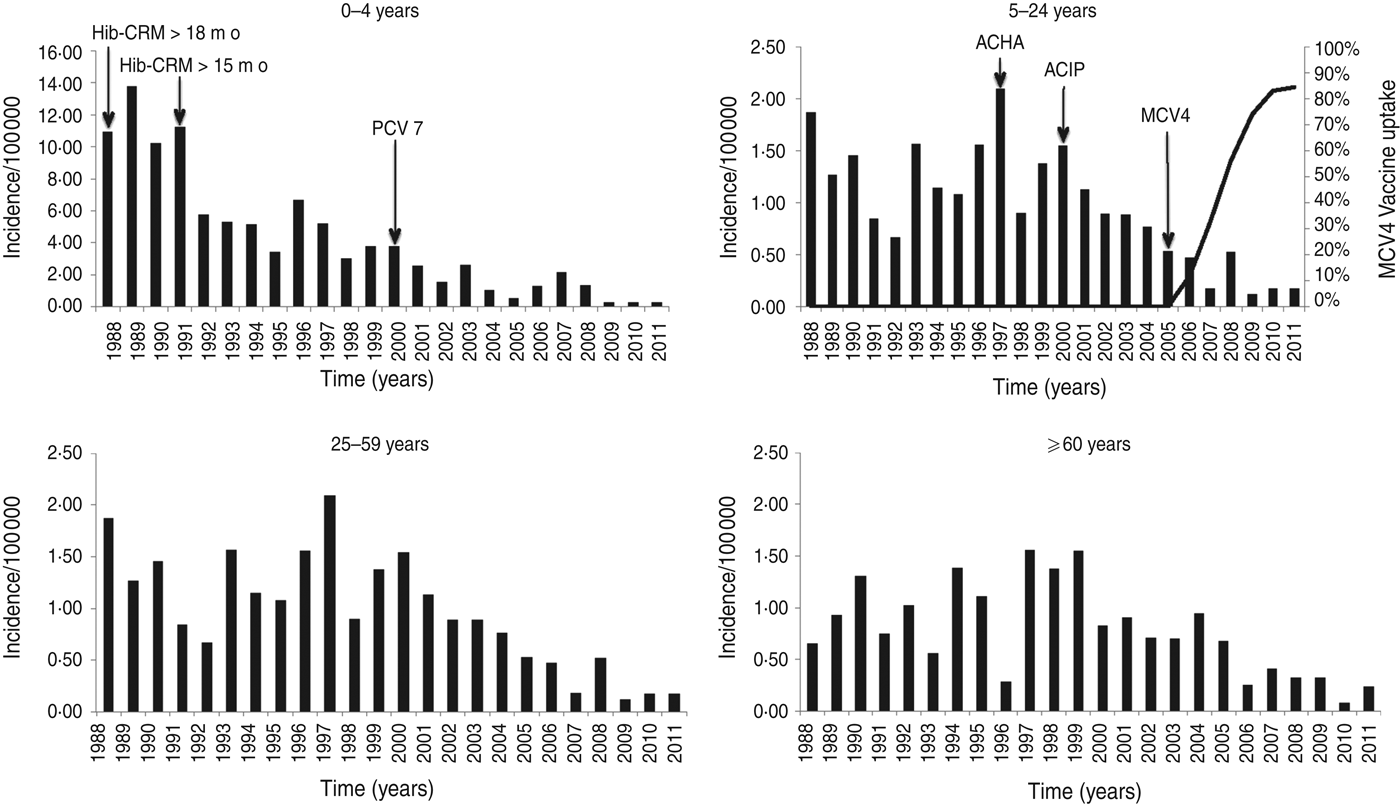
Fig. 1. Age-specific incidence rates of reported invasive meningococcal disease in Massachusetts, 1988–2011. Note the different scale on the y-axis for the 0–4 years age group. For the 5–24 years age group the introduction and uptake of MCV4, with coverage measured in 13- to 17-year-olds in Massachusetts is shown in the line graph. Arrows also indicate the American College Health Association (ACHA) recommendation for MPSV4 vaccination for college freshman (1997), Advisory Committee on Immunization Practices (ACIP) recommendation for MPSV4 vaccination for college freshman (2000), and ACIP recommendation for MCV4 in 11- to 12-year-olds (2005). Arrows in the graph of the 0–4 years age group indicate the introduction of conjugate Hib vaccine for children aged >18 months (1988), introduction of conjugate Hib vaccine for infants aged >2 months (1991) and introduction of PCV7 vaccine for children aged <23 months (2000).
