INTRODUCTION
Cryptosporidium and Giardia are the most common causes of protozoan diarrhoea worldwide, and lead to significant morbidity and mortality in both the developing and developed world. Transmission is through the faecal–oral route following direct or indirect contact with the transmissive stages of the organism [Reference Caccio1]. Parasites, like Cryptosporidium and Giardia, can be transmitted from three sources: anthroponotic, zoonotic and sapronotic. Anthroponoses have an infectious human as source and inter-human transfer is typical. Zoonoses are diseases transmissible from animals to humans, in which inter-human transfer is uncommon. Sapronoses have an abiotic substrate as source [Reference Hubalek2].
Cryptosporidium parvum and Cryptosporidium hominis (previously known as C. parvum genotype 1: the human genotype) are the most commonly reported causes of human cryptosporidiosis. C. hominis appears to be a strictly human pathogen and is therefore subject to anthroponotic transmission. The reservoir of C. parvum includes all mammals, with cattle a major host. Zoonotic transmission is therefore considered a common transmission mode [Reference Hashim3–Reference Starkey5]. Giardia duodenalis (synonyms: Giardia lamblia, Giardia intestinalis) is the only subspecies of Giardia found in humans, and is also found in the majority of domestic and wild mammals [Reference Thompson, Olson, Olson and Wallis6]. There is extensive genetic variability within G. duodenalis. Genotypes A and B, now widely referred as assemblages A and B, are the only genotypes which include humans in their host range [Reference Thompson7].
The New Zealand environment contains large numbers of farm animals, especially sheep and cattle, and widespread use of surface water as a drinking-water source. Surface water is particularly vulnerable to contamination because it is difficult to protect the catchments from feral and domesticated animals, which are known to be reservoirs of C. parvum and G. duodenalis. For example, a recent study in Western Australia revealed that animal faecal samples from irrigation catchments were regularly contaminated with zoonotic G. duodenalis (30·7%) and zoonotic Cryptosporidium (13%) [Reference McCarthy8]. However, an earlier study in the same region could not identify sheep as a source of zoonotic Giardia and Cryptosporidium so the importance of these animal reservoirs remains unknown [Reference Ryan9]. Groundwater, particularly in shallow, unconfined aquifer, is also vulnerable to pollution from the land surface [10]. Therefore, it is probable that zoonotic reservoirs contribute to human infection in New Zealand, although the importance of such reservoirs has not yet been defined.
The aims of this study were to describe the epidemiology of the two most important protozoan diseases of humans in New Zealand to better understand their impact on public health and to gain insights into the probable sources and modes of transmission that could contribute to improved interventions.
METHODS
Data from the national notifiable disease surveillance system were analysed for the period 1997–2006. In New Zealand, cryptosporidiosis and giardiasis became legally notifiable by diagnosing medical practitioners in mid-1996 so the first 10 years of notification data were available. The case definition requires a clinically compatible illness with appropriate laboratory confirmation (detection of C. parvum oocysts in faeces or Giardia cysts, trophozoites or antigen in faeces). The Institute of Environmental Science and Research Ltd (ESR) collects these data under contract to the Ministry of Health. In addition, data on hospitalization (principal diagnosis) from 1996 to 2006 were obtained from the New Zealand Health Information Service (NZHIS), which is part of the Ministry of Health. These conditions were coded as a cause of hospital admission (giardiasis: ICD-9-CM code 007.1 and ICD-10-AM code A07.1 from 1999 onwards; cryptosporidiosis: ICD-9-CM code 136.8 and ICD-10-AM code A07.2 from 1999 onwards). We also reviewed published annual summaries of outbreaks [11].
To examine the potential role of environmental sources, notified and hospitalized cases were designated as urban or rural based on their home address. Statistics NZ classification which defines seven grades of rurality, on the basis of the population number and employment status. Three of the categories are urban and four are rural. The 2001 Census classified 85·7% of the population as ‘urban’, and 14·3% as ‘rural’.
The geographical distribution of cryptosporidiosis and giardiasis in New Zealand was mapped using ArcGIS at Territorial Authority (TA) level. To examine potential zoonotic transmission, farm animal density (total number of sheep, cattle, horses and deer per hectare of grassland) was determined for every TA and regressed to the disease rates. Farm animal data are collected by Statistics NZ using an Agriculture Production Census every 5 years. TA level is the smallest area unit in which agricultural data are provided.
