Introduction
Respiratory tract infections are known to be a prominent cause of mortality and morbidity [Reference Hardelid1]. Social contacts between individuals are thought to be fundamental in the transmission of many respiratory tract infections, including pertussis, influenza and tuberculosis [Reference Mossong2]. Pertussis and influenza are highly contagious airway infections that pose serious health concerns, particularly for infants. Because they have not yet been fully vaccinated, infants, especially those <12 months of age, are at high risk for whooping cough, influenza and their serious complications. Therefore, parents should try to protect their infants by reducing their exposure to infectious individuals, vaccinating close contacts or inducing herd immunity [Reference Cherry3, Reference Blanchard-Rohner and Eberhardt4]. In recent years, Turkey, as well as many European countries, have experienced a resurgence of pertussis and its related complications [Reference Cherry3, Reference Esen5]. To protect newborns and infants, the Global Pertussis Initiative recommends appropriate pertussis control strategies, such as adolescent vaccination, maternal immunisation during pregnancy and cocooning [Reference Forsyth6]. To determine the most useful and appropriate strategies, the most probable sources of pertussis and influenza for infants should be carefully identified.
It is known that respiratory infections can be spread by simple social contacts (i.e. being in the same environment) or by intimate personal contacts (i.e. handshaking and kissing). However, social contact profiles may provide important information regarding the specific sources of infant respiratory tract infections. Qualitative and quantitative data regarding social contact profiles should also provide valuable data that will instruct parents on how to take precautions to reduce the transmission of respiratory tract infections and to determine the best vaccination strategies for targeted individuals.
Endemic diseases can have major impacts on public health. These diseases can be managed by non-pharmaceutical interventions (e.g. school closure, travel restrictions and contact tracing) or by health care interventions (e.g. vaccination, use of antiviral or antibiotic agents) [Reference Wu7]. Contact rates between individuals are often accepted as critical determinants of previously published model outcomes [Reference Wallinga, Teunis and Kretzschmar8]. In the literature, there are few studies regarding social contact modelling, and there are even fewer studies regarding social contact modelling in infants [Reference Read9, Reference Salathé10]. However, we believe that it is very important to determine infant social contact profiles since infants are at a greater risk for respiratory tract infections. While similar studies have measured social contacts in all age groups, van Hoek et al. specifically investigated the social life of infants [Reference Prem, Cook and Jit11, Reference van Hoek12]. Likewise, in our current study, we aimed to determine the best intervention policies for infectious disease control and vaccination strategies by investigating infant social contact patterns. We utilised social contact diaries to estimate the frequency and nature of social contacts of infants. We also collected information about the characteristics of the infants’ households as well as their frequencies of attendance at crowded places to determine the relationship between social contact patterns and recent reported infant respiratory symptoms. The current study is different from previously published studies in that the current study utilises a combination of infant contact patterns and recent reported infant respiratory symptoms along with social and demographic factors.
Materials and methods
Study population
A total of 1200 healthy infants (<12 months of age) were included in this prospective, cross-sectional, single-centre study, which took place in a tertiary maternal and child health hospital located in Ankara, Turkey. Infants and their families who came for well child visits between March 2015 and October 2015 were randomly asked to participate in this study. Infants between the ages 0 and 6 months participated in our study more than the older ones because they come more frequently for well child visits. The participants were given their choice of day to be interviewed.
Ethical approval
The study protocol was approved by the local Ethics Committee of Sami Ulus Hospital and was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from each parent.
Statistical analysis
Statistical analyses were performed using SPSS version 15 statistical software (SPSS Inc., Chicago, IL, USA). Descriptive statistics were expressed as numbers, percentages, arithmetic means (±standard deviations (s.d.)) and medians (minimum and maximum). Normal distributions were determined via the Kolmogorov–Smirnov test, histograms and P–P plots. Depending on the normality of the data, variables were compared with an independent samples t test or with a Mann–Whitney U test. All categorical variables were compared using the Pearson χ 2 test, Yate's correction χ 2 test and Fisher's exact test. Multivariate linear regression analysis was performed to evaluate the main effects of covariates (age, household size, frequency of attending crowded places, maternal education status) on the number of total contacts with enter method. Multivariate logistic regression analyses were performed to evaluate the effects of the covariates (i.e. age, family income, number of contacts, contacts with school children, household size and the frequency of attendance at crowded places) on the reported infant respiratory symptoms in the past week. All independent variables and their associations with the number of total contacts and reported infant respiratory symptoms in the past week were simultaneously adjusted using multivariate linear regression models and multivariate logistic regression analyses, respectively. The relationships between continuous and ordinal variables were analysed using the Kendall's τ and Spearman's ‘ρ’ correlations. Values of P < 0.05 were considered significant.
Contact diaries and questionnaire
The mother/caretaker of each enrolled infant was asked to record every contact in a contact diary, which was developed in line with previously conducted contact surveys [Reference Mossong2, Reference van Hoek12] and translated into the local language. In the diaries, the term of ‘contact’ was defined as an interaction in close proximity with three or more words directed to the infant or a physical skin-to-skin contact between infant and another person. These contact diaries were presented ‘in person’ so that the mother/caretaker could be instructed on how to properly fill in the diary. The mothers/caretakers were assigned one random day of the week in which they were to record every person having any contact with the infant between 5 am that day and 5 am the following morning. The contact diary included some information about the characteristics of each contact person, such as sex, age and relationship to the infant. For each contact, the mothers/caretakers were asked to provide information as to whether the contact involved physical touch, the social context in which the encounter was made (e.g. home, work, school, travel, shopping, other), the total duration of the contact (i.e. <5, 5–15 min, 15 min to 1 h, 1–4 or >4 h), and the typical frequency of encountering each contact (i.e. daily or almost daily, about once or twice a week, about once or twice a month, less than once a month or for the first time).
Along with the diary, the participants were asked to fill out a cross-sectional survey that was used to collect basic demographic information regarding the infants and their families (e.g. age, sex, number of siblings, age of siblings, household composition, education status of the parents, family income level, the number of individuals per infant's bedroom). This cross-sectional survey also gathered information regarding the infants’ respiratory symptoms (e.g. cough, runny nose, difficulty in breathing) and attendance at crowded places over 1 week. The term ‘crowded places’ in the survey refers to any place where more than 10 people gather (e.g. shopping malls, indoor social events, hospitals, weddings, funerals, various ceremonies or other such events). The survey also collected information regarding the infants’ attendance in a nursery as well as the influenza and pertussis vaccination status of the infants’ parents.
