Introduction
The international prevalence of psychotic disorders is approximately 0.75% [Reference Moreno-Küstner, Martín and Pastor1], and the life expectancy of people with psychosis is 10–15 years shorter than the general population [Reference Simon, Stewart, Yarborough, Lynch, Coleman and Beck2]. These illnesses are usually associated with poor quality of life and multi-morbidity [Reference Rodrigues, Wiener, Stranges, Ryan and Anderson3]. They also often lead to high societal costs, including direct costs for patients’ healthcare and costs related to productivity losses [Reference Winkler, Koeser, Kondrátová, Broulíková, Páv and Kališová4]. In low- and middle-income countries (LMICs) in Southeast Europe, an estimated 45% of patients with psychotic disorders have experienced a treatment gap (i.e., difference between the treatment they require and the treatment they receive) [5–7]. This is the result of shortages in funding and qualified staff, and a high patient load. Reducing the treatment gap in those countries through the implementation of effective and low-cost interventions is an urgent need.
DIALOG+ is an app-based psychosocial therapeutic intervention. Previously, interactions between psychotic patients and clinicians in routine face-to-face clinical meetings were guided solely by clinical judgment rather than evidence-based methods [Reference Priebe, Kelley, Omer, Golden, Walsh and Khanom8, Reference Priebe and McCabe9]. DIALOG+ was originally developed to make meetings therapeutically effective [Reference Priebe, Kelley, Omer, Golden, Walsh and Khanom8]. To do this, the intervention implements a structured self-assessment for patients during the meetings and provides guidance for clinicians on how to respond to patients’ ratings. Previous studies have shown that using DIALOG+ is effective in improving the quality of life for patients with psychosis in UK community-based settings [Reference Priebe, Kelley, Omer, Golden, Walsh and Khanom8, Reference Priebe, Golden, Kingdon, Omer, Walsh and Katevas10]. Furthermore, the effectiveness of DIALOG+ has been extensively studied in mental health care across multiple countries and in different healthcare settings [Reference Matanov, McNamee, Akther, Barber and Bird11–Reference Jovanović, Russo, Pemovska, Francis, Arenliu and Bajraktarov14].
As DIALOG+ is used in existing routine patient–clinician meetings, it does not require the formation of new services or hiring of new staff, and only requires that the existing service makes a one-off investment in computer tablets. The intervention can then be widely used by clinicians with minimal training, making it a good fit for healthcare systems with scarce resources [Reference Jovanovic, Francis, Maric, Arenliu, Barjaktarov and Kulenovic15]. Evidence from high-income settings suggests that DIALOG+ is a cost-saving intervention for people with mental disorders [Reference Priebe, Golden, Kingdon, Omer, Walsh and Katevas10]. The intervention also has the potential to deliver benefits for psychotic patients in low-resource settings. However, no study has previously evaluated its implementation in LMICs in Southeast Europe. A multi-country cluster randomised trial within the IMPULSE study was conducted to fill this empirical gap. The trial aimed to evaluate the effectiveness and cost-effectiveness of implementing DIALOG+ in five LMICs in Southeast Europe compared to standard care for patients with psychotic disorders [Reference Jovanovic, Francis, Maric, Arenliu, Barjaktarov and Kulenovic15].
The primary aim of this paper is to report the cost-effectiveness analyses of the DIALOG+ intervention versus standard care carried out in five Southeast European countries alongside the cluster randomised trial within the IMPULSE study.
Methods
Trial design
The cluster randomised trial within the IMPULSE study recruited participants from five Southeast European countries: Bosnia and Herzegovina, Kosovo (UN Resolution), Montenegro, North Macedonia, and Serbia. These countries shared similar socioeconomic and political backgrounds before the 1990s, which facilitated the trial setup and mutual learning across sites [Reference Jovanovic, Francis, Maric, Arenliu, Barjaktarov and Kulenovic15]. Eligible participants were identified through a review of medical records. Participants were eligible if they had: a primary diagnosis of psychosis or related disorder in remission with ICD-10 code F20-29 or F31; a lifetime history of being admitted to hospital at least once; a record of attending outpatient psychiatric services; and the capacity to provide written informed consent. Participants with diagnoses of organic brain disorders and/or severe cognitive deficits were excluded from the trial. Clinicians were randomised to either the intervention group (DIALOG+) or control group (standard care). Details about the trial methodology and implementation of the intervention can be found in the trial protocol [Reference Jovanovic, Francis, Maric, Arenliu, Barjaktarov and Kulenovic15]. The trial was launched in March 2019 and completed in July 2020.
