INTRODUCTION
Hepatitis B is an international health problem, with over 2 billion people estimated to have ever been infected globally [Reference Lavanchy1]. Following the development of sensitive and specific tests to detect hepatitis B virus (HBV), the natural history has been well defined. The clinical presentation of acute infection is age-dependent; being usually sub-clinical in neonates and children, but with features such as jaundice in 30–50% of adults [Reference Alter2]. Consequently, some newly acquired infections may go unnoticed and untested. Progression from acute to chronic HBV infection is also age-dependent [Reference Hyams3]. Chronic infection can lead to complications of severe liver disease. Reducing incidence by preventing transmission is thus a public health priority. Although a safe and effective vaccine has been available since the 1980s and universal immunization is recommended by the World Health Organisation [4], the UK, and northern Scandinavian countries have implemented a selective policy for immunization of groups who are at increased risk due to their lifestyle, occupation or other factors [Reference Salisbury and Ramsay5, Reference Zuckerman6]. Ongoing surveillance of acute HBV infection is vital to identifying any changes in transmission and incidence, and in determining whether this policy requires revision.
Hepatitis B prevalence and incidence varies globally [Reference Alter2]. In high-prevalence countries, which include many developing countries, perinatal or early childhood infection is most common [Reference Alter2]. In low-prevalence countries such as England, most acute infections occur in adults in key risk groups, including injecting drug users (IDUs) or men who have sex with men (MSM), and to a lesser extent, heterosexuals; perinatal infection is rare [Reference Mangtani, Heptonstall and Hall7–Reference Hahne9].
Identification and classification of acute infection relies upon laboratory testing, in conjunction with clinical history. In English laboratories, the first HBV test performed is usually hepatitis B surface antigen (HBsAg) [Reference Cooke, Main and Thursz10]. Most individuals newly identified as HBsAg positive are also tested for IgM class antibody to hepatitis B core (anti-HBc IgM) [11]. Acute infection is associated with a high level of anti-HBc IgM that declines over a few months but IgM is also often present at a low level in chronically infected individuals and can increase during ‘flares’ in liver inflammation [11–Reference Colloredo13]. Persistence of HBsAg beyond 6 months is generally considered to define chronic HBV infection [Reference Cooke, Main and Thursz10]. Specific population groups are routinely screened for HBsAg [14], these include pregnant women and blood donors [15–Reference Gunson18] although diagnostic and risk-group testing is also free and widely accessible in many healthcare settings.
Routine surveillance has been used to estimate the annual incidence of symptomatic hepatitis B during 1986–1996 (based on voluntary laboratory reports [Reference Ramsay19]) and in 2008, based on statutory and informal reports (from laboratories or clinicians) to local Health Protection Units (HPUs) [20]. However, in order to estimate the true incidence of HBV infection, adjustments are required for under-reporting and asymptomatic infection [Reference Hahne9]. Specific algorithms have been proposed [Reference Klompas21] to identify acute HBV infection using data held in medical records. The sentinel surveillance of hepatitis testing scheme was designed to collect information on the number and characteristics of people tested for markers of hepatitis B using an automated process [Reference Brant22, Reference Brant23]. We aimed to identify acute HBV infection using this centrally extracted laboratory data and therefore to provide more complete and consistent data for public health action.
This paper describes the characteristics of people being tested for anti-HBc IgM identified through sentinel surveillance, 2002–2008, and attempts to classify individuals with confirmed, probable or possible acute HBV infection. The incidence of acute HBV is estimated for 2005–2008.
METHODS
Data collection and handling
Data were collected from 23 sentinel laboratories (see Appendix) that participated during some or all of the study period; the number of laboratories participating has increased from eight in 2002. Some laboratories were able to provide retrospective data for the early period, but the most complete and consistent data comes from 19 laboratories in the period 2005–2008. The laboratories are collectively estimated to cover about 37% of the English population for primary HBV testing. These data are drawn from laboratories from all regions across England, and are therefore representative of national HBV testing. Demographic and testing data were extracted electronically from each laboratory information system (LIS) for people tested for hepatitis [Reference Brant22, Reference Brant23]. Names were replaced with a pseudo-anonymized code (soundex) [Reference Mortimer and Salathiel24]. De-duplication checks were run using (i) first name and surname (ii) soundex, date of birth and sex, within each laboratory.
