Introduction
Coccidioidomycosis is a fungal infection caused by the inhalation of Coccidioides species spores. This infection is most frequently found in areas where the soil is dry and alkaline, including the southwestern United States, parts of Mexico and Central and South America [Reference Deresinski1, Reference Anstead2]. The majority of infections are asymptomatic with symptomatic infections presenting as flu-like illness that is often self-limiting [Reference Brown3]. However, an estimated 3–5% of symptomatic coccidioidomycosis infections disseminate, and one-third of these cases are fatal [Reference Brown3]. Additionally, in a small proportion of coccidioidomycosis patients may require prolonged suppressive therapy following initial treatment course to prevent relapse [Reference Brown3]. Recently, concern has grown over a reported increase in the risk of exposure to Coccidoides spp. spores for populations in endemic areas, specifically military personnel, prisoners and solar panel workers [Reference Lee4–Reference Williams7].
In the U.S., coccidioidomycosis is a nationally notifiable disease. Data from the Centers for Disease Control and Prevention's National Notifiable Disease Surveillance System (NNDSS) have demonstrated secular shifts in coccidioidomycosis incidence. For example, coccidioidomycosis incidence has increased from 5.3/100 000 population in 1998 to 42.6/100 000 population in 2011 [Reference Tsang8]. The factors that are responsible for this alarming increase in nationally reported Coccidioidomycosis cases are poorly understood. However, climate change, and increased migration of susceptible person to endemic areas have been suggested as possible explanations for this increase [Reference Nelson9]. Of note, coccidioidomycosis is a reportable disease in California and Arizona, suggesting that the incidence increase may not be attributed to underreporting [10].
Coccidioidomycosis represents a substantial healthcare burden within California and Arizona, two highly endemic states where greater than 95% of all cases are reported [Reference Komatsu11–Reference Sondermeyer13]. Medical cost for the treatment of coccidioidomycosis is high; in Arizona, the cost for hospitalisations due to coccidioidomycosis was estimated to be $86 million in 2007 [Reference Hector14, Reference Charalambous15]. Compounding the healthcare burden of coccidioidomycosis is the difficulty in diagnosing coccidioidomycosis which is commonly misdiagnosed for bacterial community-acquired pneumonia [Reference Saubolle16]. Misdiagnoses can contribute to an increase in the cost for care due to prolonged hospital stay [Reference Saubolle16]. Antifungal treatment costs range from $5000 to 20 000 annually with critically ill patients accruing additional costs due to intensive care support over many days in the hospital [Reference Galgiani17]. Additionally, antifungal treatments are also not without adverse effects, such as hepatotoxicity, gastrointestinal discomfort and neurologic side effects such as headache [Reference Girois18].
While there are data examining risk factors for coccidioidomycosis, there are limited studies evaluating factors associated with coccidioidomycosis hospitalisation. Moreover, several of these studies utilised national administrative data sources that do not hone down on the U.S. states that are most affected by coccidioidomycosis [Reference Chu19–Reference Sondermeyer21]. Therefore, we performed an investigation examining inpatient incidence of hospitalisation for coccidioidomycosis using state-level data from large highly endemic areas.
Materials and methods
We conducted a cross-sectional study to assess the sociodemographic and comorbidity factors associated with coccidioidomycosis-associated hospitalisation vs. hospitalisation associated with other causes in California and Arizona. Hospital discharge data were obtained from the State Inpatient Dataset (SID) for both California and Arizona for years 2005–2011. The SID represents all-payer inpatient discharge information collected from nonfederal (e.g. not military, Veterans Administration or Indian Health Services) hospitals. Combining both California and Arizona SID for years 2005–2011 resulted in a cohort of over 33.0 million inpatient stays.
The study population included all inpatient discharges for The Healthcare Cost and Utilization Project (HCUP)-defined hospitals from California and Arizona for the years 2005–2011. A coccidioidomycosis case was defined as a primary or secondary diagnosis identified by the International Statistical Classification of Diseases and Related Health Problems version 9 (ICD-9) codes assigned for coccidioidomycosis (Table 1). Population denominator data for the HCUP SID datasets consist of decennial census with annual estimates for years 2005–2007 and the American Community Survey for years 2007–2011 [22]. We calculated rates of coccidioidomycosis-associated hospitalisation by race and ethnicity for the years 2005–2011 using data from the 2010 U.S. Census, assuming a stable population for our study time frame.