DIALOG+ intervention and standard care
DIALOG+ intervention
DIALOG+ is a full therapeutic intervention which aims to make existing routine patient–clinician meetings therapeutically effective. The intervention is based on the quality of life research, and embeds the concepts of a patient-centered approach and solution-focused therapy in order to provide an evidence-based structure to routine clinical meetings between patients and clinicians. The intervention consists of two parts: (1) a patient self-rating exercise of satisfaction with their life and treatment, followed by (2) a four-step solution-focused discussion that aims to address the patients’ concerns and agree on further actions.
The trial was designed so that participants in the intervention group would receive six sessions of treatment during their routine outpatient consultations over a 12-month period. In accordance with the DIALOG+ manual [Reference Priebe, Golden, Katevas, Healey and McCabe16], each session lasted between 30 and 60 minutes. In the first 3 months, participants received one session per month, followed by one session every 3 months.
Every intervention session started with the patient self-rating their satisfaction with eight life domains (mental health, physical health, job satisfaction, accommodation, leisure activities, partner/family, friendships, personal safety) and three treatment domains (medication, practical help, meetings with clinician) using the DIALOG+ app installed in computer tablets. Next, clinicians were instructed to provide positive feedback to patients for any domain that was scored highly by patients and (from session two onwards) for domains with an improvement in rating from previous sessions. After the self-rating exercise, clinicians and patients identified a maximum of three domains for discussions. These discussions were guided by a four-step approach based on the principles of solution-focused therapy. Finally, the patients and clinicians jointly agreed on actions to improve the patients’ satisfaction with the discussed domain(s). At the beginning of the next session, they reviewed those actions together [Reference Omer, Golden and Priebe17]. Each clinician in the intervention group received face-to-face training by a local research team member before the first DIALOG+ session, followed by top-up training after delivering the third session. Clinicians were also able to access individual supervision provided by the study researchers after each session. A computer tablet with DIALOG+ installed was offered to each clinician prior to the first session.
Standard care
Standard care included consultations on medication, psychological support, and discussion with patients on other aspects of care. Participants receiving standard care were offered six sessions of treatment over the 12-month trial period following the same delivery schedule as participants in the intervention group.
Impact of the COVID-19 pandemic
Although the trial intervention was originally designed to last 12 months, interruption due to the COVID-19 pandemic from March 2020 onward led to significant changes in the intervention, patient assessments, data collection, and retention in the last stage of the trial [Reference Jovanović, Russo, Pemovska, Francis, Arenliu and Bajraktarov14]. Only Serbia completed the six sessions and the last assessment (at month 12) as per protocol before the introduction of local restrictions. The other four countries adapted the DIALOG+ manual, and delivered the last two sessions (fifth and sixth) and the last assessment remotely. Because of these changes, the effect of the complete intervention at 12 months (i.e., six sessions) could not be explored. Therefore, the economic evaluation was based on the first 6 months of trial data (first four sessions), starting from the implementation of the intervention at baseline.
Study measures
Outcome measures
Three instruments were used to assess the quality of life of participants, including the 5L version of the EQ-5D (EQ-5D-5L) [Reference Herdman, Gudex, Lloyd, Janssen, Kind and Parkin18], Manchester Short Assessment of Quality of Life (MANSA) [Reference Priebe, Huxley, Knight and Evans19], and the 10-item version of Recovering Quality of Life (ReQoL-10) [Reference Keetharuth, Brazier, Connell, Bjorner, Carlton and Taylor Buck20]. Due to the COVID-19 pandemic (see section title “Impact of the COVID-19 pandemic”), only data collected at baseline and 6 months after randomisation were used in the analysis.
The EQ-5D-5L measured the primary economic evaluation outcome. EQ-5D-5L data were converted to index scores by applying the EQ-5D-5L value set. There was no country-specific value set available for any of the five participating countries, so we applied the newly published EQ-5D-5L value set for Poland [Reference Golicki, Jakubczyk, Graczyk and Niewada21] in Central Europe as the best proxy available. Quality-adjusted life years (QALYs) for participants during the first 6-month period of the trial were calculated using the area-under-the-curve method and EQ-5D-5L index scores [Reference Matthews, Altman, Campbell and Royston22]. MANSA measured the primary clinical effectiveness outcome in the IMPULSE trial. MANSA scores were calculated as the mean of the instrument’s 12 individual item scores. ReQoL-10 is a new instrument for measuring the quality of life in people with mental health conditions. For ReQoL-10 data, simple sum scores on the instrument’s 10 questions were calculated.