Data on all individuals tested for anti-HBc IgM between 1 January 2002 and 31 December 2008 were extracted along with all reported hepatitis B-specific serological markers (surface antigen, e-antigen, antibody to e-antigen, total antibody to core antigen, DNA and genotype). Quality control samples were excluded. A unique patient code identified each individual; their first test was used as baseline and number of tests counted. Age at first test was calculated and grouped. As ethnicity was not available, Nam Pehchan software [Reference Cummins25] used patient name to identify South Asians (individuals with names originating in the Indian subcontinent). A free-text field (‘clinical details’) was reviewed to identify risk exposures and/or clinical features of acute HBV, such as jaundice. For primary diagnostic samples, region and service type were identified using the requesting clinician's address. Samples referred for confirmation from another laboratory (where only a proportion of diagnostic samples, mainly from positive individuals, were sent to the sentinel centre) were classed as reference tests. Catchment areas for each laboratory were determined using the postcode of the requesting clinic/practice/setting, excluding reference samples and summing the populations for those primary-care trusts which sent samples from at least 0·5% of the population per year.
Sentinel data were matched (using a combination of name, sex, date of birth, soundex and laboratory number) to routine surveillance data from two parallel systems to obtain additional information on risk exposures. The first system was the voluntary reporting of confirmed hepatitis B infections from laboratories in England [Reference Hahne9]. The second system was established in 2007 and involved collating data from the follow-up of cases of acute hepatitis B reported to local HPUs [20] (either as statutory notification by registered medical practitioners or informal reports from local laboratories or clinicians).
Classification of HBV infection
Three laboratories used quantitative anti-HBc IgM tests, and in these centres the consultant virologist defined the IgM levels used locally (and used for the classification) to determine the stage of infection, according to the test in use, manufacturer's instructions and local evaluation. Anti-HBc IgM and HBsAg results were reviewed for every sample and classified into nine categories (Table 1), beginning with quantitative anti-HBc IgM results first as these can more accurately determine stage of infection. For the remaining laboratories that used qualitative IgM, other markers were included where appropriate. For individuals who had more than one sample that was IgM positive, the overall status was classified hierarchically in the following order: confirmed acute, probable acute, possible acute, probable chronic.
Table 1. Initial classification of the patient based on first HBV test results
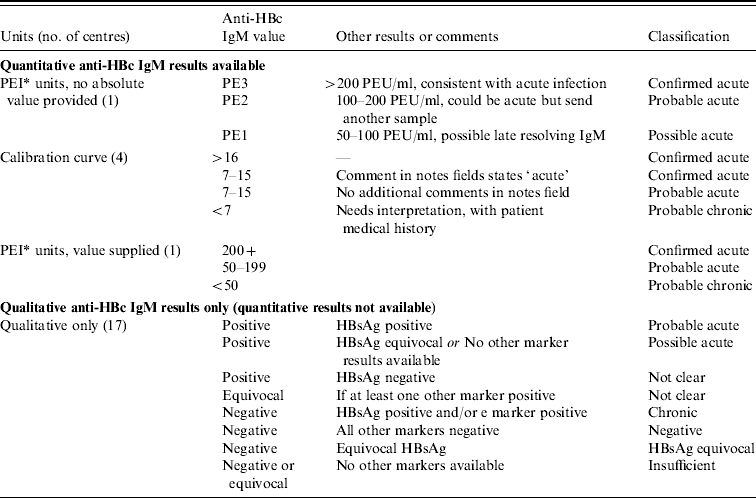
* Paul Ehrlich Institute units.
Exposure histories
Data on exposure risks were available from three sources. Questionnaires had been sent to the requesting clinician of all anti-HBc IgM positive individuals during the early period of surveillance at some sites (2002–2005) when between eight and 12 laboratories were participating prospectively. Additional information was also supplied routinely to all laboratories throughout the period, usually on the request form. Such information was gleaned from text entered onto the laboratory system in the sentinel sites, or added to routine reports to the national surveillance system. From 2007, risk factors collected on cases reported to the local HPU have been collated nationally to supplement the laboratory systems [20]. In the small number of cases where more than one risk group was available, data were coded hierarchically in line with previous routine surveillance [Reference Balogun8].