Results
Description of sample
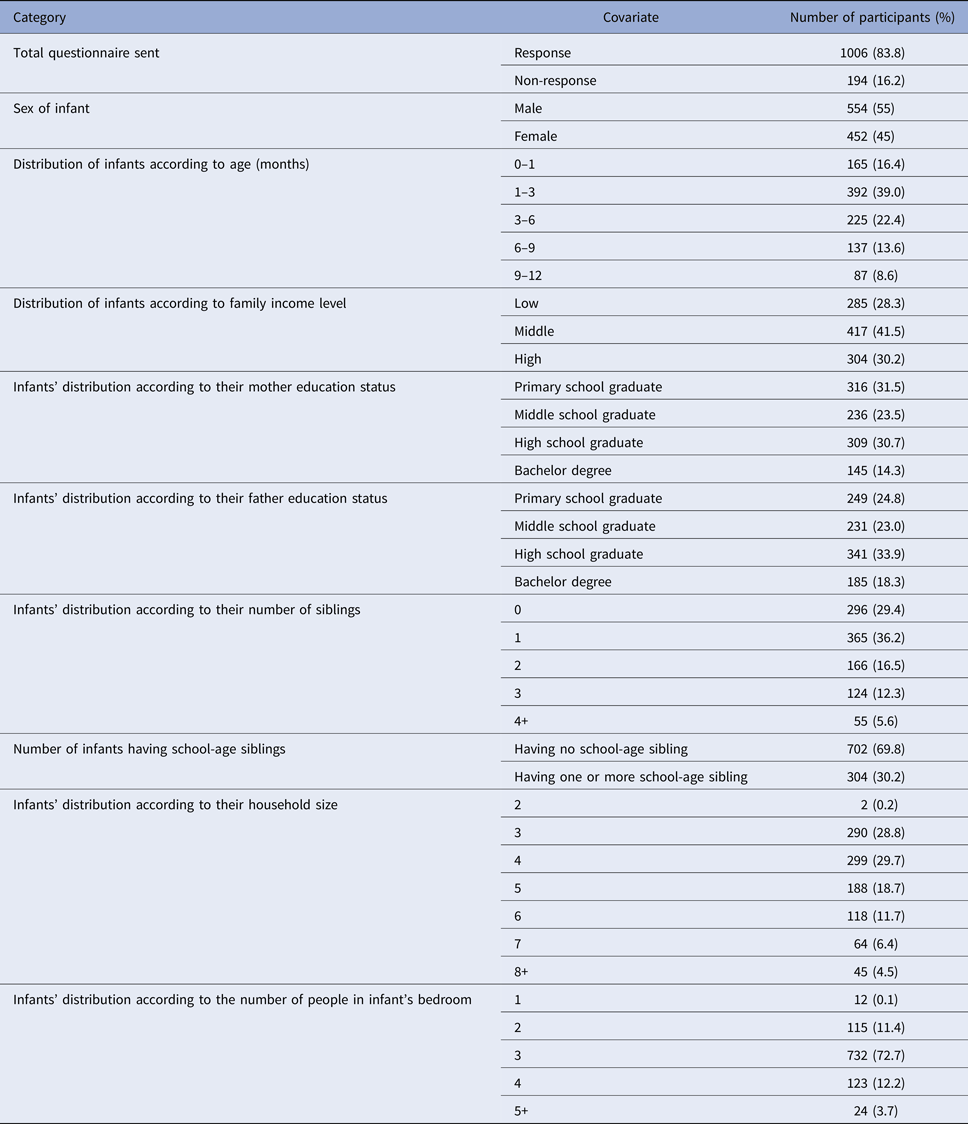
A total of 1006 diaries were collected (return rate 83.8%). Each of the collected diaries was prepared by the infants’ mothers and included every contact made during a whole day. Overall, 55% of the babies were male, their median age was 90 days and 16% were younger than 1 month. The median number of household members was 4 (range 2–19). The median number of household members sharing the infant's bedroom was 3 (range 1–10). The median number of siblings per child was 1 (range 0–7). The arithmetic mean of the siblings’ ages was 8.1 years (range 1–25), and 30% of the infants had school-age siblings. A summary of the household data and infant characteristics is presented in Table 1. None of the infants attended nursery.
Table 1. Summary of household data and characteristics of infants
According to the dairies, only 2.3% of the babies had at least one parent vaccinated against influenza, while none of the parents was vaccinated against pertussis. The parents who were vaccinated against influenza were vaccinated due to an existing chronic disease.
Attendance at crowded places
A total of 47.2% of infants attended a crowded place at least once in the past week. The median number of visits to a crowded place was 1 (range 0–7). The most frequently visited crowded places were indoor social gathering (61%), shopping malls (19%) and hospitals (12%), respectively. According to our analysis on age groups, the most frequently visited crowded place was hospitals for neonates (Fig. 1). The odds ratio (OR) of attending crowded places was higher for older infants (adjusted OR 1.27, 95% CI (1.07–1.40)). Distribution of frequency of attendance at crowded places according to age groups is shown in Figure 2.
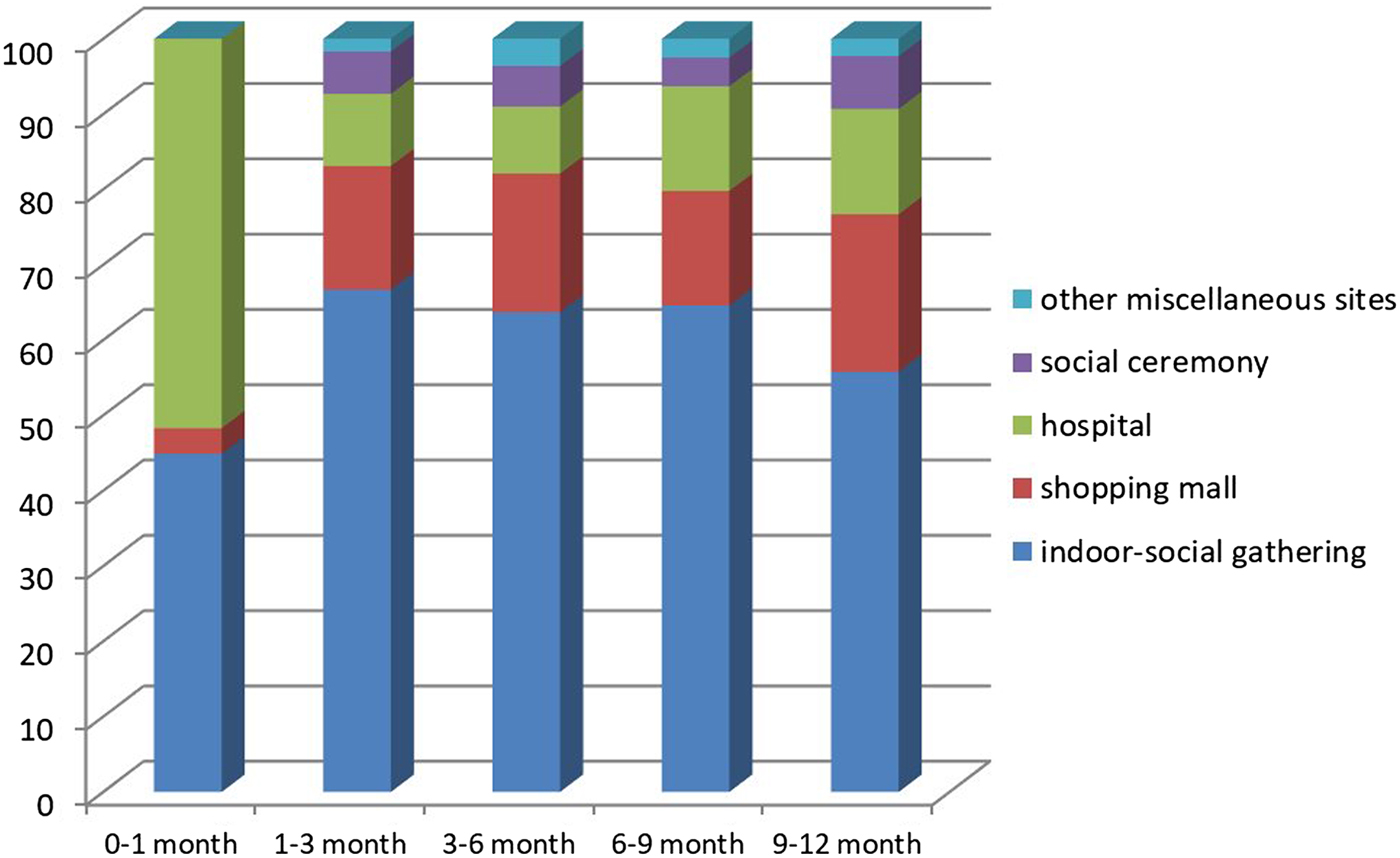
Fig. 1. Distribution of crowded place location according to infant age group.