For all three outcome measures, lower score indicates poorer quality of life. EQ-5D-5L index scores have a theoretical range between −0.590 and 1. The range is 1 to 7 for MANSA scores, and 0 to 40 for ReQoL-10 scores.
Costs data
The retrospective costs data 6 months prior to baseline and 6 months after randomisation were collected using an adapted version of the Client Service Receipt Inventory (CSRI) [Reference Beecham, Knapp and Thornicroft23]. The CSRI recorded participants’ use of inpatient hospital services, community care service, primary care service, and medication. We collected unit costs for each item from the local teams in the five participating countries. Data on participants’ socio-demographics, employment status, monthly income, number of days off from work due to mental and/or physical health issues, amount of state benefits claimed, and criminal records were also collected using the CSRI.
We developed a health economics inventory form to collect cost data for providing DIALOG+ and standard care treatments. Items included time spent by clinicians on the DIALOG+ training, time spent by clinicians and supporting staff on treatments, quantity of equipment and key materials used for providing treatments. We also collected the unit cost for each item using the inventory form.
We converted all unit costs from local currencies to euros at the year 2019 level with Purchasing Power Parity (EU28 = 1 as the reference base) adjusted [24]. Costs for each item were then calculated as a product of the quantity used and its corresponding unit cost. Finally, we summed all costs together and presented the cost data at participant and assessment time-point levels. There was no discount applied to adjust costs and outcomes data as the time horizon of the study was 6 months [25].
Outcome and cost measures used in the economic evaluation are validated scales, including EQ-5D-5L [Reference Herdman, Gudex, Lloyd, Janssen, Kind and Parkin18], MANSA [Reference Priebe, Huxley, Knight and Evans19], ReQoL-10 [Reference Keetharuth, Brazier, Connell, Bjorner, Carlton and Taylor Buck20] and CSRI [Reference Beecham, Knapp and Thornicroft23]. They were translated into the local languages by study researchers from central and local research teams before being administered to participants.
Economic evaluation
We compared participant-level costs and outcomes data between the two trial groups at each assessment time point (i.e., baseline and 6 months after randomisation). Independent t-tests were used for all comparisons. The 95% confidence intervals (CIs) were constructed using a bootstrap method with 1,000 replications. We also applied a three-level mixed-effects model to recognise the clustered nature of our data where participants nested within clinicians that nested within countries. The model controlled for baseline variables (i.e., costs or outcomes) and covariates (i.e., age of participants, ICD-10 code, and profession of clinicians).
We conducted the within-trial analyses from a healthcare perspective under the principle of intention-to-treat. Time horizon for the economic evaluation was 6 months, starting from the implementation of the intervention at baseline. This was consistent with the time horizon for the effectiveness evaluation of DIALOG+ in the IMPULSE trial [Reference Jovanović, Russo, Pemovska, Francis, Arenliu and Bajraktarov14].
Cost–utility analysis was used to conduct the base case economic evaluation. Costs included intervention costs, health service costs, and medication costs. The primary economic outcome measure used QALYs calculated from the EQ-5D-5L index scores. We estimated the incremental costs (and incremental QALYs) as the difference between the intervention and control groups over the first 6 months of the trial period, controlling for baseline values, participants’ ages, ICD-10 code, and profession of clinicians. A three-level mixed-effects model was applied. The pattern of missing values with three variables (i.e., costs at baseline, costs, and QALYs over the 6-month period) was assumed as missing at random. Multiple imputation with chained equations was applied to generate 70 imputed data sets (the largest fraction of missing information was 0.5258). The point estimate of the incremental cost-effectiveness ratio (ICER) was calculated by dividing the estimated incremental costs by the estimated incremental QALYs. To explore the uncertainty around the point estimate, we used the non-parametric bootstrap approach with 1,000 replications to estimate the 95% CI around the ICER [Reference Briggs, Wonderling and Mooney26]. The result was presented using a cost-effectiveness plane. We also constructed a cost-effectiveness acceptability curve to show the probability that DIALOG+ was cost-effective compared with standard care for a range of willingness-to-pay values for an additional QALY gained.