The proportion of N. meningitidis isolates from IMD cases which were serogrouped increased over the time of this study, with only 43·4% of 378 isolates in 1988–1991 serogrouped, 61·5% of 231 in 1992–1995, and over 95% after 1999 (Tables 1 and 2). Of those cases caused by a serogrouped isolate, serogroup C was most frequent with 325 (35·2%) identifications, followed by serogroup B (284, 30·8%), serogroup Y (273, 29·6%), serogroup W-153 (22, 2·4%) and all others (11, 1·2%); eight (0·9%) were non-typable.
Table 2. Distribution of Neisseria meningitidis in invasive meningococcal disease cases in isolates subjected to serogroup identification, by age group
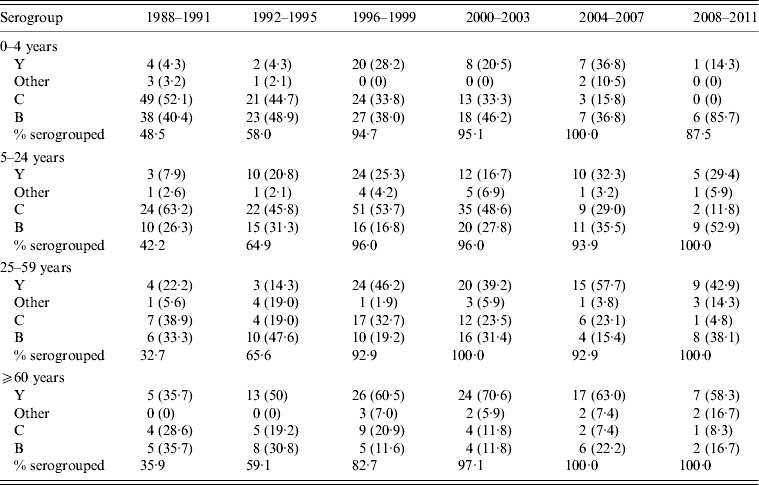
Values given are number (%).
Isolate serogroup distribution over time is summarized in Tables 1 and 2. Of the isolates subjected to serogrouping, serogroup B isolates decreased in absolute numbers, but increased in proportion from 36% of 164 isolates for 1988–1991 to 44% of 57 isolates for 2008–2011; a proportional increase in all age groups (Table 2). Serogroup C accounted for the majority of isolates in 1988–1991 (51·2%), and decreased to 7% of the serogrouped isolates in 2008–2011. The percentage of isolates of serogroup Y increased during the 1990s and 2000s, from 9·8% of isolates in 1988–1991 to 39% in 2008–2011; accounting for an increased proportion over time in all age groups (Table 2).
In children aged 0–4 years, the relative proportion of serogroup C decreased beginning in 1988 and continued until 2011 (Table 2). After 2003, serogroup C began to disappear in this age group (3/19 and 0/7 isolates serogrouped in 2004–2007 and 2008–2011, respectively) and serogroup B accounted for 6/7 (86%) isolates serogrouped in the last 4-year period. Of the IMD cases aged 5–24 years, serogroup C predominated from 1988 to 2003 (45–63%), with serogroup B (26% in 1988–1991 to 53% in 2008–2011) and serogroup Y (8% in 1988–1991 to 16–33% after 1999) emerging as a larger proportion of cases in the 1990s. IMD incidence rates were lowest in the 25–59 years age group, with predominance of serogroup Y after 1995 and a sharp reduction in the predominance of serogroup C in the 2008–2011 time period. Throughout the 24-year time period, serogroup Y became increasingly predominant in IMD cases in those aged ⩾60 years, accounting for over 50% of all serogrouped isolates in that age group after 1995.