Validation of initial status classification
As a separate validation exercise, results for the subset of anti-HBc IgM-positive individuals who had more than one sample tested (classed as ‘repeat testers’), were extracted, reviewed and compared to the initial analysis. The time (in days) between first and last test was calculated and results of all markers were reviewed sequentially to look for evolving infections. For individuals where tests were more than 6 months apart and qualitative anti-HBc IgM was persistently positive, with no evolution of the other markers, the revised classification was ‘chronic’. Where one marker changed once (e.g. anti-HBc IgM positive to negative), the revised classification was increased by one category (e.g. from possible to probable acute). Where two or more markers changed (e.g. HBsAg and anti-HBc IgM or either of these plus the e-markers) the revised classification was confirmed acute.
Statistical analysis
The characteristics of the (i) population tested for anti-HBc IgM and (ii) individuals classed as acute (confirmed, probable or possible) were investigated using χ2 and t tests for age. Trends over time were examined for individuals first reported to the sentinel surveillance system between 2005 and 2008 from the 19 participating laboratories that took part over the whole of that time period. This time-frame was selected to provide the most complete data across the largest number of sites. Reference testing was excluded from this dataset. Statistical analysis was performed using Stata (StataCorp, USA) and incidence calculations in Microsoft Excel.
Individuals with a final classification of acute (confirmed, probable or possible) infection were assumed to be incident cases. Crude annual incidence/100 000 (2005–2008) was calculated by dividing the number of acute infections by the population (mid-2007 population estimates, single year of age and sex; Office for National Statistics). Incidence was then calculated separately for South Asian individuals, identified within the laboratory data using Nam Pehchan software and compared to census-derived mid-2007 South Asian population estimates. Only diagnostic samples were included in the incidence calculations because the underlying catchment population for reference samples could not be defined. No adjustment was necessary for under-reporting. Exact 95% confidence intervals (CIs) were calculated using Poisson distribution.
Ethical approval
Ethical approval was obtained from the Northern and Yorkshire Multi-Centre Research Ethics Committee (MREC1/3/76) and the Public Health Laboratory Service (PHLS) Ethics Committee (now Health Protection Agency). Additional ethical approval was obtained locally where centres requested. Local clinicians were informed of the study.
RESULTS
A total of 55 317 people (72 106 samples) were tested for anti-HBc IgM; 547 people were excluded because their samples originated outside England, leaving 54 770 people in the analysis (6924 of these had no HBsAg results, the majority of which (75·7%) were reference requests). Based on analysis of patients who had their first positive HBsAg test undertaken in the participating laboratories over that period, 82% (46 224/56 401) of individuals were also tested for IgM. As some of the remaining individuals may have been known to be chronically infected from before the period of surveillance or from another laboratory, this suggests that ascertainment of acute infection in England is very complete.