There is no evidence-based cost-effectiveness threshold to apply in multi-country trials for LMICs [Reference Woods, Revill, Sculpher and Claxton27]. The World Health Organization has recommended using one to three times the gross domestic product (GDP) per capita of an LMIC as the cost-effectiveness threshold for the country [Reference Bertram, Lauer, De Joncheere, Edejer, Hutubessy and Kieny28, Reference Oppong, Jowett and Roberts29]. An intervention with an estimated ICER of less than three times the national annual GDP per capita is considered cost-effective. In our base case evaluation, we compared our point estimate of the ICER against one to three times the weighted GPD per capita. The weights are proportions of participants from each country out of the total trial sample size.
To check the robustness of the findings from the base case evaluation, we conducted seven sensitivity analyses. First, we ran the base case analysis with complete cases only (i.e., without missing values). Second, the seemingly unrelated regression model without robust standard error was applied to compare the impact of the model choice [Reference Gomes, Ng, Grieve, Nixon, Carpenter and Thompson30]. Third, we estimated two ICERs using the minimum (and maximum) unit costs, respectively, for all medications from each country when unit costs for some medications were reported in a range. Fourth, we undertook analyses using a broader analytical perspective, including costs due to productivity lost as a result of mental or physical health problems. In the fifth and sixth sensitivity analyses, we replaced the outcome measure EQ-5D-5L index scores with MANSA scores and ReQoL-10 sum scores, respectively. Finally, we estimated country-specific ICERs by applying the method developed by Willke and colleagues [Reference Willke, Glick, Polsky and Schulman31].
Statistical significance was determined at the 5% level (p < 0.05). All analyses were performed with the software package STATA/MP 17 [Reference StataCorp32].
Results
Characteristics of the sample
We present the characteristics of all participants at baseline in Table 1. In total, 468 eligible participants were recruited, with 236 receiving the DIALOG+ treatment and 232 receiving standard care. There were 424 participants at 6 months after randomisation. The trial recruited 81 clinicians from 11 clinics across five countries. The average age of participants in the trial was 42.59 years old (standard deviation [SD] = 11.30). More than half of the participants were male (54.3%), single (54.3%), unemployed (59.7%), not receiving any state benefits (56.8%), and reported the highest level of education as high school (60.5%). Montenegro contributed the largest trial sample (n = 122, 26.1%), followed by Kosovo (UN Resolution; n = 103, 22%), North Macedonia (n = 82, 17.5%), Bosnia and Herzegovina (n = 81, 17.3%), and Serbia (n = 80, 17.1%).
Table 1. Baseline characteristics of participants by trial group for five participating countries.

a There is one observation missing in the DIALOG+ group with N = 235.
b There are two observations missing in the DIALOG+ group with N = 234 and four observations missing in the standard care group with N = 228.
Costs for DIALOG+ and standard care interventions
The average cost of delivering DIALOG+ for each participant was €91.11 during the 6-month trial period. The majority of this cost was for clinicians’ time, with €50.92 spent on delivering DIALOG+ and €14.69 on training. The cost also included key resource use (€17.66; computer tablets, fee for translating DIALOG+ manual to local language, room booking for DIALOG+ training), and other equipment use (€6.59; cell phones, recording devices, stationery). Costs from other staff that supported the delivery of DIALOG+ were minor at €1.24 per participant. The average total cost for delivering standard care sessions during the 6-month trial period was €20.87 per participant.
Resource use and costs
Table 2 presents the quantity of resource use at the participant level over the 6-month trial period, while Supplementary Appendix 1 reports the unit costs for each resource use item. Table 3 shows the average cost per participant for resource use over the 6-month trial period. The single most costly resource was medication. On average, the medication cost for participants in the intervention group was €237.23 per participant, while the average medication cost in the control was €243.35. The total cost in the intervention group was €565.95 per participant and €497.78 per participant in the control. The difference in total cost between the groups was €68.17 (95% CI –54.26, 168.60), but this was not statistically significant as suggested by an independent t-test. While controlling for the differences in total costs and the list of other covariates at baseline, the mixed-effects models produced qualitatively similar results. The difference in total cost was estimated as €98.42 (95% CI –29.49, 208.30), although this was not statistically significant.
Table 2. Mean resource use in quantities over the first 6 months of the trial by group.