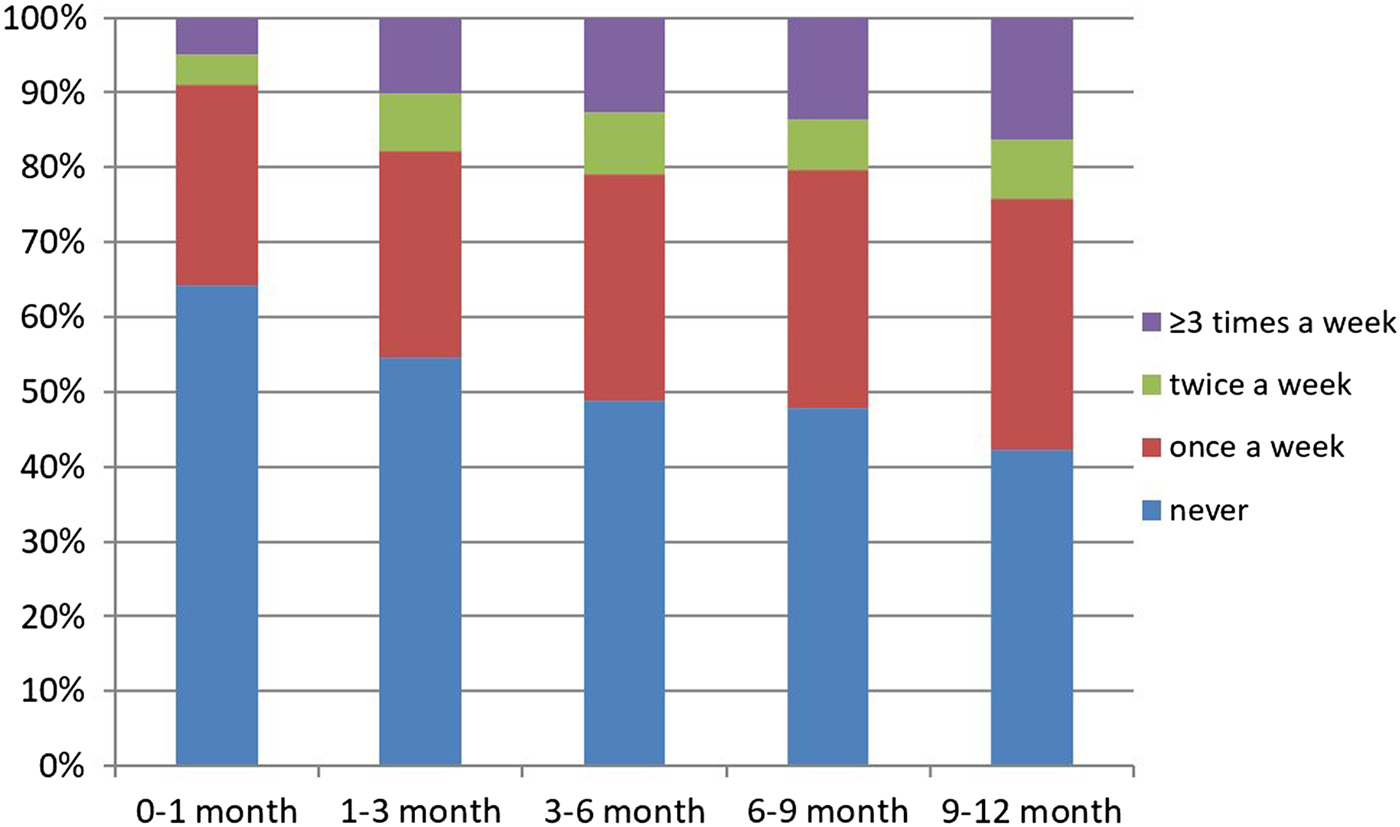
Fig. 2. Distribution of frequency of attendance at crowded places according to age group.
Further, families with higher income levels took their infants to shopping centres more frequently than those with lower income levels (OR 3.06, 95% CI (1.9–4.9)) (Fig. 3).
