Introduction
Childhood maltreatment (CM), encompassing abuse (physical, emotional, sexual) and neglect (physical, emotional) [1], affects up to 60% of adults, with 30% experiencing at least moderate CM [Reference Schulz, Schmidt, Appel, Mahler, Spitzer and Wingenfeld2–Reference Klinger-König, Streit, Erhardt, Kleineidam, Schmiedek and Schmidt4]. In general, CM emerges as a prominent risk factor for physical and mental health problems during adulthood [Reference Bengtsson, Elsenburg, Andersen, Larsen, Rieckmann and Rod5–Reference Klinger-König, Berger, Grabe, Erhardt, Streit and Völker9].
CM has been linked to various medical conditions, including cardiovascular, respiratory, and liver diseases, cancer [Reference Klinger-König, Berger, Grabe, Erhardt, Streit and Völker9–Reference Kessler, McLaughlin, Green, Gruber, Sampson and Zaslavsky11], as well as mental health problems encompassing depression and anxiety disorders, addiction, borderline personality disorder, antisocial behavior, and alexithymic personality traits [Reference Klinger-König, Streit, Erhardt, Kleineidam, Schmiedek and Schmidt4, Reference Hovens, Wiersma, Giltay, van Oppen, Spinhoven and Penninx12–Reference Humphreys, LeMoult, Wear, Piersiak, Lee and Gotlib18].
The pathogenic role of CM in this broad range of mental and physical conditions might be mediated by an increased stress vulnerability [Reference Simon and Admon19], marked by a chronic hyperarousal of the sympathetic nervous system [Reference Murphy, Nasa, Cullinane, Raajakesary, Gazzaz and Sooknarine20]. This alteration of the autonomic nervous system (ANS) might impair the individual’s flexibility to adapt adequately to changing environmental demands [Reference Thayer and Brosschot21]. This chronic imbalance between the parasympathetic and sympathetic branches of the ANS can be investigated using vagal-sensitive metrics of heart rate variability (HRV) [Reference Malik22–Reference Smith, Thayer, Khalsa and Lane24], which is defined as the timely variance between consecutive heartbeats [Reference Beauchaine and Thayer25]. Interestingly, higher HRV is associated with better coping skills, including emotion regulation, impulse control [Reference Williams, Cash, Rankin, Bernardi, Koenig and Thayer26], and self-regulation [Reference Segerstrom and Nes27], while lower HRV is linked with short-term [Reference Castaldo, Melillo, Bracale, Caserta, Triassi and Pecchia28], chronic [Reference Lampert, Tuit, Hong, Donovan, Lee and Sinha29], and social stress [Reference Lischke, Jacksteit, Mau-Moeller, Pahnke, Hamm and Weippert30] as well as stress-associated mental disorders [Reference Koch, Wilhelm, Salzmann, Rief and Euteneuer31–Reference Chalmers, Quintana, Abbott and Kemp33].
While the association of HRV and mental disorders has been extensively studied [Reference Koch, Wilhelm, Salzmann, Rief and Euteneuer31–Reference Schneider and Schwerdtfeger34], the potential long-term effects of CM on ANS dysregulation have primarily been investigated in studies with small sample sizes (N < 100) or specific subgroups (e.g., only women), limiting the generalizability of these findings [Reference Bussone, Pesca, Casetti, Croce Nanni, Ottaviani and Troisi35–Reference Meyer, Müller, Schmidinger, Bohus, Herpertz and Bertsch39]. Notably, Bakema et al. [Reference Bakema, van Zuiden, Collard, Zantvoord, Rooij and Elsenburg40] found a negative association between HRV and cumulative exposure to CM in a large population-based cohort. However, this association disappeared after adjusting for socioeconomic and demographic covariates. Meta-analyses provide further insights into this relationship. Sigrist et al. [Reference Sigrist, Mürner-Lavanchy, Peschel, Schmidt, Kaess and Koenig41] found in their meta-analysis of 32 studies examining the effects of early life maltreatment on ANS dysregulation that the association varied as a function of age and the presence of psychopathology. In contrast, Wesarg et al. [Reference Wesarg, van den Akker, Oei, Wiers, Staaks and Thayer42], focusing on the association of childhood adversities and vagal regulation, found altered HRV specifically in individuals exposed to direct childhood adversities. This specificity may arise as adverse childhood experiences encompass a broader range of negative life events compared to CM [Reference Anda, Felitti, Bremner, Walker, Whitfield and Perry43], suggesting potential long-term ANS dysregulation uniquely associated with CM exposure. Moreover, as these heterogeneous findings may stem from the characteristics of the included samples [Reference Cumming44] or differences in the research construct, further investigation is needed to clarify the specific relationship between CM and HRV, including the potential influence of covariates.
Therefore, we aimed to investigate this association in a large population-based sample. However, HRV measurements with at least 5 minutes of rest, as recommended by guidelines [Reference Malik22, Reference Quigley, Gianaros, Norman, Jennings, Berntson and de45], are rarely applied in epidemiological studies. Nevertheless, 10-second electrocardiograms (ECG) are widely available in population-based studies, and their validity has been previously demonstrated [Reference Krause, Vollmer, Wittfeld, Weihs, Frenzel and Dörr46]. Based on this, we investigated the association of CM and HRV using baseline data from the TREND cohort of the Study of Health in Pomerania (SHIP-TREND-0). Our analyses accounted for age, sex, HRV-altering medications, body weight, socioeconomic factors, lifestyle, and current depressive symptoms, while additionally considering the recency of CM.
Methods
Study population
Data were used from SHIP-TREND-0 (2008–2012; N = 4,420), the baseline from a population-based cohort study in northeastern Germany [Reference Völzke, Schössow, Schmidt, Jürgens, Richter and Werner47]. Participants were randomly selected from local registries in northeastern Mecklenburg-Western Pomerania, with 99.5% (n = 4,396) undergoing a standardized cardiological examination (10-second 12-lead ECG). Of these, 1,264 participants (28.6% of the cohort) underwent overnight polysomnography (PSG).
The study, which was conducted by trained staff, adhered to the principles of the Declaration of Helsinki, with written informed consent and approval from the Institutional Review Board of the University of Greifswald, Germany.
Data assessment
Participants underwent a standardized computer-assisted personal interview to assess sociodemographic factors, lifestyle, and medical history, followed by subsequent physical examinations, including anthropometric markers. Medication intake of the past week was registered and categorized according to the Anatomical Therapeutic Chemical (ATC) classification [48].