The analyses were carried out using Epi-Info, SPSS and Stata. Rates were calculated using population data from the 2001 Census, as this was in the middle of the 10-year period of interest and therefore provided an appropriate denominator. Rates for ethnic groups (based on prioritized ethnicity [12]), sex and urban/rural areas were directly age-standardized to the age distribution of the New Zealand population in 2001 (using the age- standardizing method of Bray [Reference Bray13]). Rate ratios (RR) and 95% confidence intervals (CI) were calculated using Stata. Trends in notification and hospitalization rates over time were tested using χ2 test for trend.
RESULTS
Incidence and impact
The public health impact of cryptosporidiosis and giardiasis in New Zealand can be assessed using the incidence of notified cases in the community, hospitalizations, deaths and outbreaks (Table 1).
Table 1. Number of notifications, hospitalizations, fatalities and outbreaks for cryptosporidiosis and giardiasis, 1997–2006

* Assumes all hospitalized and outbreak cases were also notified, therefore the true value will be less.
Incidence and trends over time
The incidence of notified cryptosporidiosis did not show a consistent trend over the 10-year period (Fig. 1). Notifications rose to a peak of 1208 cases in 2001 followed by a decline in incidence over the next 5 years. Giardiasis notification numbers decreased significantly over the 10-year period from 1998 (χ2 test for trend, P<0·001) (Fig. 1).

Fig. 1. Number of notified cases of cryptosporidiosis (––▪––) and giardiasis (![]() ) by year, 1997–2006.
) by year, 1997–2006.
Geographic distribution
Rates of cryptosporidiosis and giardiasis varied markedly by geographical area (see online Appendix, Figs A1 and A2, for maps showing average annual rate per 100 000, by quintile, for all 73 TA). High rates for cryptosporidiosis were seen in the Central North Island and in rural parts of the South Island. For giardiasis, high rates were seen in rural areas around Auckland, in Hawke's Bay, around Wellington, in the Buller and Grey District, and in Queenstown-Lakes District.
The rate of cryptosporidiosis was 2·84 times higher in rural areas (50·68/100 000) than in urban areas (17·22/100 000). There was a dose–response relationship between rurality and rates of cryptosporidiosis: higher grades of rurality were associated with higher risks of Cryptosporidium infection (Fig. 2).

Fig. 2. Rate ratios for cryptosporidiosis (––▪––) and giardiasis (![]() ), by grade of rurality (main urban area=reference value), average for 1997–2006.
), by grade of rurality (main urban area=reference value), average for 1997–2006.
There was also a higher rate of giardiasis in rural areas (53·2/100 000) compared to urban areas (42·58/100 000), although the difference was not as pronounced as for cryptosporidiosis. People in rural areas had only a 1·23 times higher risk of giardiasis than people living in urban areas and there was no dose–response relationship with rurality (Fig. 2).
Age, sex, ethnicity, deprivation
The incidence of cryptosporidiosis was highest in infants and children aged 0–4 years (136·15/100 000) followed by children aged 5–9 years (43·47/100 000). Highest rates of giardiasis were also seen in infants and children aged 0–4 years (147·01/100 000), followed by adults aged 30–39 years (69·63/100 000). Rates for males and females were similar for both diseases. The incidence of cryptosporidiosis and giardiasis was highest in Europeans (Table 2).
Table 2. Cryptosporidiosis and giardiasis notification numbers and rates (average annual rate per 100 000 population), by season, rural–urban domicile, age group, sex, ethnicity, and deprivation level, 1997–2006

* Number is the average annual number rounded to the nearest integer.
† Rate is the average annual rate per 100 000 population, calculated with the Census population counts of 2001.
‡ RR, Rate ratio calculated in relation to reference value in bold; 95% CI, 95% confidence interval calculated based on 10-year period.
§ Rates for urban/rural distribution, sex and ethnic groups were directly age-standardized to the age distribution of the New Zealand population at the 2001 Census with confidence intervals calculated according to the methods used for age-standardized data [Reference Bray13, Reference Plummer and Parkin15].
¶ Age and deprivation distribution are based on Meshblock data, the other data are based on Census Area Unit (CAU) data.
n.a., Not applicable.
The rates of both cryptosporidiosis and giardiasis were inversely related to deprivation levels with highest rates in the least deprived areas. This area-based index assigns a deprivation level on a decile scale, based on census-derived measures. Deprivation index level 1 represents the least deprived population with index level 10 representing the most deprived decile of the population (Table 2) [Reference Salmond and Crampton14].