Table 1. Demographic features and comorbidities for coccidioidomycosis-associated hospitalisation patients from the California and Arizona State Inpatient Datasets, 2005–2011

* The quartiles are identified by values of 1 to 4, indicating the poorest to wealthiest populations. First quartile represents 0-25th percentile, second quartile represents 26th-50th percentile, third quartile represents 51th-75th percentile, and the fourth quartile represents 76th -100th percentile.
Our primary outcome was coccidioidomycosis-associated hospitalisation. To assess factors that may be associated with these hospitalisations, we examined a pre-selected group of sociodemographic and patient-level comorbidity factors. These factors were chosen based on possible associations suggested by the medical literature to be associated with a higher rate of coccidioidomycosis infection or severe coccidioidomycosis infection [Reference Brown3, Reference Chu19] and the fact that they were available in the SID databases. Additionally, we examined demographic factors that we believed may be important confounders.
We hypothesised rural persons were at a higher risk given the relative lack of pavement, more contact with soil and potentially more exposure to the outdoor environment. Urbanisation was stratified into four rural–urban patient locations, urban (metropolitan), large rural town (micropolitan), small rural town and isolated small rural area. The stratification used by HCUP to generate the condensed urbanisation variable within the SID database is based on rural–urban commuting areas (RUCA) and assignment into ZIP codes using population and commuting information from the 2000-year Census [23]. Patient household income was included in our analyses to identify the presence of potential health disparities within different socioeconomic groups for coccidioidomycosis-associated hospitalisation. We hypothesised persons of lower socioeconomic status were at a higher risk attributed to financial barriers to healthcare and overall lower health status compared to persons with higher socioeconomic status. Age was stratified into six categories, (0–17, 18–29, 30–39, 40–49, 50–59 and >60 years), similar to prior work that also used administrative level data [Reference Luo20]. The race was categorised as Caucasian (non-Hispanic), African American (non-Hispanic), Hispanic, Native American or Alaska Native (non-Hispanic), Asian/Pacific Islander (non-Hispanic) and other or multiple races (non-Hispanic). Household income is represented by the quartile of the median household income based on the patient's zip code and was included as a community level socioeconomic factor. The state where the hospital is located (California or Arizona) was also included as a variable in the analysis. Comorbidities were measured using the Elixhauser comorbidity index [24–Reference Moore26]. The Elixhauser Index, a validated scale of co-morbidity from administrative databases was calculated using the Elixhauser Comorbidity Software Version 3.7 [24].
Environmental factors such as seasonal rainfall, earthquakes and soil disruptions have been shown to be associated with higher incidences of reported coccidioidomycosis cases [Reference Talamantes27, Reference Schneider28]. We hypothesised that coccidioidomycosis cases would increase following periods of increased rainfall. The incubation for coccidioidomycosis is not well established, therefore we performed four models for the lag between rainfall and coccidioidomycosis-associated hospitalisation incidence (0, 1, 2 and 3 months) [Reference Talamantes27, Reference Das29–Reference Drips31]. The average monthly rainfall was calculated using data obtained from the National Oceanic and Atmospheric Administration [32]. Due to monthly rainfall variability between Arizona and California, such as Arizona's monsoon season beginning in June and continuing through September while California experiences its rainfall season between October and April, California and Arizona, the analysis was done separately for each state. Associations were measured using Spearman's correlation.
Our study population consisted of persons hospitalised due to coccidioidomycosis, cases and non-cases. Non-cases represent hospitalised patients admitted for non-coccidioidomycosis diagnoses. Bivariate analyses were performed and all factors with a P value of <0.20 in these analyses were included in a logistic multivariable regression analysis. To assess multicollinearity among variables in our multivariable model, variance decomposition proportion (VDP) analysis was performed using the SAS macro COLLIN [Reference Kleinbaum33]. All analyses were performed using SAS® version 9.2 (SAS Institute Inc., Cary, North Carolina) [34].