Testing for anti-HBcIgM
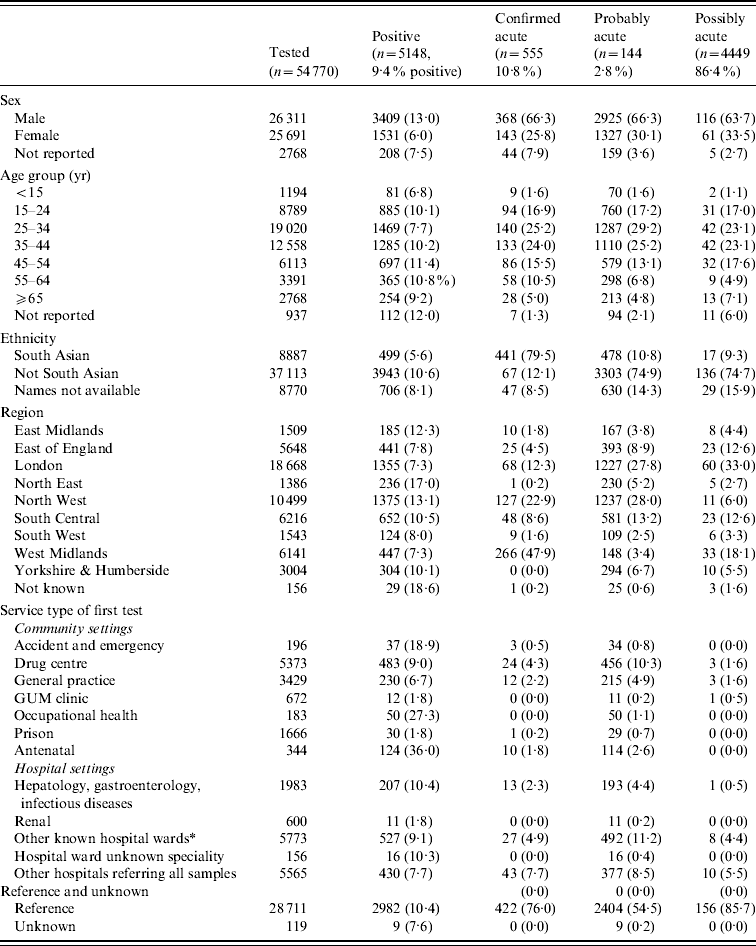
The characteristics of the people tested for anti-HBc IgM are described in Table 2. The male:female ratio was 1·02:1, except in the 15–24 years age group (0·79:1). The overall mean age was 36·6 years (s.d.=14·3, range 0–103·9); males were 4·5 years older on average than females (38·9 and 34·4 years, respectively, t test, P<0·001). Among primary diagnostic tests, almost equal numbers of people were tested in community vs. hospital settings; the largest requesters in community settings were GPs. The highest proportions of acute infections were identified in Accident and Emergency (A&E) departments and in prison services (36% and 27% were acute, respectively). Overall, anti-HBc IgM testing increased by 18% between 2005 and 2008; the increase was particularly marked in GP surgeries (30%), hospitals (26%) and genitor-urinary medicine (GUM) clinics (24%).
Table 2. Characteristics of the population tested and positive for anti-HBc IgM in sentinel laboratories, 2002–2008
GUM, Genito-urinary medicine.
* Other known hospital wards include: general medical/surgical, fertility treatment centres, paediatric, obstetrics/gynaecology, dermatology.
Acute infections
Overall, 5148 (9·4%) individuals were initially classified as having acute infections (Table 2): 555 ‘confirmed acute’, 4411 ‘probable acute’ and 116 ‘possible acute’. For a further 1607 individuals, the classification was not clear. The remainder of people tested were either probable chronic or chronic (n=42 095) or negative for all available markers (n=5920). Overall, 94% of individuals of South Asian origin were classified as having a chronic HBV infection (n=7687), compared to 88% of non-South Asians (n=28 027). The proportion of confirmed acute infections varied by region, with the West Midlands having the greatest proportion of acute infections identified by quantitative IgM testing.
There was no statistically significant difference in the mean age of those with an acute infection compared to those with a chronic infection by gender (male acute 39·0, chronic 38·5; female acute 34·6, chronic 33·9). However, a greater proportion of females aged 15–24 years were anti-HBc IgM-positive than males of the same age (25·7% vs. 13·3%, P<0·001). Except for reference samples, a similar number of acute infections were identified in community settings as hospitals (966 vs. 1191), with the highest number being identified by GPs.
Risk-factor data
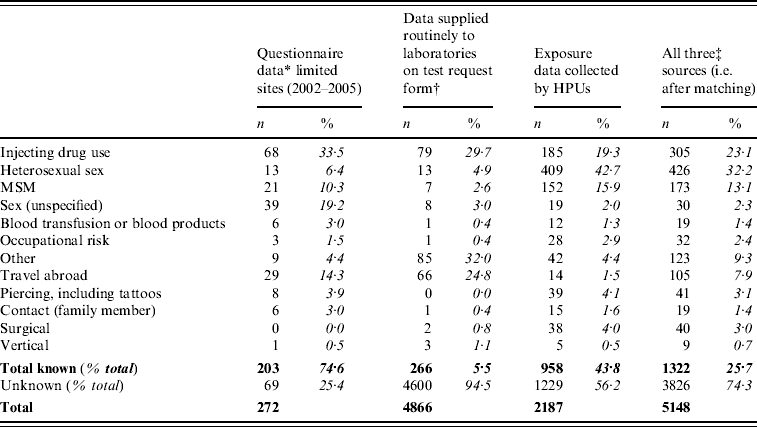
Questionnaires were received for 272 people with acute HBV infections from a subset of participating laboratories between 2002 and 2005. A risk exposure was reported for 75%; IDU was the main risk (Table 3). The reason for testing was reported as: liver disease (including jaundice or other features, 47·1%), abnormal liver function test results (21·7%), risk exposure(s) (19·1%), other (including antenatal, HIV-positive patients, 10·7%) and unknown (0·4%). Almost half the people classified with acute HBV were reported as having an acute hepatitis illness (48·5%); 21% were asymptomatic, but the majority of these had a known risk. Risk exposures could only be identified from routine laboratory data for an additional 6% of acutely infected individuals.