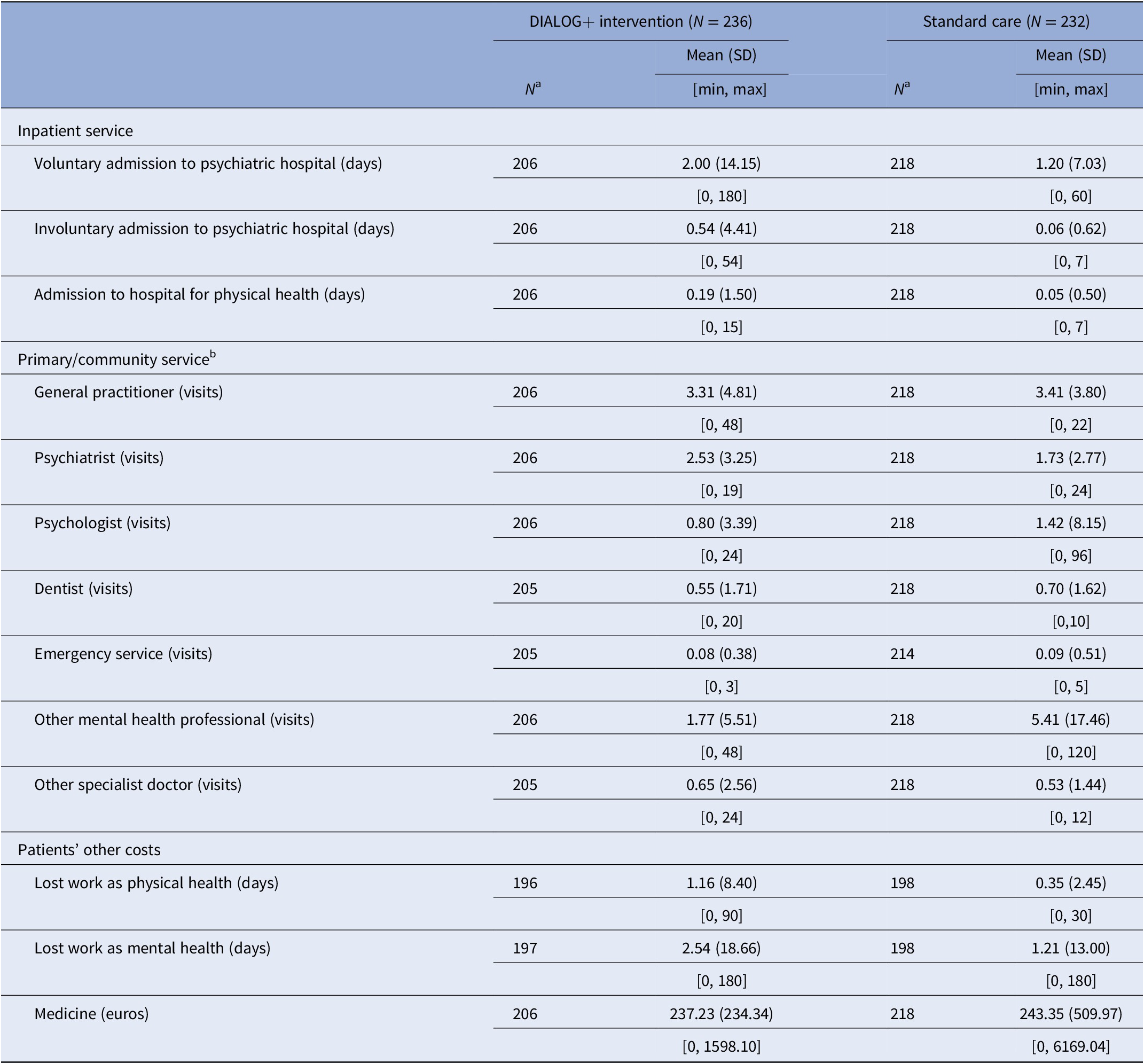
a N refers to the number of participants who responded to each question.
b Those contacts do not include care that participants received in the IMPULSE trial.
Table 3. Mean costs (euros) for resource use over the first 6 months of the trial by trial group with purchasing power parity adjusted.

a Independent t-tests are reported; 95% CI was produced using the bootstrapping method with 1,000 replications; * p < 0.05.
b Mixed-effects model with baseline cost and covariates (patients’ age, ICD code, and clinicians’ profession) controlled. 95% CI was produced using bootstrapping replication for 1,000 times with bias corrected. * p < 0.05.
c N refers to the number of participants who responded to each question.
d Those contacts do not include care that participants received in the IMPULSE trial.
We found differences between the two groups in costs for total resource use over 6 months before randomisation (Supplementary Appendix 2), and these differences were not statistically significant.