Socioeconomic factors encompassed education level and equivalized disposable income. The level of education was measured by considering the number of years of schooling and vocational training. The equivalized household income was quantified by dividing the household income (nine categories, ranging from 333€ to 5,067€) by the square root of the number of household members.
Lifestyle factors encompassed smoking status, alcohol consumption, and physical activity. Smoking status was categorized as never smoker, former smoker, or current smoker. Average alcohol consumption in grams per day (g/d) during the last 30 days was estimated based on self-reports of consumed alcoholic beverages. Regular physical activity for at least 1 hour per week throughout the year (no/yes) was determined by self-reported physical activity during summer and winter (four categories: none, less than 1 hour, 1–2 hours, more than 2 hours per week).
Electrocardiography
Cardiological examination
A 10-second 12-lead ECG was recorded using a CardioPerfect ECG system (Welch Allyn Inc., Auburn, NY, USA) with a sampling rate of 500 Hz, following standard clinical procedures [Reference Kligfield, Gettes, Bailey, Childers, Deal and Hancock49]. The examiner tried to establish a calm and relaxed atmosphere, and participants rested in a supine position in a quiet room (temperature 23 ± 2 °C). Optimal signal quality was visually checked and, if necessary, optimized.
Polysomnography
A single-night PSG was conducted as outlined by Stubbe et al. [Reference Stubbe, Penzel, Fietze, Obst, Garcia and Zimmermann50], according to the American Academy of Sleep Medicine standards [Reference Iber, Ancoli-Israel, Chesson and Quand51] using ALICE 5 PSG devices (Philips Respironics, Eindhoven, The Netherlands). Electrodes were placed carefully, and, among others, a one-channel ECG (Einthoven II) was recorded with a sampling rate of 200 Hz. All signals were checked and, if necessary, optimized. Bedtime and wake-up time were individually chosen by the participants, while a total bedtime of 8 hours was requested.
Questionnaires
Childhood trauma questionnaire
CM was assessed using the Childhood Trauma Questionnaire (CTQ [Reference Bernstein, Stein, Newcomb, Walker, Pogge and Ahluvalia52]). According to the manual, exposure to CM is defined by reporting at least one moderate exposure in any subtype of CM. In addition, childhood neglect is defined as individuals encountering at least one moderate exposure to physical or emotional neglect, while childhood abuse is characterized by at least one moderate exposure to emotional, physical, or sexual abuse.
PHQ-9
Current depressive symptoms were assessed using the PHQ-9 questionnaire [Reference Kroenke, Spitzer and Williams53, Reference Gräfe, Zipfel, Herzog and Löwe54]. For further details of the used questionnaires, see the supplement.
Data preparation
Heart rate variability
R-wave detection and calculation of HRV parameters, including the Root Mean Square of Successive Differences (RMSSD), Standard Deviation of the NN Intervals (SDNN), and low- (LF-HRV) and high-frequency HRV (HF-HRV), were performed using the HRVTool toolbox [Reference Vollmer55] with an in-house script, as outlined by Krause et al. [Reference Krause, Vollmer, Wittfeld, Weihs, Frenzel and Dörr46]. Following R-wave detection, a lead-specific template of the QRS complex was generated for each subject to validate detected R-peaks, helping to exclude ectopic beats and false detections (e.g., movement artifacts). Then, a filter was applied to remove implausible RR intervals. For multichannel ECGs, R-peaks detected on each lead were cross-validated across channels (further details in the supplement).
Three different HRV values of RMSSD were derived from the ECG sources. For all participants, ultra-short heart-rate variability (usHRV; i.e., HRV derived from less than 5 minutes) was obtained from 10-second ECG recordings. HRV was derived from a 5-minute segment of rest before sleep for those participating in PSG (see the Supplement for details). To minimize situation-specific variance, and as the HRV measurements were not directly recorded according to guidelines [Reference Malik22, Reference Quigley, Gianaros, Norman, Jennings, Berntson and de45], the obtained HRV values were averaged to yield a more trait-like HRV [Reference Laborde, Mosley and Thayer56, Reference Bertsch, Hagemann, Naumann, Schächinger and Schulz57] for conducting sensitivity analyses to validate and support the statistical findings using usHRV. Additionally, SDNN, LF-HRV, and HF-HRV were derived only from the 5-minute ECG, as their validity obtained from a 10-second ECG is questionable [Reference Baek, Cho, Cho and Woo58, Reference Munoz, van Roon, Riese, Thio, Oostenbroek and Westrik59].
Samples
ECG data from SHIP-TREND-0 (N = 4,420) included 10-second ECGs for most participants (n = 4,396). PSG data were available for 1,264 participants. Individuals with specific heart problems (i.e., heart attack, pacemaker, atrial fibrillation, and heart surgery); stroke history; or pregnant women were excluded. Thus, two final samples were analyzed: a complete sample (n = 3,438) with one 10-second ECG recording and a PSG-subsample (n = 797) with both a 10-second ECG from the cardiological examination and a 5-minute ECG of wakeful rest from PSG (flowchart in Figure 1).