Results
We found a total of 33 622 215 patients were hospitalised in Arizona and California between the years 2005 and 2011. Among these, there were 23 758 hospitalisations with an associated diagnosis of coccidioidomycosis recorded in the dataset. Primary diagnosis of coccidioidomycosis accounted for 72% of cases and a secondary diagnosis of coccidioidomycosis accounted for 28% of cases. Arizona had an over sixfold higher coccidioidomycosis-associated hospitalisation incidence rate compared to California over the study time frame, 198.9/100 000 vs. 29.6/100 000 population (Table 2). The most common coccidioidomycosis diagnosis for both states was ‘primary coccidioidomycosis’ (13 919, 58.6%). In order of decreasing frequency, the next most common diagnoses were chronic and unspecified pulmonary, progressive coccidioidal not elsewhere classified, coccidioidal meningitis and primary cutaneous coccidioidomycosis and not elsewhere specified. although the relative ranking of these diagnoses varied by state (Table 2).
Table 2. Coccidioidomycosis diagnosis rates per 100 000 persons

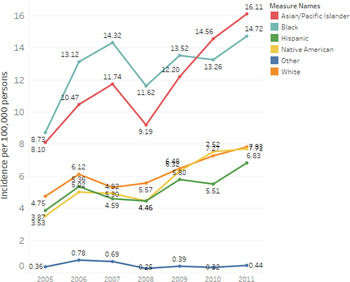
Annual incidence rates of coccidioidomycosis-associated hospitalisations differed by race/ethnicity (Fig. 1). Asian/Pacific Islanders and African Americans had the highest incidence rates across all years (Fig. 1). The average annual incidence of coccidioidomycosis-associated hospitalisation per 100 000 persons for Asian/Pacific Islanders and African Americans was 11.8/1 000 000 persons (95% CI 9.1–14.4) and 12.8/100 000 persons (95% CI 10.9–14.6), respectively. Average annual incidence among Caucasians was 6.2/100 000 persons (95% CI 5.2–7.2), 5.6/100 000 persons (95% CI 4.2–7.1) among Native American, 5.2/100 000 persons (95% CI 4.3–6.1) among Hispanics and 0.5/100 000 persons (95% CI 4.1–7.1) among other race/ethnicities.

Fig. 1. Annual coccidioidomycosis hospital incidence rates per 100 000 persons by racial and ethnic groups. Yearly coccidioidomycosis hospital incidence rates per 100 000 persons are presented for racial/ethnic groups: Asian/Pacific Islander, African American, Hispanic, Native American, Caucasian and others.
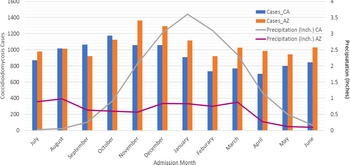
In Arizona, when examining the association of rainfall with coccidioidomycosis-associated hospitalisation, we found that monthly hospitalisations associated with coccidioidomycosis showed high-incidence periods during the winter months of October, November and December (Fig. 2). The average precipitation for these months ranged from 0.60 to 0.84 inches. September and February showed the lowest incidence of hospitalisations. In Arizona, peak precipitation during this time period occurred in August and March. We found no correlation between coccidioidomycosis-associated hospitalisations and all four lag times (0, 1, 2 and 3 months) (Table 3).

Fig. 2. Monthly hospitalisation for Arizona and California with average seasonal precipitation amounts. Bar graph represents number of cases of coccidioidomycosis by month of hospital admission for both California and Arizona. Monthly precipitations in inches is shown as a line graph for both California and Arizona. Abbreviations: CA: California, AZ: Arizona, Inch: inches.
Table 3. Seasonal precipitation and coccidioidomycosis hospitalisation for California and Arizona

In California, the incidence of coccidioidomycosis-associated hospitalisations peaked in October. Peak rainfall occurred in December, January and February (3.02, 3.60 and 3.10 inches, respectively). A significant negative correlation was found between seasonal rainfall and coccidioidomycosis-associated hospitalisations for California for the 1-, 2- and 3-month lag times but not for the zero month lag time (Table 3).