Outcome measures
Table 4 shows the participant level EQ-5D-5L index scores (and estimated QALYs), MANSA scores, and ReQoL-10 sum scores at each assessment time point (baseline and 6 months) by trial group (intervention and control). After adjusting for the baseline differences in EQ-5D-5L index scores and the list of covariates, the mixed-effect model resulted in a difference of 0.0035 QALYs (95% CI –0.0021, 0.0089) between the intervention and control groups over the 6-month period, a difference of 0.1810 points (95% CI 0.0315, 0.3158) for the MANSA, and a difference of 0.7237 points (95% CI –0.2798, 1.9375) for the ReQoL-10. All three outcome measures suggested a health improvement after 6 months of treatment with DIALOG+; however, only the difference in MANSA scores was statistically significant.
Table 4. Comparisons of EQ-5D-5L index scores, MANSA scores, and ReQoL-10 sum scores by trial group.

a Independent t-tests are reported; 95% CI was produced using the bootstrapping method with 1,000 replications; * p < 0.05.
b Mixed-effects model with baseline outcome measure and covariates (patients’ age, ICD code, and clinicians’ profession) controlled. 95% CI was produced using bootstrapping replication for 1,000 times with bias corrected. * p < 0.05.
c N refers to the number of participants who responded to each question.
d Formula used to calculate QALYs over 6 months: QALY = 0.25 X (index at baseline + index at 6 months).
Cost-effectiveness base case analysis
Table 5 reports results from the base case evaluation. Cost per QALY gained from implementing DIALOG+ was €26,347.61, achieved by dividing incremental costs of €84.17 (95% CI –8.18, 176.52) by incremental QALYs of 0.0032 (95% CI –0.0015, 0.0079). The weighted GDP per capita was €4,587, and three times this value was €13,761. Figure 1 shows the uncertainty around our point estimate of the ICER using a cost-effectiveness plane, including 1,000 pairs of incremental costs and incremental QALYs from bootstrap replications. Figure 2 presents the cost-effectiveness acceptability curve showing that the probability of DIALOG+ being cost-effective compared with standard care was 3.8% at a willingness-to-pay of €4,587 per QALY, and 18.9% at a willingness-to-pay of €13,761 per QALY. The base case analysis suggested that DIALOG+ was unlikely to be cost-effective.
Table 5. Cost-effectiveness analysis for point estimate of the ICER and sensitivity analyses.

a Measure for outcomes was ReQol-10 sum scores in sensitivity analysis 5 and MANSA scores in sensitivity analysis 6. Outcome measures for all other analyses in Table 5 used QALYs.
b For base case analysis and sensitivity analyses 1 to 4, GDP per capita was calculated as the weighted GDP per capita of the five participating countries. The weights were proportions of participants from each country out of the total trial sample size. The formula used was: (€4198.69 x 17.31 + €3036.39 x 22.01 + €4139.38 x 17.52 + €6123.57 x 26.07 + €5094.54 x 17.09)/100 = €4,587. Three times of the GDP per capita was therefore calculated using €4,587 x 3 = €13,761.
c For sensitivity analyses 7.1 to 7.5, GDP per capita was country-specific.
d For sensitivity analyses 7.1 to 7.5, we ran two regressions for each analysis including a structural cost regression and a QALY outcome regression. Country-perspective ICER was calculated using coefficients from three interactions in terms of the two regressions. We followed the method proposed by Willke et al. (1998).

Figure 1. Cost-effectiveness plane (1,000 iterations).

Figure 2. Cost-effectiveness acceptability curve.
Sensitivity analyses
Table 5 reports results from seven sensitivity analyses. The first four sensitivity analyses produced results consistent with the base case analysis: the point estimate of the ICER was above three times the weighted GDP per capita per QALY gained threshold. When ReQoL-10 sum scores were applied as the outcome measure, one score of improvement in ReQoL-10 was associated with additional costs of €119.02 (sensitivity analysis five). Analysis of MANSA scores suggested that an improvement of one score in MANSA was associated with additional costs of €523.53 (sensitivity analysis six). In sensitivity analysis seven, we attempted to estimate country-specific ICERs. DIALOG+ treatment was consistently found not to be cost-effective in four participating countries; Kosovo (UN Resolution) was the only country where the intervention was more effective and less costly than standard care.