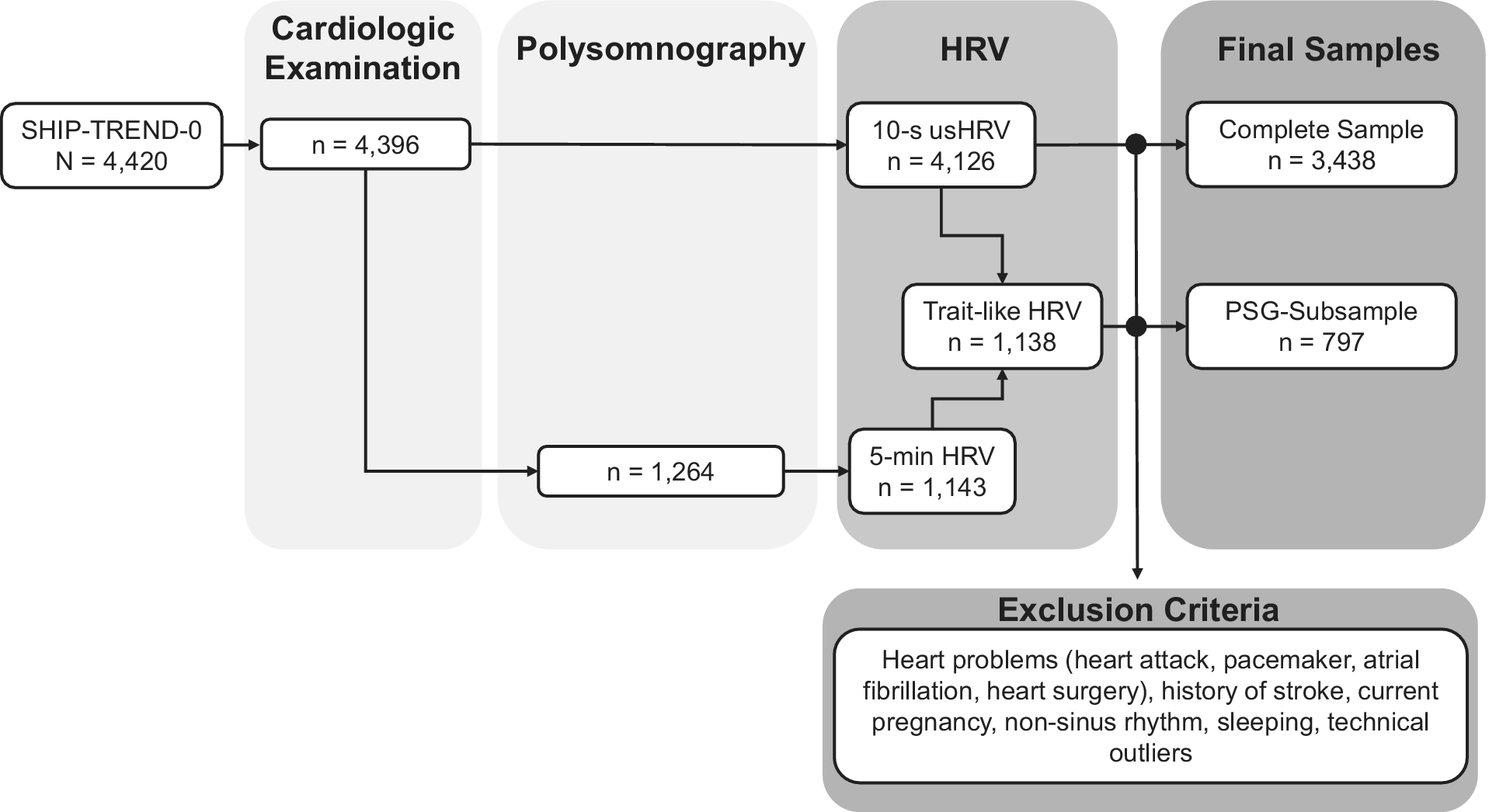
Figure 1. Flowchart of the samples and generated HRV parameters based on the ECG recordings in SHIP-TREND-0.
Statistical analysis
Multivariable linear regression models were used to estimate the effects of CM (any CM, neglect, and abuse) on HRV (logRMSSD) in the complete sample (usHRV) and the PSG-subsample (logRMSSD: HRV, usHRV, and trait-like HRV). The base model included age (nonlinear, three cubic splines – knots at the 10th, 50th, and 90th percentiles), sex, and their interaction as covariates. HRV declines with age [Reference Schumann and Bär60], while its association with RMSSD remains inconsistent [Reference Koenig, Kemp, Beauchaine, Thayer and Kaess61]. Antihypertensive (ATC codes: C02, C03, C07, C08, C09) and psychopharmacological medications (clozapine: N05AH02; tricyclics: N06AA, N06AX14), which may alter HRV [Reference Kemp, Quintana, Gray, Felmingham, Brown and Gatt32, Reference Agelink, Majewski, Wurthmann, Lukas, Ullrich and Linka62, Reference Maciorowska, Krzesiński, Wierzbowski, Uziębło-Życzkowska and Gielerak63], were added as covariates in the base model. Daytime was added as a covariate in analyses using usHRV or HRV.
We explored whether the effects of CM on HRV were mediated by body weight, lifestyle, socioeconomic factors, and current depressive symptoms. Body weight was assessed using the body mass index (BMI), encompassing underweight to overweight. Lifestyle factors included smoking, physical activity, and alcohol consumption interacted with sex. Socioeconomic factors included education level and equivalized disposable income. Current depressive symptoms were assessed using the log-transformed PHQ-9 sum score, exploring its influence on the relationship between CM and HRV, given the link between CM and increased depression risk and severity [Reference Klinger-König, Streit, Erhardt, Kleineidam, Schmiedek and Schmidt4, Reference Klinger-König, Berger, Grabe, Erhardt, Streit and Völker9, Reference Humphreys, LeMoult, Wear, Piersiak, Lee and Gotlib18]. All continuous variables, except age, were modeled linearly.
RMSSD and the PHQ-9 sum score were log-transformed to reduce skewness. Regression coefficients were considered significant at p < .05, while both p-values from the post hoc analyses of neglect and abuse were FDR-corrected [Reference Benjamini and Yekutieli64]. All analyses were additionally conducted for log-transformed SDNN, HF-HRV, and LF-HRV in the PSG-subsample based on available 5-minute ECG recordings.
All models for logRMSSD were refitted with two modifications: (1) interaction term between CM and age to explore how HRV changes with CM recency, indicated by timing since childhood, and (2) heart rate (HR) as an outcome to investigate if effects were specific to HRV.
All statistical analyses were conducted in R (4.2.1) [65] and using the rms package [Reference Harrell66]. A significance level of p < .05 was used for all analyses, and two-tailed tests were applied. Sample sizes varied between models. Continuous variables were z-transformed within each model; thus, the reported regression coefficients are standardized. Visualizations are conditioned on median (continuous) and mode (categorical) values.
Results
Characteristics of the samples
The complete sample encompassed 3,438 participants, with an average age of 49.7 years (SD = 14.8), and 54.7% were women (see Table 1 for details; Supplementary Table S1 for the sex-stratified sample in the supplement). The PSG-subsample (N = 797 participants, 52.1% women) was largely comparable. However, the mean age was slightly higher (M[SD] = 51.7[13.4]), as were the BMI, educational status, equivalized disposable income, PHQ-9 sum score, and intake of antihypertensives. In contrast, more participants in the complete sample were currently smoking, were less physically active, and had a slightly higher resting HR.