In bivariate analysis, we found numerous predictors associated with coccidioidomycosis-associated hospitalisations that were included in our multivariable analysis (Table 4). In multivariable analysis, among demographic factors, coccidioidomycosis-associated hospitalisation were inversely associated with female gender (adjusted odds ratio (aOR 0.40 (95% CI 0.40–0.41)). Persons in the age group (40–49) years were more likely to have coccidioidomycosis-associated hospitalisation compared to our referent group: age 18–29 years, aOR = 1.50 (95% CI 1.43–1.59)). Similarly, other non-elderly adult age categories were positively associated with coccidioidomycosis-associated hospitalisation: age 30–39 years: (aOR 1.42 (95% CI 1.35–1.50)); and age 50–59; (aOR 1.07 (95% CI 1.02–1.30)). However, children age 0–17 years: (aOR 0.17 (95% CI 0.16–0.18)) and adults age >60 (aOR 0.51 (95% CI 0.49–0.54)) years were less likely to have coccidioidomycosis-associated hospitalisation. In term of race, compared to Caucasians in our multivariable model, we found that coccidioidomycosis-associated hospitalisations were associated with African American race (aOR 1.98 (95% CI 1.89–2.06)), Hispanic ethnicity (aOR 1.32 (95% CI 1.27–1.36)), Native American/Alaska Native race (aOR 1.26. (95% CI 1.18–1.35)) and other race/ethnicity (aOR 1.07 (95% CI 0.94–1.22)).
Table 4. Predictors of coccidioidomycosis hospitalisation (SID CA & AZ, 2005–2011)

Compared to those living in urban areas, coccidioidomycosis-associated hospitalisation was associated with residence in a large rural area (aOR 2.28 (95% CI 2.19–2.39)) and inversely associated with isolated rural areas (aOR 0.95 (95% CI 0.84–0.91))). Compared to those residing in higher (fourth quartile) income areas, coccidioidomycosis-associated hospitalisation was lower in those residing in first quartile (aOR 0.87 (95% CI 0.84–0.91)), second quartile (aOR 0.78 (95% CI 0.74–0.81)) and third quartile areas (aOR 0.87 (95% CI 0.83–0.81)). Coccidioidomycosis-associated hospitalisation was associated with Arizona residence (referent group: California) (aOR 6.42 (95% CI 6.25–6.60)).
Higher Elixhauser score was associated with coccidioidomycosis-associated hospitalisation (aOR = 1.02 (95% CI 1.02–1.03)) for each point in the Elixhauser score). Persons with AIDS and obesity had reduced odds of coccidioidomycosis-associated hospitalisation (aOR 0.75 (95% CI 0.58–0.99) and aOR 0.78 (95% CI 0.74–0.83), respectively). Uncomplicated diabetes (aOR 1.47 (95% CI 1.41–1.52)) and chronic pulmonary disease (aOR 1.59 (95% CI 1.54–1.65)) showed an increase in the odds of coccidioidomycosis-associated hospitalisation. Coccidioidomycosis-associated hospitalisation was associated with an increased mortality compared to the referent group who were alive at hospital discharge. (referent group: non-coccidioidomycosis-associated hospitalisation) (aOR 1.12 (95% CI 1.03–1.22)).
Discussion
Using very large HCUP administrative datasets we had several novel observations related to coccidioidomycosis-associated hospitalisation, including relative incidence, association with important clinical, demographic and climatic factors, specifically rainfall. To the best of our knowledge, this is the first study to evaluate sociodemographic and comorbidity predictors of hospitalisations associated with coccidioidomycosis using a large inpatient population-based dataset.
We found many sociodemographic predictors such as gender, age, race/ethnicity and place of residence that had relationships with coccidioidomycosis-associated hospitalisation. Not surprisingly, African Americans had the highest odds of coccidioidomycosis-associated hospitalisation of any racial/ethnic group. African-American race is associated with a higher risk of disseminated infection and greater disseminated disease's severity [Reference Ruddy35]; thus the association with coccidioidomycosis-associated hospitalisation (compared to Caucasians) was expected. Using a California inpatient dataset, Sondermeyer et al. similarly found an increased relative risk for hospitalisation among African Americans consistent with our findings [Reference Sondermeyer21]. We found an increase in the odds of coccidioidomycosis-associated hospitalisation among persons in the age group 40–49 years and a lower odds for elderly patients. The increase for this age group may be attributed to the occupation. An occupational hazard for coccidioidomycosis infection has been shown among construction and agricultural workers who may belong to this age group [Reference Das29]. Elderly patients may be hospitalised for other age-related conditions such as cardiovascular disease, malignancy and injury due to falls.