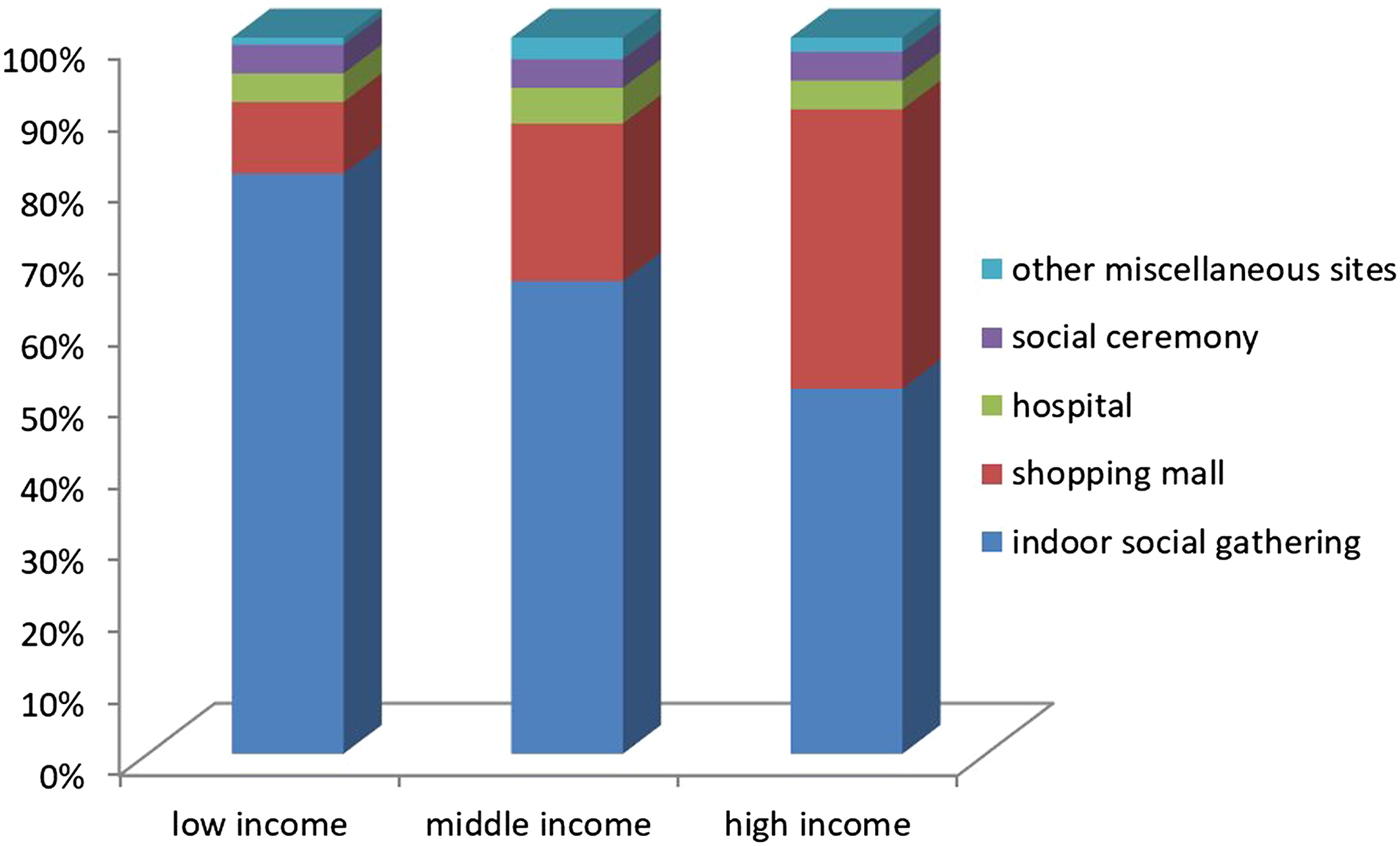
Fig. 3. Distribution of crowded place location according to family income level.
Characteristics of the contacts
General numerical data on the contacts
A total of 4706 contacts were recorded in the dairies. The mean number of contacts per infant per day was 4.6 ± 2.2 (range 1–18). A histogram showing the total number of contacts per day/per infant for each age group is shown in Figure 4.
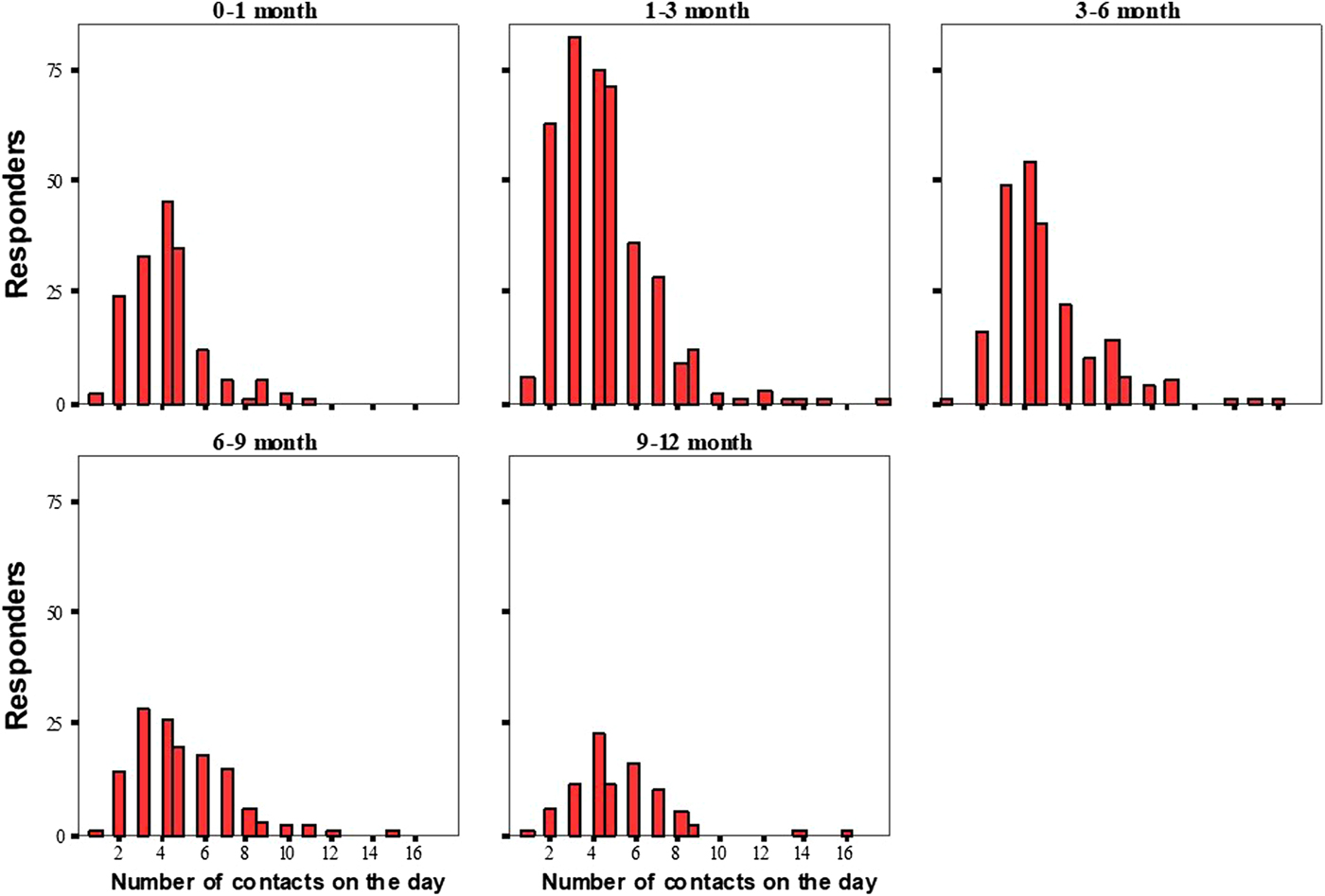
Fig. 4. Histogram of the total number of contacts per day/per respondent for each age group.
A total of 58.4% of the reported contacts were with females, and the mean age of the contacts was 28.7 ± 15 years. Of the contacts, 89.5% were physical, and 84.6% of the babies were in contact with the people other than their parents.
The age of the contacts ranged from 0 to 85 years, and only four of the recorded contacts were other infants <1 year old (Fig. 5a). The distribution of the contacts’ relationships with the infants according to the age of contacts is shown in Figure 5b.