Table 1. Sample characteristics
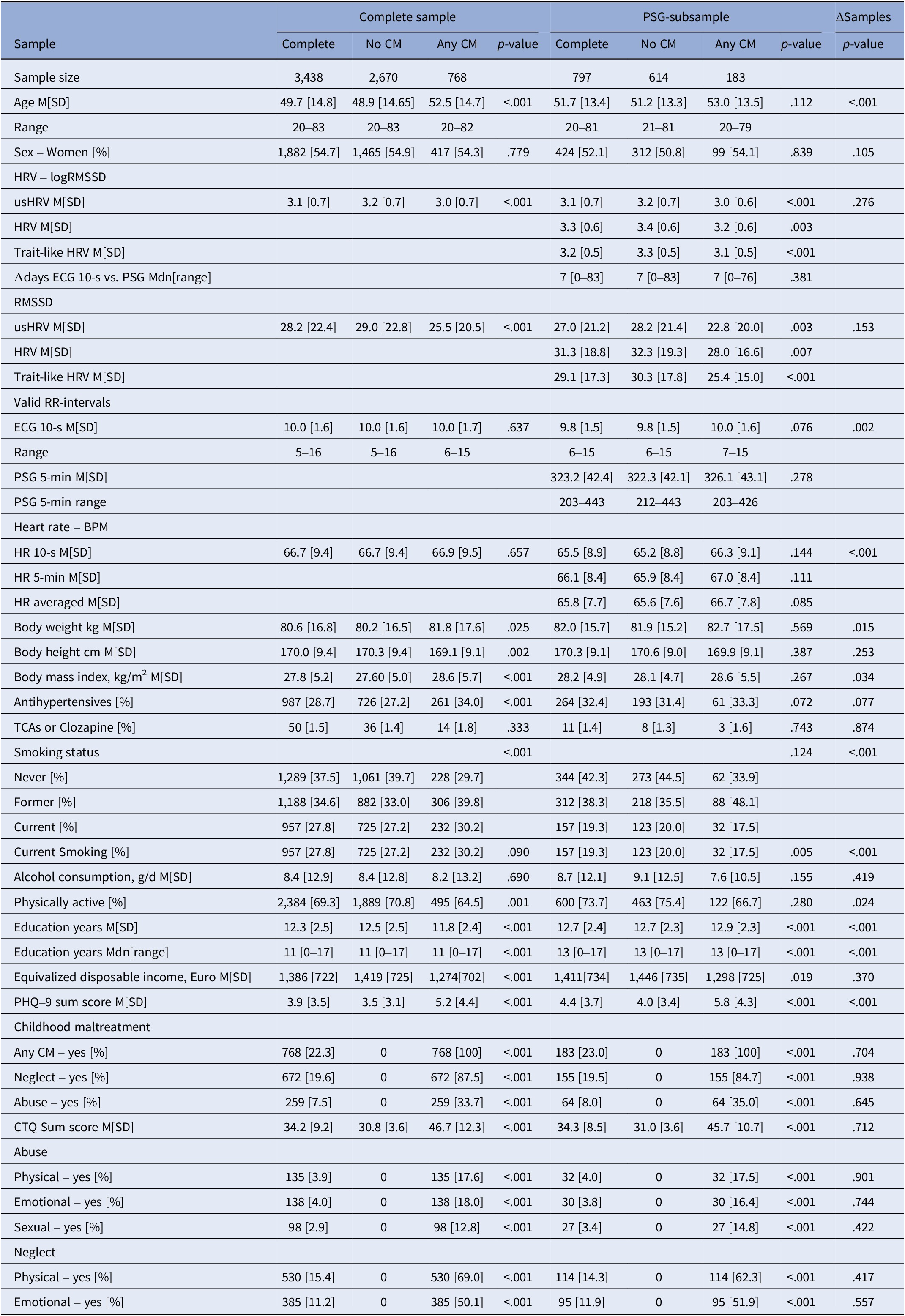
Abbreviations: BPM, beats per minute; CM, Childhood Maltreatment; HR, heart rate; HRV, heart rate variability derived from polysomnography before falling asleep (5 min); logRMSSD, logarithmized root mean square of successive differences between heartbeats; M, mean; Mdn, median; PHQ-9, Patient health questionnaire; TCAs, tricyclic antidepressants; trait-like HRV, averaged usHRV and HRV; usHRV, HRV derived from 10-second ECG.
In total, 22.3% of the participants reported any CM, whereas neglect was more prevalent (19.6%) than abuse (7.5%). These characteristics were similar in the PSG-subsample (any CM 23.0%; neglect 19.5%; abuse 8.0%).
Individuals exposed to CM had lower usHRV across both samples, as well as lower HRV and trait-like HRV in the PSG-subsample, with no differences in HR. In the complete sample, participants reporting CM were older, more obese, used antihypertensive medication more often, were less physically active, had fewer years of education, lower equivalized disposable income, and more current depressive symptoms. In the PSG-subsample, these trends were similar but mostly nonsignificant.
Association of HRV and CM
Overall, exposure to CM was found to be associated with lower HRV in the complete sample (usHRV: β = −0.20 [95%-CI: −0.28, −0.12], p = 1.2e-06) and the PSG-subsample (trait-like HRV: β = −0.33 [95%-CI: −0.49, −0.16], p = 1.1e-04; Table 2; Supplementary Table S2 for results of usHRV and HRV of the PSG-subsample).
