INTRODUCTION
Q fever is a zoonosis caused by Coxiella burnetii, an obligate intracellular Gram-negative bacterium. C. burnetii has strong zoonotic potential and a large reservoir, including both wild and domestic mammals, birds and arthropods. The majority of Q fever outbreaks in humans are related to domestic ruminants [Reference Maurin and Raoult1].
Clinical disease in Q fever is variable ranging from asymptomatic in 60% of cases to influenza-like illness, pneumonia, hepatitis and meningo-encephalitis. A minority of cases, ranging from 2% to 5%, become chronically infected after acute illness [Reference Raoult, Marrie and Mege2].
The Netherlands has been troubled for three consecutive years (2007–2009) by a rise in the numbers of Q fever patients related to areas with dense populations of dairy goats. Which factors caused this outbreak are not completely clear. Proximity to aborting small ruminants and a susceptible population are thought to be the likely causes of this epidemic [Reference Roest3]. However, it is still not clear whether pathogen-related factors such as the existence of a more virulent C. burnetii strain may have favoured the extensive Dutch spread. Thorough genotyping data on C. burnetii are limited due to a lack of culture facilities and human material at that time [Reference Huijsmans4]. In 2010, after rigorous interventions in dairy goat and sheep farms, the number of Q fever patients has substantially decreased. However, it is expected that Q fever may remain an endemic infectious disease in The Netherlands.
Acute Q fever is a notifiable disease in The Netherlands and national data on the incidence of acute Q fever are collected from regional laboratories and clinicians. In order for a case of Q fever to be notified at least one clinical and one laboratory criterion must be present (Table 1).
Table 1. Case definition of a confirmed Q fever case formulated by the National Institute of Public Health and the Environment (RIVM). One clinical and one laboratory criterion must be present to confirm a Q fever case
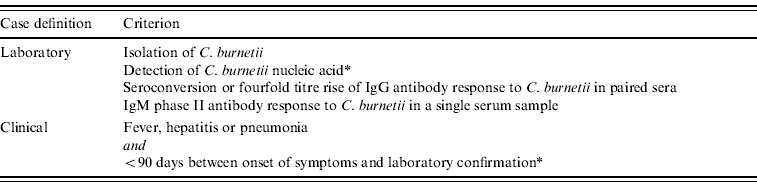
* Added to the notification criteria in 2010.
The appropriate diagnostic approach for acute Q fever depends on the interval between the onset of symptoms and presentation for diagnostic testing. Within 2–3 weeks after onset of disease symptoms, only the polymerase chain reaction (PCR) test is positive in acute Q fever [Reference Schneeberger5]. Subsequently immunoglobulin M (IgM) phase I/II antibodies can be detected by serological testing followed shortly by IgG phase I/II. Anti-phase II antibodies predominate during acute Q fever in contrast to anti-phase I antibodies which persist in chronic Q fever [Reference Maurin and Raoult1, Reference Wegdam-Blans6].
Diagnostic screening strategies were implemented to cope with large numbers of Q fever diagnostics during this outbreak. A recently published general algorithm for acute Q fever diagnostics suggests the use of either an enzyme-linked immunoassay (ELISA) IgM phase II or a PCR test depending on the time of onset of disease [Reference Wegdam-Blans6].
However, in most cases the microbiological diagnosis of acute Q fever depends on its serology. Three serological methods are commonly used: indirect immunofluorescence assay (IFA), complement fixation assay (CFA) and ELISA. In general IFA is considered to be a reference test for diagnosing Q fever [Reference Maurin and Raoult1] but it is a labour-intensive technique and must be performed by an experienced technician to ensure reliable results. This is in contrast to the ELISA, which can easily be scaled-up but has a lower sensitivity. CFA is a very specific test, but is less sensitive than the IFA as well as being laborious [Reference D'Harcourt7, Reference Field8].
In a few per cent of patients suspected of acute Q fever, a solitary positive serology result for IgM phase II is detected. Such results may point to a presumptive diagnosis of acute Q fever.
We therefore determined and compared the positive predictive value (PPV) of solitary positive IgM phase II detected with indirect IFA and ELISA for acute Q fever.
MATERIALS AND METHODS
A dataset of the serological results of patients suspected of acute Q fever, collected in 2008 and 2009 in a laboratory in a tertiary referral hospital was analysed. A change in screening practices, first using IFA for IgM and IgG antibodies against both phase I and phase II in 2008 (Focus Diagnostics, USA) and subsequently using ELISA for IgM antibodies to phase II in 2009 (Serion Immundiagnostica, Germany) enabled us to compare the outcome of both tests. A positive screening ELISA IgM phase II was confirmed with IgM and IgG IFA for both phase I and II antibodies in 2009.