Discussion
The main cost-effectiveness analysis suggested that DIALOG+ is slightly more costly and slightly more effective than standard care over the first 6 months of the trial period. The point estimate of the ICER was higher than the willingness-to-pay value at three times the weighted GDP per capita of the five participating countries. Regarding the uncertainty of this point estimate, our results suggested that the probability was low (18.9%) that DIALOG+ was cost-effective compared with standard care at the provider’s willingness-to-pay threshold. We conducted sensitivity analyses to explore the impact of missing values, estimation methods, key parameters for costs, and evaluation perspectives. None of these analyses challenged the main finding. In country-specific analyses, we found DIALOG+ was more effective and more costly in four of the five participating countries (and the point estimate of the ICER was not cost-effective). Kosovo (UN Resolution) alone showed DIALOG+ as more effective and less costly than standard care. This result should be interpreted with caution as the trial was not powered to detect country-specific treatment effects (in particular, for the EQ-5D-5L measure). Cost analyses shared similar limitations. Additionally, a few unit costs for resource use in Kosovo (UN Resolution) were proxied by the lowest unit price among the other four participating countries due to absence of an official local data source. Country-specific costs for total resource use per participant and outcomes by trial group is reported in Supplementary Appendices 3 and 4, respectively.
In this trial, we observed modest improvements of quality of life measured by three instruments. Only the difference in MANSA scores (i.e., the primary clinical effectiveness outcome in the IMPULSE trial) between the intervention and control groups was statistically significant [Reference Jovanović, Russo, Pemovska, Francis, Arenliu and Bajraktarov14]. The primary economic evaluation relied on QALYs derived from the EQ-5D-5L data as the outcome measure. It should be noted that the EQ-5D-5L has been criticized for its sensitivity regarding people with psychotic disorders and severe and complex nonpsychotic disorders [Reference Brazier33]. It has been argued that a condition-specific instrument might be more sensitive in reflecting changes in quality of life in these populations than a generic instrument like the EQ-5D-5L.
DIALOG+ has previously been applied in community care settings in the UK for patients with psychosis [Reference Priebe, Golden, Kingdon, Omer, Walsh and Katevas10]. The UK study found that the treatment was less costly than standard care, which was not in line with the results from our IMPULSE study. The study did not collect EQ-5D-5L data, which was one of the limitations reported by its authors. We, therefore, were unable to make a direct comparison between IMPULSE and the UK study of patients’ self-reported EQ-5D-5L and QALYs.
Evidence of cost-effectiveness analyses of treatments for severe mental illness in Southeast Europe is scarce [Reference Jovanovic, Francis, Maric, Arenliu, Barjaktarov and Kulenovic15]. Treatments are predominantly provided in large psychiatric hospitals with limited community-based alternatives. However, a recently published economic evaluation in the Czech Republic showed that it is cost-effective to discharge patients with chronic psychotic disorders to community care compared with care in psychiatric hospitals [Reference Winkler, Koeser, Kondrátová, Broulíková, Páv and Kališová4]. This finding supports one of the aims of introducing DIALOG+ in the LMIC settings, namely, to provide effective and cost-effective mental health treatment for psychotic patients through community-based services.
To our knowledge, this study reports the first cost-effectiveness evaluation of implementing (non-pharmacological) psychosocial treatments for people with psychosis in Southeast Europe. A strength of this study is the trial data that we collected. The challenges around data collection and lack of country-specific unit cost data in multi-country randomised controlled trials are well documented in the literature [Reference Oppong, Jowett and Roberts29]. It has widely been observed in economic evaluations of multi-country clinical trials that the analyses applied unit costs from one country to all participating countries due to lack of unit cost data from all individual countries [Reference Oppong, Jowett and Roberts29, Reference Reed, Anstrom, Bakhai, Briggs, Califf and Cohen34]. A concern with this approach is around the possibility of generating biased (over/under) estimates for costs. In the IMPULSE trial, we collected resource use and outcomes data at the patient level, as well as country-specific unit costs for each resource item used. This strategy for data collection enabled patient-level data analyses with multi-country costing.
This study has several limitations that should be considered. First, there were no country-specific value sets for the three outcome measures (EQ-5D-5L, MANSA, ReQoL-10). As we observed minimal improvements in QALYs for EQ-5D-5L data, the impact of value set choice on the estimated ICERs could, therefore, be very limited. We reported the results of cost-effectiveness analyses in this paper using ReQoL-10 and MANSA to enable comparisons with future research. Another consideration is around the generalizability of our findings. This issue is well documented for economic evaluations of multi-country randomised controlled trials [Reference Oppong, Jowett and Roberts29, Reference Sculpher, Pang, Manca, Drummond, Golder and Urdahl35]. We showed different results in cost analyses from the application of the DIALOG+ in the UK [Reference Priebe, Golden, Kingdon, Omer, Walsh and Katevas10]. Care should be taken when interpreting our findings to inform decision-making in a different context or/and for a different population. A final limitation of the study relates to the COVID-19 pandemic. The trial was designed to last 12 months, but only the first 6 months of data was interpretable due to disruptions in the study’s delivery relating to pandemic restrictions [Reference Jovanović, Russo, Pemovska, Francis, Arenliu and Bajraktarov14].