Table 2. Association of childhood maltreatment with heart rate variability
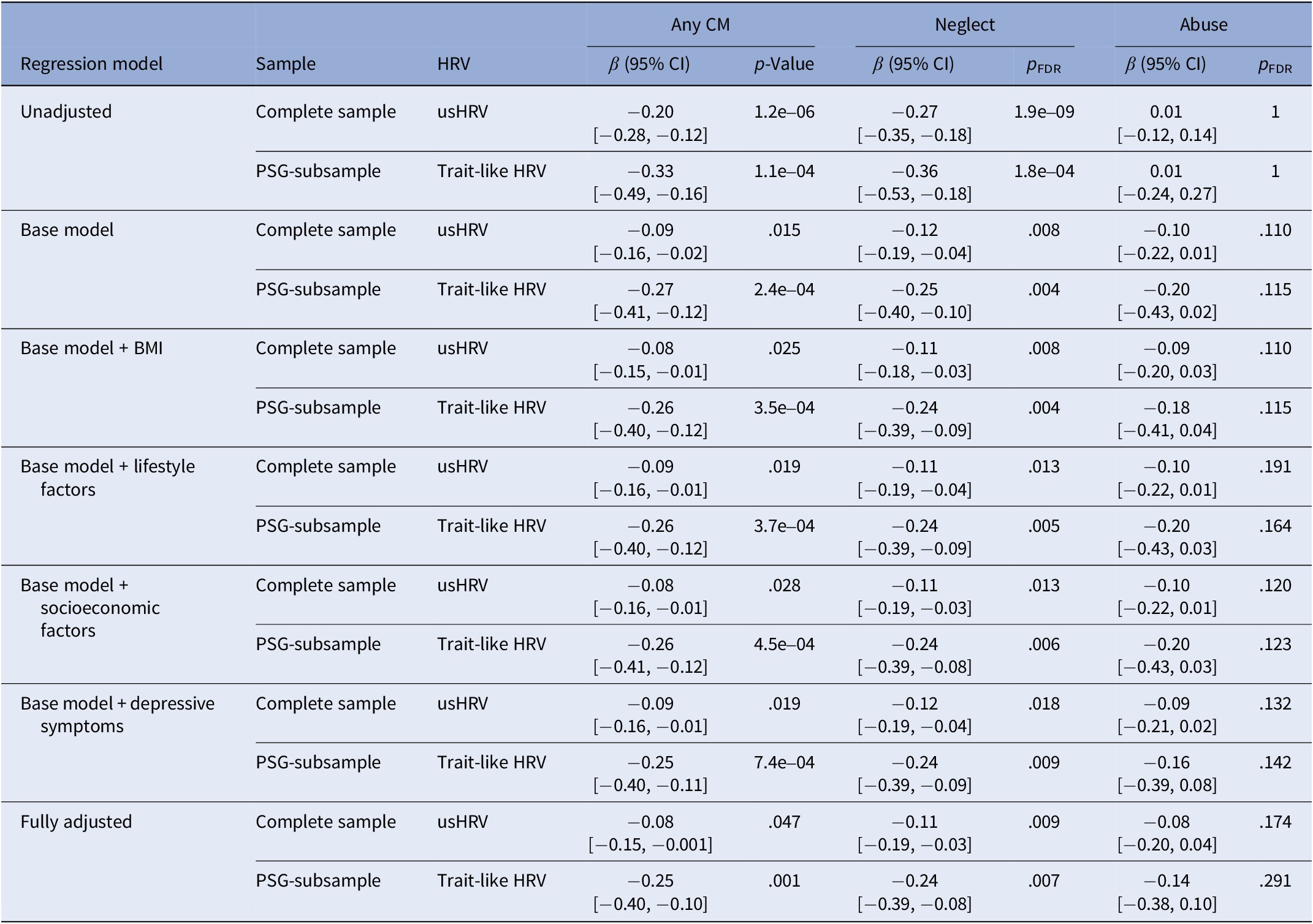
Note: Base model adjusted for age, sex, antihypertensive, and psychopharmacological medication (tricyclic antidepressants and clozapine), the interaction of sex with age and daytime in the case of usHRV; BMI, body mass index; lifestyle factors included smoking status (never, former, current), regular physical activity (less/at least 1 hour per week), alcohol consumption (in g/d) and its interaction with sex; socioeconomic factors included education and equivalized disposable income; depressive symptoms were assessed using the sum score of the PHQ-9 questionnaire; CI, confidence interval; FDR, false discovery rate to adjust for multiple testing; usHRV, HRV derived from 10-second ECG; trait-like HRV, HRV based on the average of usHRV and HRV.
Base model
Adjusting for age, sex, and antihypertensive and psychopharmacological medications, the negative association between HRV and CM was attenuated but remained significant in the complete sample (usHRV: β = −0.09 [95%-CI: −0.16, −0.02], p = .015) and in the PSG-subsample (trait-like HRV: β = −0.27 [95%-CI: −0.41, −0.12], p = 2.4e-04).
Adjusted models
Additional adjustments for socioeconomic factors, BMI, lifestyle, and depressive symptoms did not explain the association between CM and HRV. None of these factors, either individually or in combination, accounted for the observed effects.
Association of HRV and neglect
HRV was lower in individuals exposed to childhood neglect in the complete sample (usHRV: β = −0.27 [95%-CI: −0.35, −0.18], p = 1.9e-09) and the PSG-subsample (trait-like HRV: β = −0.36 [95%-CI: −0.53, −0.18], p = 1.8e-04).
Base model
Adjusting for age, sex, and HRV-altering medication attenuated the negative association between HRV and neglect but remained significant in the complete sample (usHRV: β = −0.12 [95%-CI: −0.19, −0.04], p = .008) and the PSG-subsample (trait-like HRV: β = −.25 [95%-CI: −0.40, −0.10], p = .004).
Adjusted models
Again, additional adjustments did not explain these effects.
Association of HRV and abuse
No association between HRV and childhood abuse was found in the complete sample (usHRV: β = 0.01 [95%-CI: −0.12, 0.14], p = 1) or in the PSG-subsample (trait-like HRV: β = 0.01 [95%-CI: −0.24, 0.27], p = 1), in either model.
Association of HR, SDNN, HF-HRV, and LF-HRV with CM
Exposure to CM showed no association with HR in either the complete sample or the PSG-subsample (Supplementary Table S3). In the PSG-subsample, no consistent associations were found between CM and SDNN, HF-HRV, or LF-HRV (Supplementary Tables S4–S6).
Association between HRV and the recency of CM
Exploring the interaction between CM and age at assessment, indicating CM recency, revealed no significant association in the complete sample (all p > .05; Table 3 and Supplementary Table S7). However, in the PSG-subsample, the interaction trended toward significance in the unadjusted model (trait-like HRV: p = .065) and reached significance when adjusting for covariates (base model: p = .029, BMI: p = .014; lifestyle factors: p = .024; socioeconomic factors: p = .004; depressive symptoms: p = .03, fully adjusted: p = .001). In the PSG-subsample, CM exposure was associated with reduced trait-like HRV in younger participants, an association that diminished at midlife but seemed to reemerge in older participants (Figure 2).