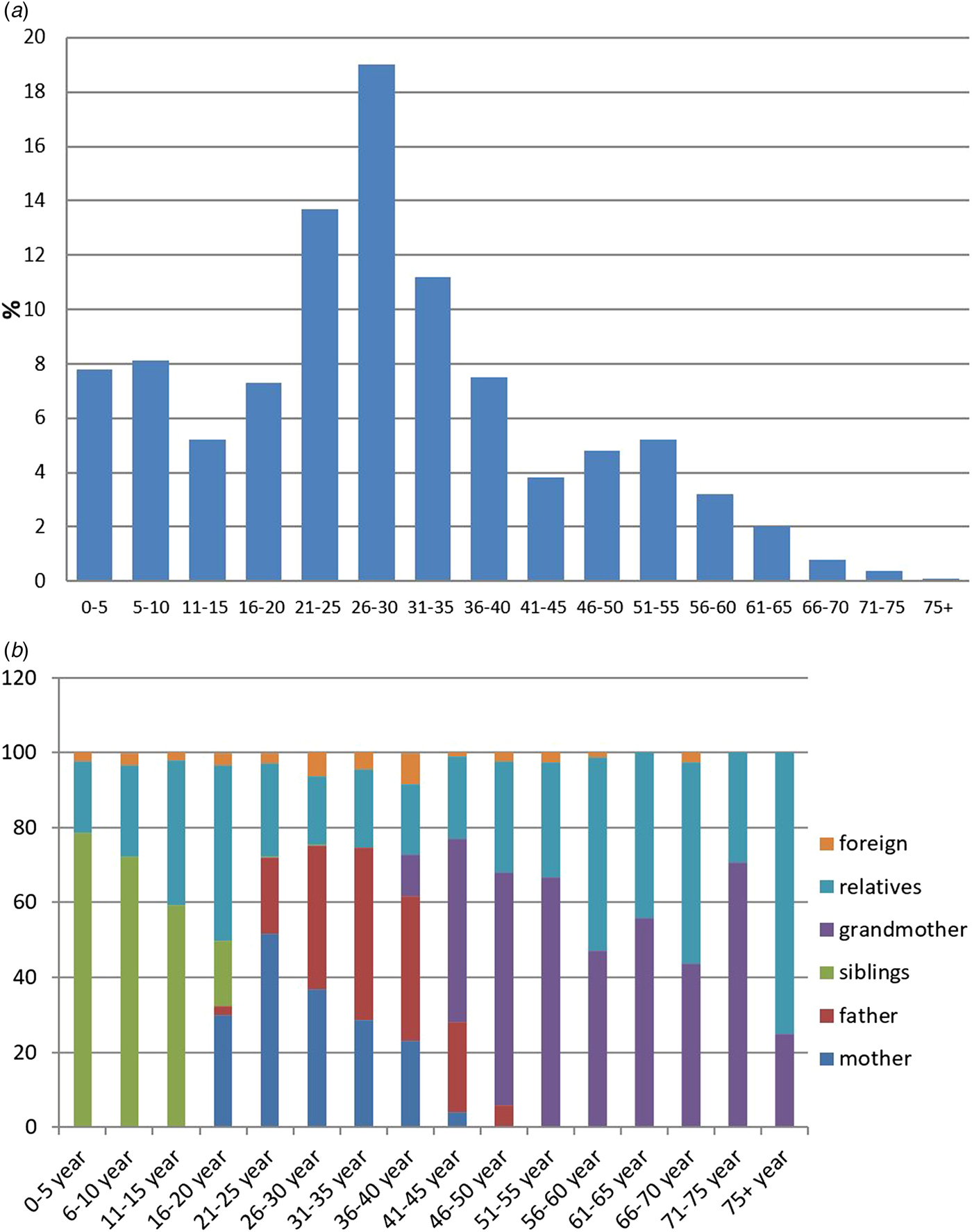
Fig. 5. (a) Age distribution of those who contacted infants; (b) distribution of contact relationship with infants according to contacts’ age group.
Distribution of contacts
Of the contacts lasting more than 4 h, 68.2% were with the mothers and 14% were with the grandmothers. Figure 6 shows the distribution of contacts according to contact duration. Of all the recorded contacts, 70.3% were with household members and 29.7% were with non-household members. Of the babies, 50.3% had contacts with non-household individuals.
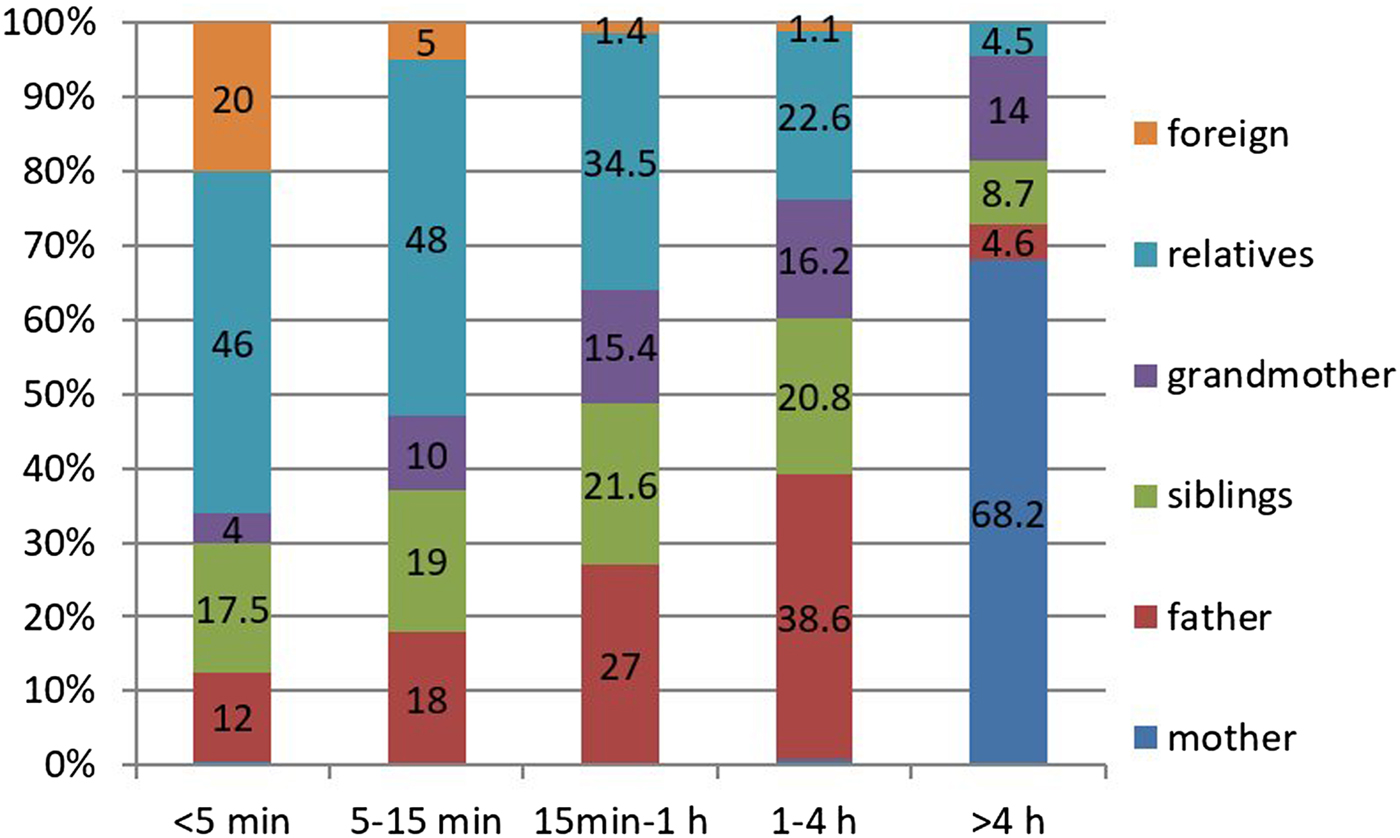
Fig. 6. Distribution of contacts according to contact times.