Table 3. Risk exposures reported for people with acute infections in England, 2002–2008 (percentage shown as of known/reported)
HPU, Health protection unit; MSM, men who have sex with men.
* Questionnaires were mailed to all positive individuals during the pilot and first phase of the study.
† Free-text clinical details that are entered from the test request form are extracted from the sentinel laboratory information system and classified as explained in the methods. Similar information is also obtained from laboratories contributing to the national voluntary system.
‡ Three sources: questionnaires, data supplied to laboratories, and data collected by HPUs.
Sixty percent (3075/5148) of people with acute infections were matched to a record in one or both routine surveillance databases and this increased the overall proportion with risk exposures to 26% (Table 3). The largest reported risk exposure was now heterosexual sex, closely followed by drug use.
Incidence of acute infection
Between 2005 and 2008, of the 19 centres who contributed data for the complete period 2005–2008, 615 acute infections were identified on average each year. However, there was an annual decline of 15% (Table 4). Excluding reference testing, 972 acute infections in people with known age group and sex were identified in primary diagnostic testing in these 19 laboratories (age and/or sex was missing for 37) (Table 4). Based on the initial classification, the crude annual incidence was therefore estimated to be 1·3/100 000 person-years (95% CI 1·1–1·5); 1·9 (95% CI 1·6–2·2) and 0·7 (95% CI 0·6–0·9) in males and females, respectively. Incidence was highest in males aged 30–34 years (4·61/100 000) (Fig. 1). Estimated incidence in individuals classified as South Asian (n=277) was 10·91/100 000 person-years (95% CI 9·23–12·81).
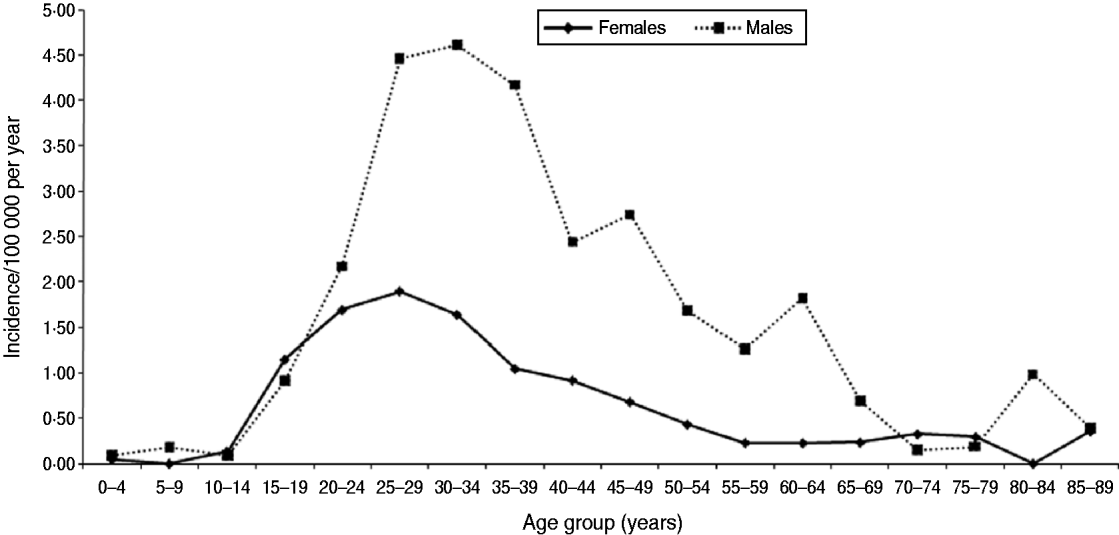
Fig. 1. Age group and sex-specific hepatitis B annual incidence in England (2005–2008).