Inclusion criteria were sera that initially tested positive only for IgM phase II either with IFA and/or ELISA. In addition at least one follow-up serum sample had to be available to study seroconversion. Exclusion criteria were: inconclusive initial IgM II results or follow-up sera taken within 10 days of the initial sample.
Serology
IFA and ELISA were performed according to the manufacturer's instructions. A false positive may result when rheumatoid factor (complexed IgG) is present in the specimen. Therefore, pretreatment of the serum to remove free and complexed IgG antibody was performed on each serum sample. We considered a cut-off value of 1:32 for both phases I and II, IgM and IgG in the IFA to be positive. A seroconversion for Q fever was documented if the initial IgG phase II sample tested negative but was positive in a follow-up sample [Reference Jager9].
Patients
All samples were obtained from patients that had been referred by hospital physicians or by their family physicians for Q fever diagnostics. In total 93 samples from 2008 met our inclusion criteria. In 2009 a total of 86 sera were included. For each patient we recorded age, sex and time interval between first and follow-up samples.
Data analysis
Baseline characteristics of cases from 2008 and 2009 were compared using the χ2 test or t test depending on the nature of the variable.
The PPV of a solitary IgM phase II result was calculated for IFA and ELISA separately, taking a seroconversion to IgG phase II as the reference method for a diagnosis of Q fever. The χ2 test was used to compare proportions of seroconversion of data from 2008 and 2009. Significance was designated at P<0·05. Data were analysed using SPSS 16.0 software (SPSS Inc., USA).
RESULTS
Serology results
In 2008, 5014 ‘first samples’ from patients referred for the first time for Q fever diagnostics, were evaluated by IFA testing. In total, 720 samples tested positive for at least IgM phase II antibodies. Of these, 93 (1·9%) were solitary IgM phase II-positive sera. In 2009, 813/9524 first samples evaluated for acute Q fever diagnostics with an IgM phase II ELISA were positive, of which 86 (0·9%) were solitary phase II IgM positive. Two follow-up serum samples, one in both years, were taken within 10 days and were excluded. The mean time interval between the first and the second sample was 35·6 days and 43·1 days in 2008 and 2009, respectively. In 2008 a positive initial IgM phase II serum sample detected by IFA seronconverted for Q fever-specific antibodies in 60 of these 92 cases, resulting in a PPV of 65·2%. In 2009 overall – irrespective whether IFA confirmation was positive or negative – a seroconversion response to Q fever was observed in 43/85 ELISA samples, resulting in a PPV of 50·6% (Fig. 1). Difference of PPV between both tests was just significant (P=0·049).
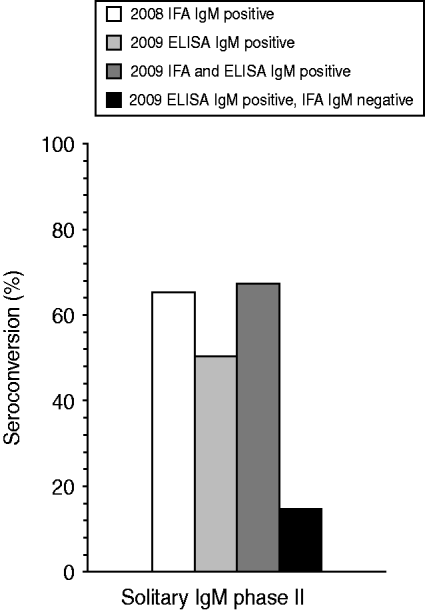
Fig. 1. Percentage of seroconversion to acute Q fever in 2008 and 2009 for serum samples being initially only IFA IgM phase II positive (n=92), ELISA IgM phase II positive (n=85), IFA and ELISA IgM phase II positive (n=58), or ELISA IgM phase II positive and IFA IgM phase II negative (n=27). A seroconversion for Q fever is defined as an initial sample being IgG phase II negative but positive in the follow-up sample.
A total of 27 initially included IgM phase II ELISA-positive samples tested negative in a subsequent IFA confirmation test. Four (15%) of these 27 samples seroconverted to Q fever. In 58 samples both a positive ELISA and IFA IgM phase II response was detected. In 39 (67%) of these 58 samples a follow-up sample resulted in seroconversion to Q fever (Fig. 1).
Patient characteristics
Included patients from 2008 and 2009 were comparable with respect to age, sex and mean time interval between the first and second blood sample. In 2008, 53 (58%) of the 92 patients included were male compared to 43 (51%) of 85 patients in 2009. Their mean age was 51 years (range 8–92 years) compared to a mean age of 49 years (range 5–85 years) in 2009.