Age-specific analysis on contacts
Characteristics of contacts with infants ⩽1 month old (neonates) or 1–12 months old are shown in Table 2. Contacts with household members, daily contacts and contacts lasting <5 min are more frequent in neonates than in other infants. The median total contact time, adolescent contacts, non-household contacts, non-parent contacts and number of contacts with children attending at school are presented in Table 3 (based on age groups). The regarding percentages of the infants are also included in the table. We see that contacts with school-age children have a significant increase as the age of the infants gets higher. The median number of adolescent contacts was zero for all age groups.
Table 2. Characteristics of the contact events with regards to infants ⩽1 and 1–12 months old
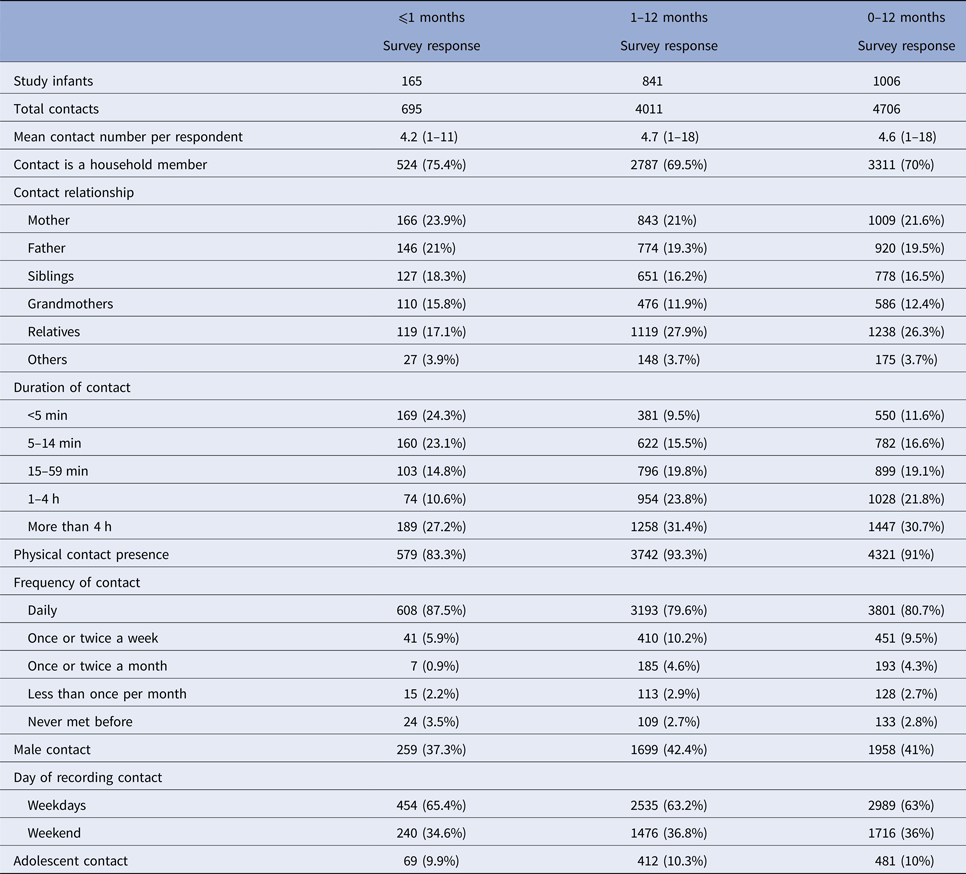
Table 3. Percentages of infants and median number of contacts with respect to contacting groups
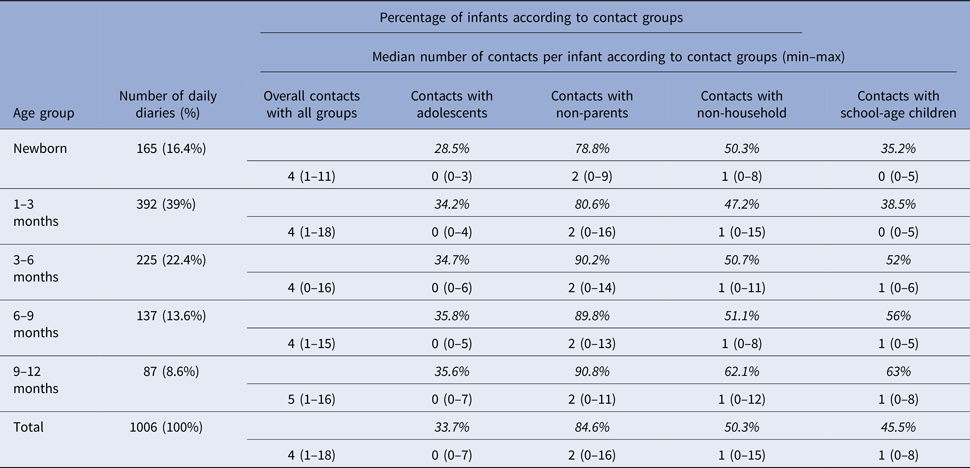
The percentages in italics indicate the percentages of infants having contacts with the specified group (adolescents, non-household and school-age children). Since there is overlapping in these groups (i.e. household adolescent contacts and non-household adolescent contacts may exist at the same time), the sum of percentages is >100%.
Other findings on contact numbers
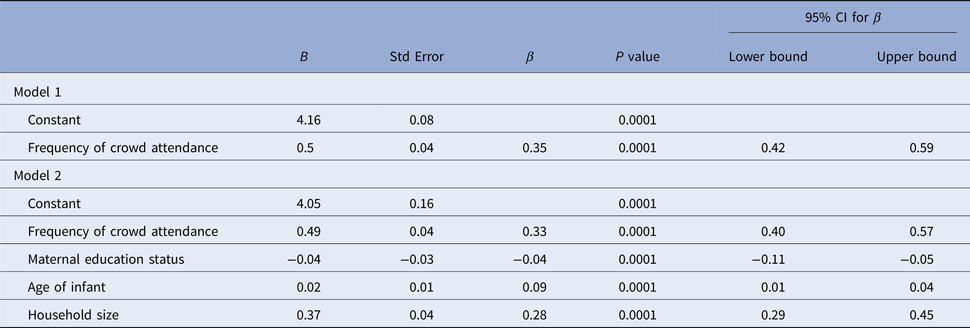
There was a positive weak correlation between age and number of contacts (Spearman's ρ = 0.149; P = 0.0001). Although there was no significant difference in the contact durations of mothers with regards to infant age (P > 0.05), there was an increased contact duration of fathers as infants became older (Spearman's ρ = 0.350; P = 0.0001). There was also a reverse and significant weak correlation (Kendall's τ = −0.155, P = 0.0001) between maternal education status and the number of non-parent contacts. However, there was no correlation with paternal education status (Kendall's τ = −0.076, P = 0.33). In linear regression model, contact numbers seemed to increase along with age, household size and frequency of attending at crowded places, and on the other hand, contact numbers decrease as maternal education status gets higher (P = 0.001) (Table 4).