Table 4. Acute infections identified by year and estimated annual incidence, in a subset of 19 laboratories supplying data for the entire period 2005–2008
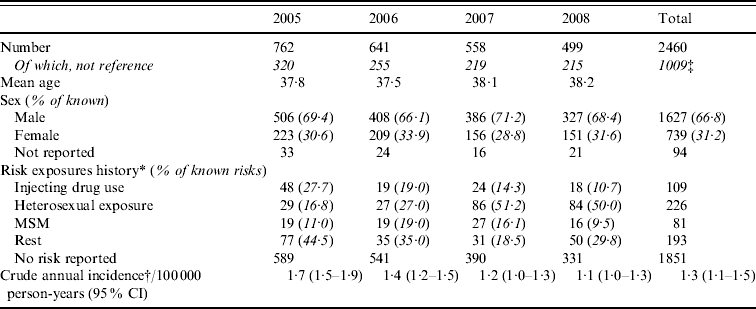
MSM, Men who have sex with men; CI, confidence interval.
* All three sources of risk exposure history combined (routine and sentinel for 2005–2006 and routine, sentinel and HPU data 2007–2008). Main risks only presented (see Table 3 for all categories).
† Reference tests excluded.
‡ Thirty-seven patients did not have a reported age group or sex.
Validation of initial status by review of individuals having multiple tests (repeat testers)
Between 2002 and 2008, 9163 (17%) patients were tested more than once for anti-HBc IgM; 6438 twice, 1556 three times, 563 four times and 606 more than five times. The mean number of days between anti-HBc IgM tests was 385 days (range 1–2504, median 157 days). Seventy-eight percent and 87% of individuals had an inter-test interval of less than 1 and 2 years, respectively. Of the 6755 patients initially classified as acute (n=5148) or those that could not be classified (n=1607) based on a single specimen, 1969 (29%) had been tested more than once (Table 5). Of those initially classified as acute, the majority (1375/1593, 86%) had a final classification of acute. Overall 217/1593 (14%) had results that were more consistent with chronic infection (where the markers remained constant over a 6-month period) but cases classified as possible acute were no more likely to be reclassified as chronic compared to cases initially classified as probable acute. An additional 166/376 (44%) infections initially classified as ‘not clear’ were consistent with acute infection.
Table 5. Results from the validation exercise of individuals with initial classification of acute infection or unclear, where tested more than once between 2002 and 2008
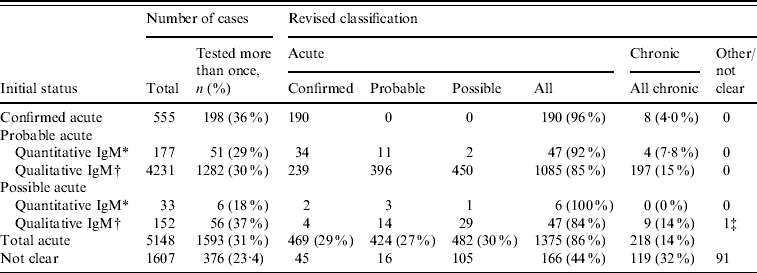
* Based solely on quantitative anti-HBc IgM test results.
† Based solely on qualitative anti-HBc IgM test results.
‡ False positive anti-HBc IgM result. All markers negative 14 days later.
DISCUSSION
Automatically extracted laboratory data can be used to enhance routine surveillance and provide consistent data on the incidence of hepatitis B. These analyses of sentinel laboratory surveillance show that the number of patients with acute hepatitis B infection is low and declined between 2005 and 2008. Routine testing for anti-HBc IgM is commonly performed but appears to be concentrated in a few large laboratories in England, as samples found to be HBsAg positive are referred to the sentinel laboratories from other local laboratories.
More males have acute HBV infection than females in England [Reference Balogun8, Reference Hahne9, Reference Polakoff and Tillett26], yet a similar number were tested for anti-HBc IgM, suggesting that males are at higher risk of acquiring infection. Sexual transmission in MSM has been recorded in Europe [Reference Hahne27, Reference Diel, Helle and Gottschalk28] and historically, a high proportion of acute infections in England had an IDU risk exposure [Reference Mangtani, Heptonstall and Hall7, Reference Balogun8, Reference Polakoff29], and IDUs are more likely to be male [Reference Hickman30]. Acute infection was most commonly detected in those tested in prisons, drug services and A&E departments.