Table 3. Association of HRV and childhood maltreatment with interaction of age
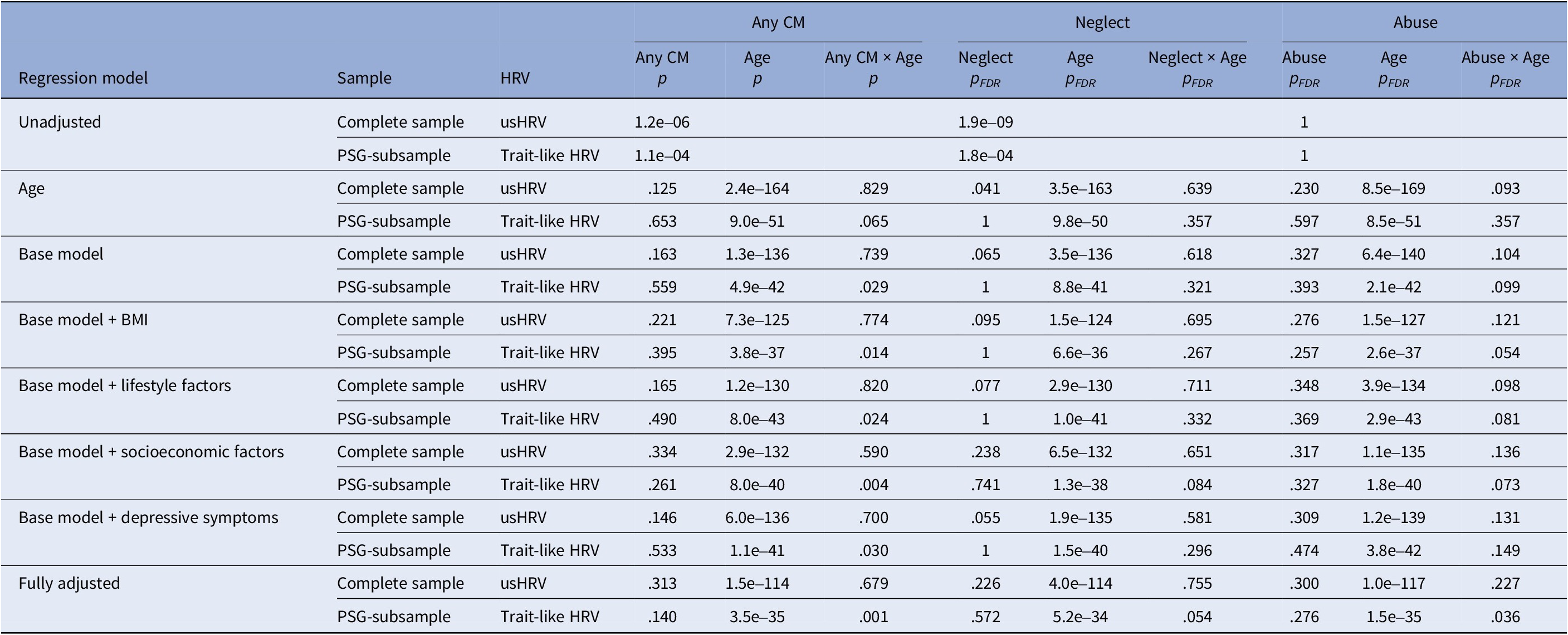
Note: Base model adjusted for age, sex, antihypertensive, and psychopharmacological medication (tricyclic antidepressants and clozapine), the interaction of sex with age and daytime in the case of usHRV; BMI, body mass index; lifestyle factors included smoking status (never, former, current), regular physical activity (less/at least 1 hour per week), alcohol consumption (in g/d) and its interaction with sex; socioeconomic factors included education and equivalized disposable income; depressive symptoms were assessed using the total score of the PHQ-9 questionnaire; FDR, false discovery rate; usHRV, HRV derived from 10-second ECG; trait-like HRV, HRV based on the average of usHRV and HRV.
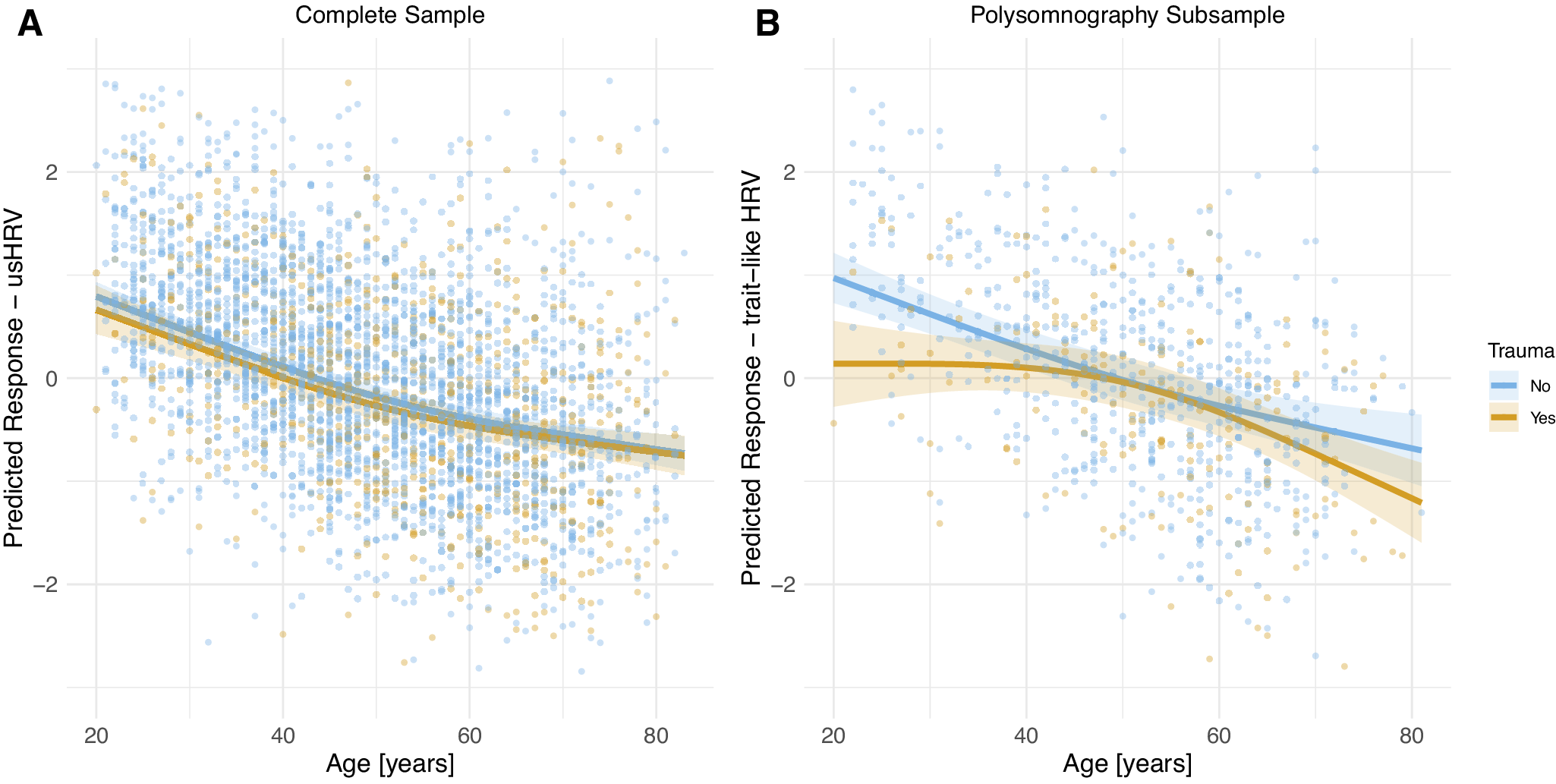
Figure 2. Predicted HRV (z-transformed logRMSSD) as a function of age and exposure to childhood maltreatment in the complete sample (A) and the PSG-subsample (B), both based on the fully adjusted model.