Interestingly, we found that lower income patient household (first through third quartile) had a lower odds for coccidioidomycosis-associated hospitalisation. This finding surprised us and the reasons for this association are unclear. It is possible that higher-income populations have better insurance and thus might have their coccidioidomycosis infection managed as an outpatient with a primary care provider, or perhaps are diagnosed earlier, preventing the need for coccidioidomycosis-associated hospitalisation.
Hospitalised patients in Arizona were at a much higher risk for coccidioidomycosis-associated hospitalisation than patients residing in California. Our findings are consistent with overall coccidioidomycosis incidence (hospitalised and non-hospitalised); a 2016 study found that Arizona accounted for 66% of cases while California accounted for 31% [Reference Luo20]. Of concern, Arizona has one of the fastest-growing populations in the United States, [36] which suggests that coccidioidomycosis-associated hospitalisation is likely to further increase in cases due to this influx of potentially susceptible persons. Our data suggest that not only are those residing in Arizona at higher risk for infection but at much higher risk for coccidioidomycosis-associated hospitalisation compared to Californians.
We also found that several comorbidities that were associated with coccidioidomycosis-associated hospitalisation, including uncomplicated diabetes and chronic obstructive pulmonary disease. Supporting our findings, a prior study showed diabetes to be a contributing cause of death in coccidioidomycosis patients, [Reference Santelli37] suggesting diabetes may be associated with more severe coccidioidomycosis infection and coccidioidomycosis-associated hospitalisation. Additionally, patients with AIDS were at a lower risk for coccidioidomycosis compared to patients without AIDS. A study coinciding with the time frame of this study suggests a decrease in the incidence and severity of coccidioidomycosis within the HIV-1 infected population may be attributed to the introduction of potent antiretroviral therapy [Reference Masannat38]. Our observation of lower risk of coccidioidomycosis-associated hospitalisation may be because persons with HIV are seen frequently by primary care practitioners for management of their HIV given their very high rates of insurance from Ryan White funding [39]. If persons with HIV or AIDS had coccidioidomycosis, they may have been diagnosed earlier, which precluded more serious illness and need for hospitalisation.
Interestingly, in our multivariable model, obese patients were at reduced risk of coccidioidomycosis-associated hospitalisation, which was an unexpected and novel finding. Possible reasons for this relationship are not readily apparent. Perhaps obese patients who were hospitalised were more likely to be hospitalised due to complications of obesity, such as cardiovascular disease. Higher comorbidity score was associated with coccidioidomycosis-associated hospitalisation (aOR = 1.02 (95% CI 1.02–1.03)) for each point in the Elixhauser score), suggesting that more severe forms of coccidioidomycosis requiring hospitalisation are more common in those with underlying co-morbidities. Studies examining coccidioidomycosis-associated death, which is obviously another severe complication of coccidioidomycosis, found several comorbidities associated with death [Reference Rosenstein40]. Our study is the first study to include all 29 comorbid conditions present in the Elixhauser comorbidity index score. Although we did not exclusively look at fungal meningitis per se, a recent study showed that fungal meningitis due to either cryptococcus, coccidioidomycosis, histoplasmosis or candidiasis was also associated with more comorbid conditions [Reference Charalambous15]. Together these data suggest that higher comorbidities are associated with more severe coccidioidomycosis infection requiring hospitalisation.