Of cases with known risks, the proportion attributed to IDU or MSM exposure appeared to decline over the period. Heterosexual transmission is now the main recognized transmission route in England, although as exposure information was missing for 74% of acute cases, it is not clear whether this relative increase simply reflects improved ascertainment. In the early part of the study, data on risk factors were collected directly from clinicians and was more complete. It is important that individuals are asked about their risk exposures for HBV acquisition and that this information is passed to the testing laboratory to ensure that the correct tests are performed and appropriate interpretation is given. There is evidence that the surveillance system established in 2007, collating data from HPUs nationally, has improved the reporting of risk exposures.
One of the challenges in this study is that different assays were used in the sentinel laboratories. Although all laboratories participate in external and internal quality assurance schemes, inter-laboratory comparison of anti-HBc IgM levels determined in local laboratories does not occur. While some laboratories report results as ‘Paul Ehrlich Institute (PEI) units’, most do not calibrate their assays against the original Paul Ehrlich external standard, but rely upon the kit manufacturers' standards to generate an estimate of units of anti-HBc IgM. There is a need for a true international standard for anti-HBc IgM and for inter-laboratory comparisons (quality assurance) to standardize testing to provide more certainty of classification. Using serial samples helped to demonstrate the validity of the initial classification by enabling some differentiation between individuals with evolving acute infections and those who were chronically infected (persistent HBsAg); quantitative anti-HBc IgM data greatly enhanced this process, as changing levels could be identified. As this process was labour intensive and only possible retrospectively in under a third of cases, it is not suitable for routine ongoing surveillance. However, it does confirm that our initial classifications based on anti-HBc IgM are valid for monitoring trends in hepatitis B incidence.
Laboratory reports of acute infection have been used to estimate HBV incidence on many occasions [Reference Balogun8, Reference Hahne9, 31, Reference Ramsay, Rushdy and Harris32]. Our estimates suggest that HBV incidence fell between 2005 and 2008 and is at its lowest level in recent years. By using data directly from testing laboratories, under-reporting – a common problem with passive surveillance systems – was removed. Data are collected efficiently by automated extraction and information on the number of people tested for IgM provides reassurance about the completeness of ascertainment. The true rate of diagnosed acute infections may be slightly higher, as we were unable to classify all infections, or lower than presented, as the review of people tested more than once suggested that a small proportion of people classified as acute were probably chronically infected. The true incidence, however, is substantially higher because many individuals with acute hepatitis B are never tested, mainly because they are asymptomatic or have non-specific symptoms. Previous authors have adjusted for untested asymptomatic infections [Reference Hahne9], which more than doubled their age- and sex-specific incidence. Using the latter approach, the estimated incidence in this study would have been 4·8/100 000 overall (6·8 and 2·9/100 000 for males and females, respectively). This is still lower than Hahne's national estimates for the period 1995–2000 (7·4/100 000) [Reference Hahne9]. While the current study only covers one third of the population of England, it is clear that HBV incidence in England is among the lowest in the world [Reference Custer33] and that it has declined in recent years. This decline may be due to increased immunization of key risk groups, such as IDUs (e.g. through the prison immunization programme [Reference Hutchinson34–36]) or MSMs attending GUM clinics.
While country of birth was not available, our restricted ethnicity analyses indicated that in people with hepatitis B infections South Asians were more likely to be chronically infected than non-South Asians, replicating findings in other countries [Reference Robinson37, Reference Gjorup38]. However, the incidence in South Asians was also estimated to be almost ten times higher than in non-South Asians, suggesting that exposure to a higher prevalence population, including through travel to their country of origin, may increase the risk. This finding is in agreement with previous analyses [Reference Balogun8], that suggested higher incidence and different risk-factor profiles for this population. As with the previous analysis, the numerator is derived from name analysis, and the denominator from self-assigned ethnicity, but this is unlikely to have led to this degree of inflation of incidence Targeted awareness, testing and immunization of families with origins in higher-prevalence countries may be required. Efforts to reduce HBV incidence, through immunization, has led to a declining incidence in a number of countries [Reference Hong39–Reference Salleras41]. Better classification of ethnic status and/or country of birth would enhance the understanding of inequalities in acute hepatitis B in England.