DISCUSSION
In our study we evaluated seroconversion rates to acute Q fever to calculate a PPV, given a positive solitary IgM phase II blood sample. Overall, Q fever seroconversion rates in 2008 and 2009 were low at 65% and 51%, respectively. This suggests that isolated IgM phase II serology is not sufficient for diagnosing and notifying a confirmed case of Q fever. This relatively low seroconversion rate might be explained by cross-reactivity with sera from patients who are experiencing other infections as seen in other studies [Reference Field8, Reference Villumsen10].
We considered a cut-off value of ⩾1:32 for IFA in both phases I and II to be positive. This value is higher than that recommended by the manufacturer (⩾1:16). A higher cut-off value negatively effects the sensitivity of a test, but results in a higher PPV [Reference Villumsen10, Reference Dupont, Thirion and Raoult11]. Even with a cut-off value of 1:32 the PPV in 2008 and 2009 of a solitary positive IgM phase II result was low.
Differences found between IFA (2008) and ELISA (2009) IgM phase II seroconversion results may have been influenced by the introduction of PCR testing in 2009 for Q fever, and a decrease in diagnostic delay and raised awareness among clinicians and the general public about Q fever. In 2009 PCR was introduced as a screening assay for patients with a history of disease of <2 weeks. Evaluation of C. burnetii specific PCR during this early period of infection shows a superior diagnostic outcome in detecting C. burnetii infection compared to isolated IgM phase II serology [Reference Schneeberger5, Reference Turra12]. Therefore PCR is the preferred first step for early acute Q fever diagnosis. However, by the end of the first 2 weeks after onset of disease, both PCR and IgM phase II serology may both be positive [Reference Schneeberger5, Reference Fournier and Raoult13]. PCR was positive in 90% of cases of solitary IgM in patients that seroconverted and negative in all samples that did not lead to seroconversion [Reference Schneeberger5]. As the diagnostic delay on Q fever decreased during each consecutive year of the epidemic in The Netherlands from 2007 to 2009 [Reference Hoek14] this may have resulted in a lower recovery of solitary IgM phase II serology in 2009, which may have influenced the seroconversion rate. A raised awareness over the years of the epidemic among clinicians and the general public about Q fever may have resulted in more people consulting their general physician with aspecific influenza-like symptoms leading to more referrals of Q fever diagnostics in 2009 compared to 2008.
On the other hand, the study was conducted during a period when the incidence of cases was extremely high which improves the PPV of every test. In a standard diagnostic setting with low incidence of acute Q fever the proportion of aspecific isolated IgM compared to specific IgM is higher resulting in even lower PPVs. Differences in specificity of the two diagnostic tests also result in different PPVs. Both IgM phase II ELISA as well as the IFA are known to have some degree of false-positive test results [Reference Wegdam-Blans6, Reference Field8], in which ELISA is significantly less specific (our observations).
During a large-scale outbreak of Q fever – as experienced in The Netherlands during three consecutive years (2007–2009) – the laboratory diagnosis of acute Q fever is expected to have become less accurate because of high seroprevalence due to persisting IgM. After an acute Q fever infection IgM phase II serology can be positive for many months [Reference Dupont, Thirion and Raoult11, Reference Dupuis15]. Matters are complicated by the fact that about 60% of Q fever patients are asymptomatic and symptomatic patients have in general non-specific symptoms [Reference Maurin and Raoult1, Reference Raoult, Marrie and Mege2]. As a result, during an outbreak, it is very difficult to differentiate between acute Q fever and Q fever which was contracted months before based on IgM phase II serology alone.
Laboratory diagnosis is indispensable for a diagnosis of Q fever given its non-specific clinical presentation in patients. Correct diagnosis is important for the treatment and follow-up of patients, as well as for accurate surveillance of the disease. The latter is mandatory for Q fever in the European Union. A person with compatible clinical symptoms and a laboratory confirmation of solitary IgM phase II antibodies is usually considered a probable or confirmed Q fever case. The Centers for Disease Control and Prevention (CDC) classifies this as a probable Q fever case, whereas the case definition from the European Union (EU) and The Netherlands (i.e. National Institute of Public Health and the Environment) regards this as a confirmed Q fever case [16–Reference Forland18].
About 65% and 51% of solitary IgM phase II results could be confirmed in a follow-up serum sample in our study. The latter case definition may have resulted in a overestimation of notifiable Q fever cases by abouty 4–5%.
We conclude that isolated IgM phase II serology results are not sufficient for diagnosis and notification of a confirmed case of Q fever. We therefore recommend that for serological diagnosis of acute Q fever, apart from a positive IgM, a seroconversion of IgG should be recorded for confirmation, preferably in combination with compatible clinical symptoms.
ACKNOWLEDGEMENTS
We thank Jamie Meekelenkamp for providing additional information on the serological results.
DECLARATION OF INTEREST
None.