Similar, although less pronounced, patterns were observed for neglect and abuse in the PSG-subsample, but p-values survived FDR correction only in the fully adjusted model of neglect (HRV: p = .014; Supplementary Figure S1) and abuse (trait-like HRV: p = .036; Supplementary Figure S2).
Discussion
This study examined the long-term impact of CM on ANS dysregulation by exploring its relationship with vagal-sensitive metrics of HRV in a population-based sample. We revealed reduced HRV in adults exposed to CM, mainly driven by neglect. After adjusting for age, sex, and HRV-altering medication, the association was attenuated but remained stable when further accounting for BMI, lifestyle, socioeconomic factors, and current depressive symptoms. Notably, this is the first time these associations have been identified in a population-based sample using ultra-short HRV obtained from a 10-second multichannel ECG.
In detail, CM was associated with reduced 10-second usHRV in the complete sample and trait-like HRV in a subsample, indicating tonic disinhibition of the sympathetic branch of the ANS. This finding is in line with previous studies reporting reduced HRV in individuals exposed to CM [Reference Bussone, Pesca, Casetti, Croce Nanni, Ottaviani and Troisi35, Reference Krause-Utz, Walther, Kyrgiou, Hoogenboom, Alampanou and Bohus38–Reference Bakema, van Zuiden, Collard, Zantvoord, Rooij and Elsenburg40]. However, our findings differ from those reported in an earlier population-based study [Reference van Ockenburg, Tak, Bakker, Gans, de and Rosmalen67] and in two meta-analyses [Reference Sigrist, Mürner-Lavanchy, Peschel, Schmidt, Kaess and Koenig41, Reference Wesarg, van den Akker, Oei, Wiers, Staaks and Thayer42], likely due to methodological differences (e.g., assessment of HRV, HRV-parameters), sample characteristics, and the construct of CM in contrast to adverse childhood experiences. For instance, van Ockenburg et al. [Reference van Ockenburg, Tak, Bakker, Gans, de and Rosmalen67] investigated adverse childhood experiences rather than specifically CM, encompassing a broader spectrum of negative life events, some of which might be more easily buffered, for example, by sensitive and responsive caregiving. Similarly, the meta-analysis by Wesarg et al. [Reference Wesarg, van den Akker, Oei, Wiers, Staaks and Thayer42] found no association with broader adverse childhood experiences but with CM.
The association between HRV and CM may be mediated by socioeconomic disadvantages, poor lifestyle, and mental health problems, which are more likely to emerge following exposure to CM [Reference Klinger-König, Streit, Erhardt, Kleineidam, Schmiedek and Schmidt4, Reference Klinger-König, Berger, Grabe, Erhardt, Streit and Völker9, Reference Hughes, Bellis, Hardcastle, Sethi, Butchart and Mikton68], as well as by HRV-altering factors such as age, sex, physical fitness, and medication. HRV declines with age [Reference Krause, Vollmer, Wittfeld, Weihs, Frenzel and Dörr46, Reference Schumann and Bär60] and is altered by medications such as antihypertensives, clozapine, and tricyclics [Reference Kemp, Quintana, Gray, Felmingham, Brown and Gatt32, Reference Agelink, Majewski, Wurthmann, Lukas, Ullrich and Linka62, Reference Maciorowska, Krzesiński, Wierzbowski, Uziębło-Życzkowska and Gielerak63]. Adjusting for these factors attenuated the association between HRV and CM but remained stable after further adjustments for socioeconomic status, lifestyle, current depressive symptoms, and BMI. This is interesting as CM is associated with unhealthy lifestyle factors (e.g., alcohol use, smoking, physical inactivity), depressive symptoms, and low socioeconomic status [Reference Klinger-König, Streit, Erhardt, Kleineidam, Schmiedek and Schmidt4, Reference Hughes, Bellis, Hardcastle, Sethi, Butchart and Mikton68, Reference Jaffee, Ambler, Merrick, Goldman-Mellor, Odgers and Fisher69], all of which can alter HRV [Reference Koenig, Kemp, Beauchaine, Thayer and Kaess61, Reference Thayer, Yamamoto and Brosschot70–Reference Seeman, Epel, Gruenewald, Karlamangla and McEwen74]. Thus, our findings suggest small but substantial long-term ANS dysregulation in those exposed to CM, even after decades.