Future research might consider producing value sets or conducting mapping exercises to convert scores from MANSA and ReQoL instruments to health utilities in LMIC settings. Furthermore, we found limited research evidence on country-specific cost-effectiveness thresholds in LMICs [Reference Vončina, Strbad, Fürst, Dimitrova, Kamusheva and Vila36]. The empirical evidence and methodological research in this area are much needed. Finally, we did not find an agreed approach for estimating country-specific cost-effectiveness of an intervention in multi-country clinical trials. Additional research is required in this area in order to inform policy makers regarding resource allocation decisions at the country-specific level.
Conclusion
This paper reports an economic evaluation of the DIALOG+ intervention alongside the IMPULSE trial. Within the trial, DIALOG+ was shown to be more costly and also more effective for patients with psychosis compared with standard care. The probability of DIALOG+ being a cost-effective treatment at the willingness-to-pay threshold of three times the weighted GDP per capita of the five participating countries was low.
Trial Registration
ISRCTN11913964.
Ethical Approval
All procedures in the trial were approved by the following six ethics committees including Bosnia and Herzegovina (Klinicki Centar Univerziteta u Sarajevu – Eticki Komitet 03-02-4216, Eticki komitet JU Psihijatriska bolnica Kantona Sarajevo & JU Zavod za bolesti ovisnosti Kantona Sarajevo 02.8–408/19); Kosovo (UN Resolution) (Hospital and University Clinical Service of Kosovo – Ethics Committee 2019–85); Montenegro (Javna Zdravstvena Ustanova Klinicki Centar Crne Gore – Eticki komitet 03/01–29304/1, ZU Specijalna Bolnica za Psihijatriju ‘Dobrota’ Kotor – Eticki komitet, Eticki Komitet JZU Dom Zdravlja ‘DR Nika Labovic’ Berane 01–47); Republic of North Macedonia (Eticka Komisija za istrazuvanje na luge, Medicinski Fakultet pri UKIM vo Skopje 03–24219); Serbia (Eticka komisija Medicinskog fakulteta u Beogradu 2650/XII-20 and Eticka komisija Specijalne bolnice ‘Dr Slavoljub Bakalovic’ Vrsac 01–36/1); and the United Kingdom (Queen Mary University of London QMREC2204a, 16 October 2018).
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1192/j.eurpsy.2022.2310.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, YF, upon reasonable request.
Acknowledgments
We are thankful to all the patients and clinicians who participated in the IMPULSE trial. We are also grateful for the useful comments from participants in the IMPULSE trial meetings on the earlier versions of this manuscript.
Author Contributions
Conceptualization: Y.F., N.J.; Data curation: Y.F., C.R., M.R., S.R., A.D.K., L.I.S., J.K., S.M.-S., L.N., I.R., E.S.-M., F.U., M.Z., N.J.; Formal analysis: Y.F., C.R.; Methodology: Y.F.; Writing–original draft: Y.F.; Writing–review—editing: Y.F., C.R., M.R., S.R., A.D.K., L.I.S., J.K., S.M.-S., L.N., I.R., E.S.-M., F.U., M.Z., L.V., A.B., N.J.
Financial Support
The study is funded by the European Commission’s Horizon 2020 research and innovation program (grant agreement no. 779334). The opinions expressed in this paper are those of the authors, not of the funder.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
- CI
-
confidence interval
- CSRI
-
Client Service Receipt Inventory.
- EQ-5D-5L
-
The 5-level EQ-5D version.
- GDP
-
gross domestic product.
- ICD-10
-
International Classification of Diseases, Tenth Revision.
- ICER
-
incremental cost-effectiveness ratio.
- LMICs
-
low- and middle-income countries.
- IMPULSE
-
Implementation of an effective and cost-effective intervention for patients with psychotic disorders in low and middle-income countries in Southeast Europe.
- MANSA
-
Manchester Short Assessment of Quality of Life.
- QALY
-
Quality-adjusted life year.
- ReQoL-10
-
Recovering Quality of Life, a short 10-item version.










Comments
No Comments have been published for this article.