Urban rural distribution and seasonal patterns
Cryptosporidiosis showed marked seasonality with 55% of notified cases occurring over the spring period (September–November in New Zealand) and only 11% occurring in summer time (December–February) (RR 4·92, see Table 2). This consistent spring peak was predominantly seen in rural areas (see online Appendix, Fig. A3). A smaller late summer/early autumn (February–April) peak was also present in some years (1998, 1999, 2001), predominantly in urban areas. By contrast, giardiasis showed little seasonality with only a moderately elevated rate in autumn (RR 1·17) and a slightly lower incidence in spring (RR 0·91) (Table 2). There was no difference in seasonality between urban and rural areas (see online Appendix, Fig. A3).
Animal density
We regressed average annual rates of cryptosporidiosis and giardiasis per 100 000 population with farm animal density at TA level. Farm animal density was defined as the number of farm animals (sheep, cattle, horse and deer) per hectare of grassland. Farm animal data were obtained from Statistics New Zealand's Agricultural Production Census of 2002. Cryptosporidiosis showed a small positive correlation with farm animal density at the TA level (Fig. 3). Giardiasis notification rates showed no correlation with animal density.

Fig. 3. Cryptosporidiosis [▪; linear (–––); R 2=0·2323] and giardiasis [![]() ; linear (
; linear (![]() ); R 2=0·0795] notification rates (average annual cases per 100 000 for 1997–2006) correlated with farm animal density (beef, sheep, horses and deer per hectare in 2002) by territorial authority.
); R 2=0·0795] notification rates (average annual cases per 100 000 for 1997–2006) correlated with farm animal density (beef, sheep, horses and deer per hectare in 2002) by territorial authority.
Self-reported risk factors
The most commonly reported exposure for people notified with cryptosporidiosis was contact with farm animals (59·4%). Using untreated drinking water (38·7%) and attending school or childcare (43·4%) were also frequently reported risk factors. For giardiasis the most commonly reported exposures were using untreated drinking water (35·3%), contact with other symptomatic cases (34·9%), and recreational water (32·8%). Overseas travel during the incubation period appeared to make an important contribution to giardiasis risk (being reported by 19·1%), but little contribution to cryptosporidiosis (5·7%) (see online Appendix, Table A1, for a full list of self-reported risk factors and exposures for notified cases of these protozoan diseases).
DISCUSSION
This analysis of the descriptive epidemiology of cryptosporidiosis and giardiasis in New Zealand, based on the first 10 full years of notification data, shows that these diseases have important similarities and differences. Cryptosporidiosis distribution is consistent with animal reservoirs acting as an important source of infection (i.e. zoonotic). By contrast, the epidemiology of giardiasis suggests that most transmission originates from human sources (i.e. anthroponotic). These differences have important implications for prevention and control of these diseases.
Impact of cryptosporidiosis and giardiasis in New Zealand
Both cryptosporidiosis and giardiasis are relatively common diseases in New Zealand. The annual notification rate for cryptosporidiosis was 22·0/100 000 population and 44·1/100 000 population for giardiasis. The rates for both diseases were higher than those reported by other developed countries (Table 3).
Table 3. Rates of cryptosporidiosis and giardiasis reported in New Zealand and other developed countries, 2005
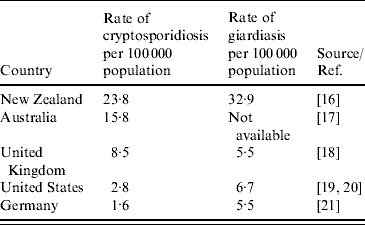
Both diseases have low numbers of hospitalizations and deaths. There was only one death attributed to cryptosporidiosis and two deaths due to giardiasis in 10 years. Less than 3·6% of the notified cryptosporidiosis cases and 1·7% of the giardiasis cases were hospitalized. However, the high notification rates of both diseases indicate that they are important health issues, infection may be particularly serious for some vulnerable subpopulations (e.g. elderly, immunocompromised people) and they have relatively high outbreak potential. Furthermore, both diseases result in high economic costs. Infectious intestinal diseases are estimated to cause up to 823 000 cases of illness per year in New Zealand [Reference Lake22] with total economic cost of about NZ$216 million in 2000 [Reference Scott23]. Cryptosporidium and Giardia result in about 2400 notified cases per year, which corresponds to economic costs of approximately NZ$1.5 million per year (using an estimated cost per case of $599, based on the average cost of a case of intestinal infectious disease of $462 in 2000 updated to 2008).