After introduction of MCV4 vaccine in 2005, coverage of 13- to 17-year-olds in Massachusetts reached 84·4% by 2011 (Fig. 1).
IMD due to serogroups represented in meningococcal vaccines licensed in the USA (A, C, Y, W-135) began to decrease in incidence after 1999 in IMD cases aged 5–24 and 0–4 years. Less marked decreases occurred in older age groups as well, but there was an overall decrease in vaccine serogroup IMD incidence in all age groups after 2005 (see Table 2).
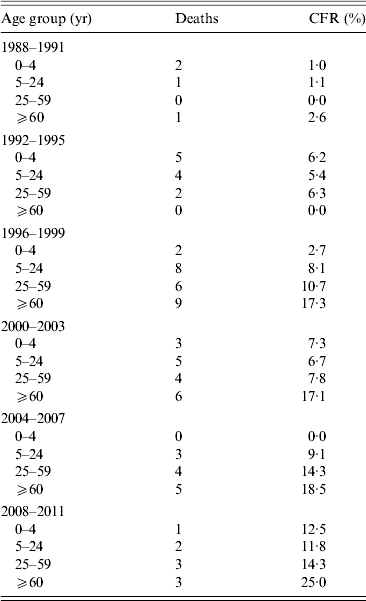
There was an increase in the CFR of IMD over time (Table 3). The CFR was highest in the ⩾60 years age group (2·6–25%).
Table 3. Case-fatality rate (CFR) in invasive meningococcal disease cases in Massachusetts by age group and time period
DISCUSSION
Between 1988 and 2011, reported cases of IMD declined in all age groups in Massachusetts. The decrease in incidence was most marked in children aged <5 years, with an abrupt drop after 1991 followed by continued reductions in this age group. Cases also decreased in other age groups, but most of that decline was observed in later years. Similar declines in IMD during this time period have been observed elsewhere [Reference Thigpen1, Reference Moura3, Reference Rosenstein4, Reference Jackson and Wenger12, Reference Wang13], except in situations of community outbreaks and/or epidemic clones of N. meningitidis [Reference Jackson14–Reference Diermayer17]. Outbreaks of IMD were not identified in Massachusetts during the period of observation.
The reason for the marked drop in reported cases of IMD in the 0–4 years age group after 1991 is not clear. The reduction in IMD is not likely a surveillance artifact, as it is not seen in other age groups. The incidence rates for IMD in this age group for 1988–1991 were consistent with those reported by Loughlin et al. [Reference Loughlin, Marchant and Lett18] in a similar population of children in Massachusetts in the 1980s. Moura et al. [Reference Moura3] hypothesized that a marked decrease in serogroup B meningococcal disease in children aged <5 years in New York City may have been related to the introduction of H. influenzae type b (Hib) vaccine conjugated to serogroup B meningococcal outer membrane protein (OMP). Significant declines in incidence of IMD in younger children were also observed in other countries in the mid-1990s [Reference Howitz, Samuelsson and Mølbak19, Reference Ben-Shimol20], coincident with their implementation of conjugate Hib vaccine programmes [Reference Ladhani21].
Massachusetts is a universal vaccine state supplying every dose of every childhood vaccine to all children in the Commonwealth. The Hib conjugate vaccine provided by the state since 1991 has been the diphtheria CRM197 protein conjugate vaccine, and essentially no meningococcal OMP conjugate Hib vaccine was used in Massachusetts. If one were to hypothesize the introduction of conjugate Hib vaccine to be related to the marked reduction in IMD incidence in children aged <5 years in Massachusetts and elsewhere, then the immunological mechanism would need to be predicated on a mechanism other than an immune response to meningococcal OMP.