The association between climate and coccidioidomycosis incidence is based on the ‘grow’ and ‘blow’ hypotheses [Reference Drips31]. This hypothesis argues that Coccidioides arthrospores are present at a higher concentration in the soil after heavy rains and as the soil dries up wind or soil disruption spreads the arthrospores [Reference Jones41, Reference Tamerius42]. Interestingly, we found no correlations between coccidioidomycosis hospitalisations and seasonal precipitation levels for Arizona and paradoxically negative, albeit weak, correlations between coccidioidomycosis hospitalisations and seasonal precipitation levels for California. Our results support the more recent hypothesis that additional environmental factors such as wind, temperature and human activities play a more influential role than precipitation alone in explaining relationships between climate and incidence of coccidioidomycosis infections [Reference Weaver43]. Alternatively, it could be that coccidioidomycosis-associated hospitalisation incidence follows a different pattern than overall coccidioidomycosis infection incidence and hence we are describing previously undescribed unique patterns.
Our study had limitations. First, our case definition included patients with a primary or secondary diagnosis code for coccidioidomycosis. Inclusion of a secondary diagnosis could result in an overestimation for the incidence of disease due to diagnostic coding error since the hospitalisation may have been due to a non-coccidioidomycosis primary diagnosis and the patient may have had chronic coccidioidomycosis that did not trigger the hospitalisation. However, among cases included based on a secondary diagnosis of coccidioidomycosis the two most prevalent primary diagnoses were HIV and pneumonia. Since HIV infection without complications is not a reason for hospitalisation and pneumonia could be related to coccidioidomycosis pulmonary infection, these patients' hospitalisations may be due to coccidioidomycosis. Additionally, our investigation followed previously reported methods for epidemiological study inclusion criteria [Reference Sondermeyer21]. Second, administrative databases are prone to missing or misclassifying diseases and comorbidities. Thus, selection bias may have been introduced, although it is difficult to know which direction any bias may have tilted our findings. Additionally, a limitation of comorbidities is that coccidioides may be underdiagnosed or patients have less aggressive investigations if milder cases, or those with intact immune systems, which may skew the incidence to patients with comorbidities. Future studies should evaluate the accuracy of billing coding for coccidioidomycosis. Third, we examined only inpatients for our investigation. Many severe infections may have been treated as outpatients and we could not measure the burden of hospitalisation compared to overall disease. The outpatient population was excluded from our study because information on outpatients is not collected in the SID. Fourth, our study population did not include those hospitalised in Indian Health Services facilities or federal hospitals, such as veterans. Thus, our population was not truly representative of all hospitalised patients in California and Arizona given we missed these populations. Finally, our comparison group is those who are hospitalised without coccidioidomycosis. Thus, we cannot estimate risks of coccidioidomycosis hospitalisation among the general population. Instead, our population was that of hospitalised patients and we found characteristics that were significantly high or low among those hospitalised with and without coccidioidomycosis.
Our investigation has strengths. First, ours is the first study to examine the association between coccidioidomycosis-associated hospitalisation and comorbidity measures including the relatively new Elixhauser index. Secondly, to our knowledge, our study is novel in that it utilised a large population-based cohort consisting of hospitalised patients from two highly endemic areas, Arizona and California where prior studies used both inpatient or outpatient cohorts from individual endemic states. Finally, we examined a large period of time from multiple SID databases, thus avoiding relationships that may be present only in low or high incidence years and the peculiarities specific to more epidemic years.
In conclusion, we found multiple sociodemographic predictors and comorbidities associated with coccidioidomycosis-associated hospitalisation in an inpatient dataset consisting of individual-level data obtained from the State Inpatient Datasets for Arizona and California. Disseminated coccidioidomycosis confers a high mortality rate and significant cost (an estimated $55 000 per hospital visit [Reference Freedman44]). Therefore, early identification of populations at highest risk for coccidioidomycosis-associated hospitalisation may be helpful for limiting disease-associated morbidity, mortality and cost. Our results contribute to a better understanding of the inpatient coccidioidomycosis population. Studies like ours bring attention to healthcare provides of the potential disease burden attributed to coccidioidomycosis. Future research may wish to consider improved methods of early diagnosis and treatment modalities in high-risk populations to prevent coccidioidomycosis-associated hospitalisation.
Conflict of interest
None.
Data availability statement
The data that support the findings of this study are available from HCUP. Restrictions apply to the availability of these data, which were used under agreement for this study. Data are available from Deborah Kupferwasser with the permission of the HCUP.