Table 4. Factors associated with the number of total contacts according to linear regression model
CI, confidence interval.
For model 1, R 2 = 0.1 (P < 0.001); for model 2, ΔR 2 = 0.08 (P < 0.001) (models with P < 0.001 are taken as statistically significant).
Respiratory symptoms in the past week
The rate of respiratory tract infection in the past week was 5.6% (n = 57). However, this study did not take place during the main period for influenza transmission. There were significant weak correlations between the existence of at least one reported respiratory symptom in the past week and the number of household contacts, household size, age of infant, family income level, frequency of visits to crowded places, number of total contacts, number of non-parent contacts, number of contact children attending school and the presence of contact with school children. There was no correlation with the number of non-household contacts (Table 5). According to the developed regression model contacts with school children, frequency of attendance at crowded places and age were determined to be significant effective factors for reporting respiratory symptoms (Table 6).
Table 5. Relationship between reported respiratory symptoms in the past week with the number of household contacts, family income level, frequency of entrance into crowded places, number of total contacts, number of non-parent contacts, number of non-household contacts and contacts with school-age children
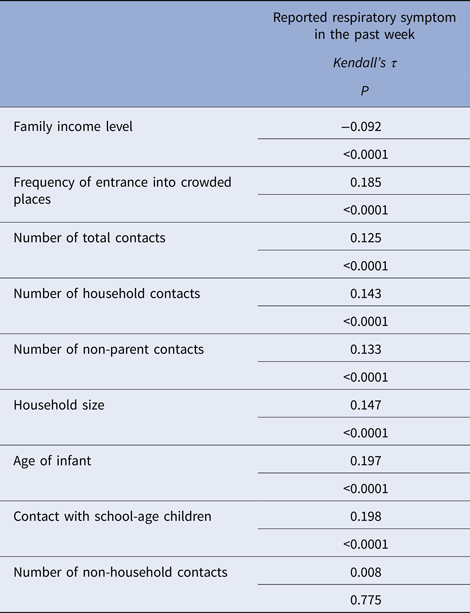
Table 6. Factors associated with reporting respiratory symptoms in the past week
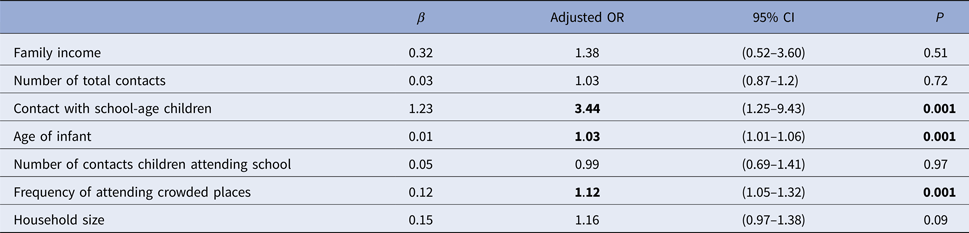
CI, confidence interval; OR, odds ratio (factors having p < 0.001 are statistically significant).
Discussion
This study describes the social contact patterns of infants, which were determined by the data collected from social contact diaries filled out by mothers and caretakers. These diaries also collected information regarding recently reported infant respiratory tract infection symptoms, allowing us to investigate the associations between these symptoms and contact patterns, household crowding and attendance at crowded places. To our knowledge, the current study is the largest cross-sectional survey on infants. We recorded 4706 contacts among 1006 participating infants. The current study not only covers a large number of infants, but it also has a remarkably high response rate (84%), assuring good representation for the targeted infant population.
Understanding infant contact patterns may be helpful for identifying the most appropriate strategies for coping with whooping cough and other droplet-borne respiratory infections. Data from the current study reveal that infants <12 months old had the longest contacts with their mothers, followed by contacts with their grandmothers, fathers and siblings, respectively. Moreover, 70.3% of all infant contacts were with the members of their households. Therefore, these results suggest that the best way to protect infants from respiratory tract infections is to vaccinate (i.e. cocoon) the members of their households.
To protect infants who are at the greatest risk for severe pertussis, the Advisory Committee on Immunization Practices (ACIP) recommends cocooning, which involves the vaccination of everyone who has close contact with the baby [Reference Zlamy13]. In a meta-analysis including eight studies of whooping cough cases, Wiley et al. found that the main pertussis sources for infants were their mothers and fathers, followed by grandmothers [Reference Wiley14]. Another study in the Netherlands that included infants <6 months old who were admitted to the hospital due to pertussis reported that 53% of those infants’ household members were also diagnosed with pertussis; the most common source of pertussis included the infants’ brothers, mothers and fathers [Reference de Greeff15]. Also Kara et al. showed that household contacts play an important role in the transmission of pertussis to infants and mothers were the main sources of infection [Reference Kara16]. The results of these previously published studies are similar to those of our current study, in that they promote the philosophy of the cocooning strategy.
On the other hand, we also found that nearly half of the infants in our study had non-household contacts. Likewise, the aforementioned study conducted in the Netherlands reported that one-third of the infants hospitalised due to whooping cough contracted the disease from non-household individuals [Reference de Greeff15]. In addition to known difficulties of cocooning (such as high cost, individuals’ need to be vaccinated at least 2 weeks prior to contact with the baby, the necessity of all those in close contact with the infant need to be vaccinated, etc.), existence of several contacts with non-household individuals found in this study and the previous ones’ suggest that cocooning may be insufficient and inefficient in protecting infants [Reference Urwyler and Heininger17, Reference Blain18]. These drawbacks should be kept in mind when determining the optimal vaccination approaches for infants.
Infants may also contract diseases from adolescents who have not been vaccinated. In a study involving 214 adolescents with prolonged cough, 7% of the participants had pertussis positivity [Reference Karagul19]. Also, seroprevalence studies have shown that immunisations over the course of acellular pertussis were terminated within a mean of 5 years [Reference Wendelboe20]. Our current study revealed that babies had limited contacts with adolescents; in fact, the majority of the contacts in our current study were with those in the 26–30 age group. In the current study, only 10.2% of contacts were with adolescents, and the median daily adolescent contact number was 0. Therefore, vaccination of adolescents is not a priority in protecting infants, particularly against whooping cough and influenza. In parallel to our current findings, a study by Choi et al. comparing 10 different pertussis vaccination schemes revealed that adolescent vaccination was ineffective in protecting infants [Reference Choi21].