The study shows that cases of acute HBV infection identified through automated laboratory extraction can supplement national surveillance to provide a more complete picture of ongoing acquisition of HBV in the population. This method could be adapted by other surveillance programmes to assist in the ascertainment and classification of acute HBV infection in their region. The study also emphasizes the requirement for both laboratories and clinicians to improve their investigation and diagnosis of possible acute hepatitis B. Quantification of HBsAg [Reference Gerlich, Glebe and Schuttler42], detection of anti-HBe seroconversion, and quantification of IgM against a standard can greatly improve the diagnostic accuracy and allow early classification of acute infection. The single most appropriate marker, that of anti-HBc avidity [Reference Rodella43], is not currently routinely available in England. Nevertheless, the availability of a full range of HBV markers and serial samples, collected through this sentinel surveillance scheme, has improved on the classification of HBV status provided through routine surveillance.
ACKNOWLEDGEMENTS
We thank all the staff in the testing laboratories, including the IT, medical and scientific staff who supported this study on an ongoing basis (see Appendix). We thank Dr Sam Lattimore for his comments, Darren Lyons for his administrative support at Leeds and Emily Tweed for her earlier work on the database.
The sentinel surveillance of the hepatitis testing study was funded by the English Department of Health (study reference AIDB 2/30) until September 2009.
APPENDIX: Sentinel surveillance study group members
(* indicates members who participated during the data period but are no longer active)
Hamid Jalal, Melanie Matthews, Rachael Smith* (Addenbrookes Hospital, Cambridge). Chas Ashley, Peter Muir (Bristol Regional HPA Laboratory). Rolf Meigh (Castle Hill Hospital, Cottingham, Hull). Mark Atkins, Mark Green, Lesley Mayoh (Chelsea and Westminster Hospital, London). John Croall (Chester Microbiology Laboratory, Countess of Chester Hospital, Chester). Tony Vicca (Diana Princess of Wales Hospital, Grimsby). Ferial Ahmad*, Imad Ibrahim* (Ealing Hospital, London, 2002–2003 only). Koye Balogun*, Lisa Brant, Sarah Collins, Mary Ramsay (Health Protection Agency, Centre for Infections, London). Samreen Ijaz, Siew Lin Ngui, Richard Tedder (Sexually Transmitted and Blood Borne virus laboratory, Health Protection Agency, Centre for Infections, London). Manoj Vallapil, Jeff Taylor*, Clive Taylor* (Health Protection Agency, Newcastle laboratory, Newcastle General Hospital, Newcastle). Elizabeth Boxall, Janet Mowbray (Health Protection Agency West Midlands laboratory, Heart of England Foundation Trust, Birmingham). David Lewis, Antony Hale (Leeds Teaching Hospitals NHS Trust, Leeds). Martin Hurrelle (Health Protection Agency, Leeds laboratory, Seacroft Hospital, Leeds). Ralph Henderson, David Johnson, Mark Zuckerman (King's College Hospital, London). Alan Blackley, Paul Klapper, Ken Mutton*, Keith Paver*, Andrew Turner* (Manchester Medical Microbiology Partnership, Manchester Royal Infirmary, Manchester). Shuja Shafi, Dilip Zala (Northwick Park Hospital, London). Will Irving, Lisa Prichett, Simon Pugh* (Queen's Medical Centre, Nottingham). Josephine Silles, Geoff Benge*, Bharat C. Patel* (HPA Collaborating centre, North Middlesex University Hospital, London). Louise Hesketh (Microbiology Laboratory, Royal Preston Hospital, Preston). Lynne Ashton, Ian Hart (Royal Liverpool Hospital, Liverpool). Tony Oliver, Adele Lee, Ines Ushiro-Lumb* (Barts and The London NHS Trust, London). Hasan Al-Ghusein, Phil Rice (St George's Hospital, London). Graham Hewitt, Gillian Underhill (St Mary's Hospital, Portsmouth). Mike Kidd, Peter Luton, Emma Aarons* (University College Hospital, London). Mark Baker, James Nash (William Harvey Hospital, Ashford, Kent).
DECLARATION OF INTEREST
None.