A second reduction in IMD in Massachusetts in children aged <5 years after 2000 occurred coincident with the introduction of PCV7 (diphtheria CRM197 protein conjugate vaccine) and its widespread use. Complex interactions occur in bacterial species colonizing the nasopharynx. For example, N. meningitidis carriage appears to be positively related to carriage of Streptococcus pneumoniae and H. influenzae [Reference Bakir22]. To the extent that specific conjugate vaccines reduce carriage of these organisms [Reference Dagan23, Reference Takala24] they may reduce likelihood of N. meninigitidis carriage as a consequence. Filice et al. [Reference Filice25] found that 45% of subjects carried bacteria from a minimum of nine different species that were inhibitory to N. meningitis serogroup A, suggesting that ecological variations in oral-pharyngeal flora may also be associated with resistance to meningococcal carriage and therefore IMD. They postulated that cross-reacting antibodies in response to normal flora and bacterial interference from colonization with other bacteria as the cause of the virtual disappearance of serogroup A IMD in the USA. Changes in amount and type of antibiotic use over time may also modify normal flora in ways that favour or inhibit colonization with meningococci, but outpatient antibiotic utilization and its impact on normal flora is difficult to characterize and quantify [Reference Huttner and Samore26].
Declines in the incidence of IMD in older age groups followed and paralleled American College Health Association proactive efforts to encourage use of meningococcal polysaccharide vaccine in college students in 1997 [Reference Collins27], public awareness and concerns about IMD, and similar recommendations of the ACIP (2000) for college students living in dormitories [28]. This decline in IMD was also observed at the national level [Reference Harrison5, Reference Cohn29]. Paralleling this generalized downward trend in IMD after 2000 in Massachusetts was the near disappearance of serogroup C in all age groups.
The routine use of quadrivalent meningococcal conjugate vaccine (MCV4) began in 2005 after licensure of the vaccine and ACIP recommendations [Reference Bilukha and Rosenstein30]. In Massachusetts, there was also a mandate for universities and colleges to offer meningococcal vaccine to students who were about to enter dormitories. MCV4 was targeted at older children and use was associated not only with a reduction in IMD due to vaccine serogroups in the targeted age group, but also in the 0–4 years age group. Use of conjugate vaccine is associated with decreased upper airway colonization with meningococci, as well as prevention of invasive disease, which is a probable explanation of decreased incidence of IMD in age groups not targeted for immunization with MCV4 [Reference Caugant, Tzanakaki and Kriz31].
There is an increase in CFR over time that is partially, but not fully, explained by the increase in the age of cases during that time period, as it is observed in all age groups. However, there are small numbers of cases and deaths throughout the time period, especially in the later period.
There are several limitations to this study that should be noted. The first is that surveillance data were collected at the time of disease event and are often incomplete. Surveillance information accuracy is dependent primarily on original report, with limited data added subsequently. For example, more serogroup data may have been available at the beginning of the period under analysis than was entered into the surveillance system. MAVEN was not utilized prior to 2006, so all data on cases occurring previously were retroactively entered from a closed legacy database. Limited information was available for parts of the study period. Data not already in the data system (such as antibody levels, details of immunization, etc.) could not be retrieved from other sources. Isolates of N. meningitidis were not subject to differentiation beyond serogroup, so unrecognized transmission of a clonal strain may have occurred at the community level during the period of observation. This might explain the cyclic counter-trends in incidence observed in the mid to late 1990s.
Recent declines in IMD incidence are almost certainly related to successful efforts promoting immunization with specific meningococcal vaccines, but the incidence of infections due to serogroup B have also declined with vaccines not containing a serogroup B component. The possibility that factors other than deployment of meningococcal vaccines (introduction of unrelated vaccines, antibiotic use, etc.) might have contributed to the reduction of IMD through non-specific mechanisms (immune or related to alteration of normal flora) deserves further consideration. Such studies go beyond the capacity of most public health surveillance systems. If such mechanisms of protection against IMD are actual contributors to reduced incidence of disease, then they might contribute to efforts to reduce IMD in higher incidence areas of the world where specific immunization efforts are more difficult.
ACKNOWLEDGEMENTS
We thank Laura White at Boston University School of Public Health, Boston, Massachusetts for assistance with the statistical analysis. This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
DECLARATION OF INTEREST
None.