In the literature, there are other contact-diary studies with some similarities and differences to ours. The largest contact-diary study included eight European countries with a total of 660 children aged between 0 and 4 years; the mean number of contacts per child was 10.2 [Reference Mossong2]. Melegaro et al. reported the median number of contacts per child (0–5 years old) as 8 [Reference Melegaro22]. It should be noted that the aforementioned studies reported age assortativeness. In our current study, however, we did not encounter the concept of age assortativeness, and our participants had remarkably fewer mean contacts per infant (4.6); this is because only four infants in our study had contact with a baby in the same age group (0–12 months). Likewise, in another social contact study including 115 infants under 1 year of age, the mean number of daily contacts was 6.68, and the authors reported no age assortativeness [Reference van Hoek12].
Another significant finding in our current study is that 89.5% of contacts were also accompanied by physical contacts. In the POLYMOD social contact study including participants ranging in age from 0 to 70+ years, 66% of all contacts were accompanied by physical contacts, although physical contacts were not specified by age [Reference Mossong2]. In a study conducted in Vietnam with participants ranging in age from 0 to 60+ years, Horby et al. found that 40% of daily contacts were accompanied by physical contacts [Reference Horby23]. That these previously published studies report a lower frequency of physical contact can be explained by the fact that touching is necessary to satisfy the needs of infants. In our current study, the total number of contacts was weakly positively correlated with the age of infants in a monthly manner. This may be explained by the fact that local traditions dictate that touching the baby before it is 40 days old is inadmissible. Also in our current study, according to the regression model, contact numbers are seemed to increase along with age, household size and frequency of attending at crowded places. Similar to our findings, Melegaro et al. showed that the number of contacts get higher as individuals spend more time in crowded places [Reference Melegaro22]. On the other hand, contact numbers decrease as maternal education status gets higher indicating that more educated or wealthy mothers keep their babies more isolated. However, there was no meaningful relationship between the total infant contacts and the education status of their fathers.
Furthermore, in the current study, none of the babies older than 6 months were vaccinated for influenza, and the rate of influenza vaccination for at least one of the parents was 2.3%, with none of the parents being vaccinated for pertussis. In a study conducted in Australia by Newcombe et al., the rate of influenza vaccination was 41% in paediatric patients with underlying disease and 14% in healthy children [Reference Newcombe24]. In the USA, it is suggested that all caretakers be vaccinated; however, the immunisation rate is only about 30% [Reference Shah and Messina25]. On the other hand, O'Leary et al. reported that 61% of mothers and at least one close contact of their newborn received the influenza vaccine with the recommendation of an obstetrician specialist [Reference O'Leary26]. Further, in another study in which paediatric practitioners suggested that caretakers be vaccinated against influenza, the vaccination rate increased from 23.7% to 85.6% [Reference Cooper White27]. Results of previous studies, as well as those of the current study, indicate a need to improve awareness of the importance of vaccinations for caretakers.
In our current study, several infants had respiratory symptoms reported in the past week; it was found that the greatest impact on the increase in the risk of infection was contact with school children. The frequency of attendance at crowded places and the age of the infant also affected respiratory symptoms. Although household crowding provides an adequate environment for the spread of respiratory infections, we found no significant relationship between household crowding and the frequency of reported respiratory symptoms [Reference Smith28]. This may be because even in crowded households, there were relatively few individuals sharing a bedroom with the infant. Similarly, in a study of the African meningitis belt, Ferraro et al. showed no relationship between household crowding and respiratory symptoms; however, their results suggested a clear association between social mixing and reported respiratory symptoms [Reference Ferraro29]. The relationship between attendance at crowded indoor places and respiratory tract infections has previously been shown in some studies in the literature [Reference Gordon30, Reference Wei and Li31]. In our current study, the frequency of attendance at crowded places increased reported respiratory infection symptoms by an OR of 1.12 (95% CI (1.05–1.32)); indoor social gatherings were the most commonly attended crowded place. For neonates, hospitals were the most visited crowded places. Because of this, vaccination of all paediatrics health practitioners should be a priority for the protection of neonates. Another finding in our current study is that second-most visited crowded places were shopping malls. Families with higher income levels were found to take their babies to malls more frequently than those with lower income levels, with an OR of 3.06 (95% CI (1.9–4.9)). In the study conducted by Amodio et al., it was found that shopping centres had very high air pollution, especially in the food courts, and that there was not enough air refreshment [Reference Amodio32]. Therefore, it is absolutely important that paediatricians should be informed about the fact that shopping centres, which are the indoor places with the highest risk of spreading respiratory infections, are not suitable for babies under 1 year. Numerous reports document the central role of school-aged children in spreading influenza [Reference Guclu33, Reference Mikolajczyk34]. In our current study, we also showed that contacts with school-age children increased the number of reported symptoms of respiratory tract infections with an OR of 3.44; therefore, influenza vaccination of school-age children can be considered a recommended intervention.
In addition, there are some other noteworthy points in this study. We had a relatively high response rate at the end of 6 months because most of the participants were provided with the questionnaires by hand at the hospital with a brief description about how to fill them out. In return, we received them by hand with face-to-face checking of each of the returned questionnaires. The final sample was large enough to observe significant findings. In our data, infants under 6 months were oversampled because families generally take babies in this age group to their well child visits much more frequently. However, this situation may be valuable in the sense that infants between 0 and 6 months have not yet completed their vaccination procedures against pertussis and influenza, and therefore, they constitute the main risk group. Looking at the household characteristics of the participants, there is a wide range of household size (ranging from 2 to 19), number and age of siblings, as well as income level and educational status of the families. This indicates that our data encompass a representative population.
There are also some limitations in our study. The families were asked to denote if their infants had some respiratory tract infection symptoms; however, these symptoms were based on families’ declarations and not on laboratory findings. Additionally no data were collected about the infants’ spent time in crowded places. Another limitation is that contact definitions consisting of only conversation and physical touch may fail to address the interactions with shared objects that would permit fomite transmission. In the future studies, appropriate questions could be included within the diaries to detect such information.
In this infant contact pattern study, there was a diverse range of contacts, a representative population and a high respondent rate. Because of this diversity seen on the contacts, addressing specific main target population of vaccination is not an easy task. However, when developing an appropriate vaccination strategy, one should carefully keep in mind that we found that the longest contacts are with the mothers, and that almost half of the infants have contacts with non-household people. Moreover, in our current study, attendance at crowded places and contacts with school-age children are risky situations causing reported infant respiratory symptoms. Hence, we suggest that all school-age children as well as mothers should be vaccinated against influenza and that babies should be kept away from crowded places as much as possible.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268818001048.
Acknowledgements
Special thanks are for Prof. Ufuk Beyazova for inspiring the authors to start working on this challenging study topic and for her unique comments and suggestions which improved our manuscript a lot.
Funding source
This study did not receive any financial.
Declaration of interest
None.