Similarities in the epidemiology of cryptosporidiosis and giardiasis in New Zealand
High rates of both protozoan diseases were seen in infants and young children (0–9 years). This age distribution is common to other developed countries [Reference Caccio1, Reference Meinhardt24] Young children are more susceptible to parasitic infections [Reference Fraser25, Reference O'Ryan26] and have more regular visits to a doctor, which may also increase the chance of being diagnosed and notified.
The high rates in Europeans for both diseases are surprising. Maori and Pacific people generally experience a higher incidence of infectious diseases in New Zealand [27]. Notification data also show that both diseases have an inverse relationship with deprivation level (Table 2). This finding is also unexpected as most infectious disease are associated with socioeconomic deprivation [Reference Castelli and Carosi28]. These findings may reflect poor access and use of primary health-care services by more deprived populations, which include a disproportionate number of Maori and Pacific people. This conclusion is supported by detailed analyses of hospitalization data which show higher admission rates for cryptosporidiosis and giardiasis for people living in more deprived areas [Reference Snel29, Reference Snel30]. Hospitalizations also showed less variation by ethnicity than was seen for notified cases [Reference Snel29, Reference Snel30].
Differences in the epidemiology of cryptosporidiosis and giardiasis
Cryptosporidiosis was strongly associated with living in rural areas, with a rate about 2·8 times higher than for urban populations. Cryptosporidiosis rates also showed a small but positive correlation with farm animal density. These observations provide evidence that farm animal reservoirs contribute to the high rates in rural areas in New Zealand (i.e. zoonotic disease). Other studies in New Zealand support this theory. C. parvum is dominant in rural areas and its main reservoir is in animals, especially cattle, suggesting zoonotic transmission [Reference Learmonth31, Reference Learmonth32].
The regions with the highest rates of cryptosporidiosis in the North Island have a concentrated dairy cattle farm industry, which would be consistent with cattle acting as an important source of human disease. However, the highest rates of cryptosporidiosis were found in the South Island, which is dominated by intensive sheep farming. This observation suggests that sheep may also be an important source of infection.
Giardiasis rates showed only a weak relationship with rurality and no correlation with animal density. Farm animals are not likely to be a major source of infection. The literature reports that Giardia is also present in farm animals, but the extent of transmission to humans remains unclear [Reference Ryan33–Reference Olson35]. It is reported that, in rural New Zealand, both domestic and wild animals provide a significant reservoir of G. intestinalis cysts in areas without substantial human activity [Reference Chilvers36].
Cryptosporidiosis in New Zealand showed a striking seasonal pattern, with the largest number of notifications occurring in spring. The breeding season of both cows and sheep is during early spring [Reference Garcia and Holmes37, Reference Barrell38]. During this period there is extended contact between humans (farmers) and (young) farm animals, which provides an opportunity for zoonotic transmission of C. parvum from young farm animals. It is known that only calves aged <2 months are major hosts for C. parvum. Less than 1% of post-weaned and adult dairy cows excrete C. parvum oocysts in their faeces [Reference Fayer39]. These observations all support the important role of young animals as a source of cryptosporidiosis.
By contrast, giardiasis showed little seasonality which might suggest only a small contribution from zoonotic transmission. However, a recent study showed that G. intestinalis can be carried by adult animals [Reference Trout34, Reference Trout40]. Therefore, G. intestinalis (including zoonotic subtype assemblage A) may be carried all year round in a proportion of adult farm and domestic animals, which could help explain the absence of seasonality in human disease.
Until 2001, there was also an autumn peak in cryptosporidiosis incidence in urban areas. This spatio-temporal pattern would be consistent with anthroponotic transmission of C. hominis through contaminated swimming pools. Another study in New Zealand reported that C. hominis is dominant during autumn, corresponding with the late part of the swimming season in this country [Reference Learmonth31, Reference Learmonth32]. A swimming pool was also identified as the source for a large cryptosporidiosis outbreak in late summer/early autumn in 1998 [Reference Baker41]. The publicity following that outbreak may have contributed to improved regulations and filtration systems in public swimming pools resulting in the subsequent disappearance of this ‘swimming pool’ peak after 2001.
Previous studies on giardiasis in New Zealand concluded that seasonal patterns are present, with a late summer/early autumn peak (March/April) [Reference Hoque42–Reference Hunt44]. There was only a slight seasonal pattern visible in our data, with a relatively small increased risk for giardiasis in autumn (RR 1·17). We speculate that this higher risk may have been caused by anthroponotic transmission related to outbreaks from swimming pools, as was also seen with cryptosporidiosis. Similarly, this peak largely disappeared after 2001 presumably as a consequence of improved management of swimming pools.
Giardiasis notifications showed high rates in people aged between 30 and 39 years. This age group is more likely to have contact with young children, as parents and/or caregivers, and therefore is more often involved with childcare and/or nappy handling. Contact with young children and nappies are known to be risk factors for giardiasis [Reference Hoque45, Reference Hoque46]. This finding supports anthroponotic transmission.
Our study found that notified cases of cryptosporidiosis and giardiasis reported somewhat different patterns of exposures prior to becoming ill. The relative importance of these exposures has been investigated in a case-case analysis reported elsewhere [Reference Wilson47]. That analysis (using campylobacteriosis notifications as the comparison group) found significantly elevated crude odds ratios for giardiasis and overseas travel, whereas cryptosporidiosis was associated with contact with farm animals and sick animals. Both diseases had elevated risks associated with contact with sick people, human faecal matter, consumption of untreated drinking water and recreational water [Reference Wilson47].
Implication for prevention and control
Prevention of cryptosporidiosis should focus on reducing transmission in the rural environment, particularly from farm animals to humans during spring. Such measures could include general advice about hand washing after contact with farm animals and contaminated environments. Children should receive special attention, because disease risk appears so concentrated in this group. Parents and caregivers should be targeted during the spring period when disease risk is highest. Spread through water also requires monitoring [Reference Hoxie48]. The New Zealand Ministry of Health should continue efforts to improve the quality of drinking-water supplies and operation of swimming pools to reduce the risk of cryptosporidiosis.
Prevention of giardiasis should focus on measures to reduce person-to-person transmission. Based on existing evidence, such measures should include continuing efforts to improve hand washing, nappy handling, and other hygiene measures [Reference Yoder and Beach20, Reference Hoque45]. Travel health advice relating to enteric infections may also be useful. As with cryptosporidiosis, continuing efforts to improve the quality of drinking-water supplies and operation of swimming pools are worthwhile strategies.
Limitations of this analysis
This analysis has the limitations associated with use of routinely collected surveillance data. Notifications represent a small proportion of the estimated total cases of acute gastrointestinal illnesses (AGI), including giardiasis and cryptosporidiosis, in the community. It is estimated that only 2·0% of AGI cases in New Zealand are eventually notified [Reference Lake49]. In addition, descriptive data on their own have limited ability to identify the probable sources of infection. However, they may provide an indication of the relative importance of some sources, particularly if the distribution of cases is markedly different from that expected.
Further research
There is considerable potential to extend the simple univariate analyses presented here. A multivariate model could, for example, help to identify the separate contribution of ethnicity and deprivation to the observed giardiasis and cryptosporidiosis rates. Spatial analysis could also investigate how these diseases were related to animal density, drinking-water quality and other exposures of interest at a much finer level of spatial resolution.
The higher rates of cryptosporidiosis in rural areas deserve further investigation. Such research could focus on identifying the modes of transmission in this setting, particularly the relative importance of direct contact with farm animals and contaminated environments, and the role of contaminated drinking water. Future research is also needed to identify sources of the high burden of giardiasis in New Zealand, particularly since this finding cannot be attributed to the high numbers of farm animals in this country. Work by Hoque et al. identified environmental and social risk factors for giardiasis in New Zealand, although the exact sources remain unclear [Reference Hoque46]. While nappy changing was associated with a fourfold increased risk for giardiasis this risk factor is unlikely to explain the high rates of infection in New Zealand [Reference Hoque45]. Further research on water supplies and water quality would also be useful to identify whether this source is important in New Zealand. Advances in molecular characterization of these parasites also have much to offer. For example, the use of a new generation of molecular diagnostic tools is likely to produce a more complete picture of zoonotic cryptosporidiosis [Reference Xiao and Feng50].
ACKNOWLEDGEMENTS
The Institute of Environmental Science and Research Ltd (ESR) supplied the notification data and the New Zealand Health Information Service supplied the hospitalization data. Jane Zhang extracted the hospitalization data and Simon Hales helped in the construction of the maps in ArcGIS.
NOTE
Supplementary material accompanies this paper on the Journal's website (http://journals.cambridge.org/hyg).
DECLARATION OF INTEREST
None.