The observed association was particularly driven by neglect rather than abuse. Although abuse is generally considered more harmful and has been associated with altered HRV [Reference Bussone, Pesca, Casetti, Croce Nanni, Ottaviani and Troisi35, Reference Thurston, Carson, Koenen, Chang, Matthews and Känel37], its relatively low prevalence in our sample (7.5%, n = 259) may have reduced the statistical power. Additionally, the characteristics of abuse may vary in timing, whereas neglect, defined as the experience of deprivation, is inherently chronic [Reference Leeb, Paulozzi, Melanson, Simon and Arias75]. Thus, chronic stress induced by neglect might cause tonic sympathetic disinhibition, especially if it occurs during vulnerable developmental periods in childhood [Reference Lupien, McEwen, Gunnar and Heim76], leading to ANS dysregulation rooted in the prefrontal-amygdala axis in the brain [Reference Smith, Thayer, Khalsa and Lane24]. As this may manifest as a disruption in a child’s development, it can impair homeostasis and adaptive behavioral control [Reference Smith, Thayer, Khalsa and Lane24], as well as emotional processing and executive functioning [Reference Bussone, Pesca, Casetti, Croce Nanni, Ottaviani and Troisi35, Reference McLaughlin77]. This might have reduced stress resilience [Reference Chen, Suchting, Thayer and Fagundes78], facilitating the emergence of mental and physical health impairments in adulthood.
Our analysis revealed an age-dependent effect of CM on ANS dysregulation within the PSG-subsample. HRV was reduced in young adults exposed to CM, diminished by midlife, only to reemerge in older participants. Interestingly, this pattern was not observed in the complete sample, likely due to differences in sample characteristics, such as the PSG-subsample having fewer smokers, being more physically active, and possessing higher education. These factors might mediate a selection bias, particularly given the time-intensive nature of the additional PSG examination. Furthermore, the age effects may have been obscured in the complete sample, as only usHRV derived from 10-second ECGs was available as a surrogate.
Moreover, our PSG-subsample findings differ from two meta-analyses [Reference Sigrist, Mürner-Lavanchy, Peschel, Schmidt, Kaess and Koenig41, Reference Wesarg, van den Akker, Oei, Wiers, Staaks and Thayer42] that found the association between adverse childhood experiences and ANS dysregulation to increase with age. Unlike these studies, which used mean ages across samples, we examined CM exposure within one large sample. Therefore, our results suggest that CM-related ANS dysregulation peaks in early adulthood, when exposure to CM is still recent, potentially affecting crucial personal, professional, and social development. However, this effect diminishes by midlife, perhaps due to compensatory factors or benefits from more stable life circumstances, before re-emerging in old age, possibly as CM-induced early life ANS dysregulation exerts a toll on the individual’s health [Reference Danese and McEwen79]. This later-life effect may be underestimated due to selection bias, as severely affected individuals might be less likely to participate in epidemiological studies or even due to survival bias.
Moreover, post hoc analyses indicated that this effect might stem from abuse and neglect. While our main analyses found ANS dysregulation primarily associated with neglect, these findings highlight the need for further research.
Limitations
We used ECGs recorded during rest to derive HRV, although they were only approximately recorded according to current guidelines [Reference Malik22, Reference Quigley, Gianaros, Norman, Jennings, Berntson and de45]. This might raise concerns about the validity of our results. However, we previously demonstrated that a 10-second HRV can be a valid surrogate in large cohorts [Reference Krause, Vollmer, Wittfeld, Weihs, Frenzel and Dörr46]. Additionally, we replicated our results using the average of two HRV values obtained from two different ECGs within a subgroup, aiming to derive a more reliable measure of HRV as a trait and minimizing situation-specific variance [Reference Laborde, Mosley and Thayer56, Reference Bertsch, Hagemann, Naumann, Schächinger and Schulz57]. In this regard, it is worth noting that our sample was phenotyped using identical assessments under continuous quality control procedures, resulting in a homogeneous sample, in contrast, for example, to a meta-analysis.
Our null finding on abuse should be interpreted cautiously, as previous studies found ANS dysregulation and adverse childhood experiences to vary as a function of psychopathology [Reference Sigrist, Mürner-Lavanchy, Peschel, Schmidt, Kaess and Koenig41] and to be associated with abuse [Reference Bussone, Pesca, Casetti, Croce Nanni, Ottaviani and Troisi35, Reference Stevens, Williams, Thayer and Zalta80]. In this regard, post-traumatic symptoms should be considered in future studies.
Moreover, our results should be replicated in other population-based cohorts, such as the German National Cohort, as previous findings on the association between HRV and CM were heterogeneous, possibly due to differences in HRV parameters, sample characteristics, and definitions of CM versus childhood adversity. Since HRV measures besides RMSSD in this study showed no substantial association, future research should focus on HRV metrics that provide insights into the overall autonomic regulation of cardiac function.
Additionally, investigating HRV’s mediating effect on somatic outcomes like cardiovascular disease and mental health outcomes such as anxiety and depression, especially related to childhood neglect, could clarify how CM translates into long-term health risks.
CM was assessed using the self-report questionnaire CTQ, a valid and reliable tool, although retrospective reports of CM can be biased [Reference Baldwin, Reuben, Newbury and Danese81]. Replicating our findings with cross-validated data, such as interviews, is therefore advisable. For variables like alcohol consumption, medication, BMI, depressive symptoms, and income, only current data were available, limiting insights over the lifespan.
Summary and conclusion
Our results suggest long-lasting effects of ANS dysregulation following exposure to CM, especially neglect, based on HRV data derived from 10-second multichannel ECGs in a population-based sample. These effects remained robust, unaffected by socioeconomic, lifestyle factors, BMI, and current depressive symptoms, highlighting the need for prevention and intervention to reduce individual suffering and socioeconomic costs.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1192/j.eurpsy.2025.10040.
Data availability statement
The data from the SHIP study cannot be made publicly available due to the informed consent of the study participants; however, it can be accessed through a data application form available at https://fvcm.med.uni-greifswald.de/ for researchers who meet the criteria for access to confidential data.
Acknowledgments
The authors would like to sincerely thank everyone who participated in SHIP-TREND.
Author contribution
M.S., E.K., and H.J.G. designed the project. All co-authors contributed to the acquisition, analysis, or interpretation of the data. M.S., E.K., and H.J.G. drafted the manuscript, while all other coauthors critically revised it. MS and EK conceived and conducted the statistical analyses with support from J.K.K., M.V., S.F., and A.W., M.D., L.K., S.B.F., B.S., R.E., H.V., and H.J.G. were involved in data curation, administrative, technical, or material support, or funding acquisition. H.J.G. and E.K. supervised the project. All authors have read and approved the submitted version of the manuscript.
Financial support
The SHIP is part of the Community Medicine Research Net (CMR, www.medizin.uni-greifswald.de/icm) of the University Medicine Greifswald, which is supported by the German Federal State of Mecklenburg-West Pomerania and the German Federal Ministry of Education and Research (BMBF, grant numbers 01ZZ96030, 01ZZ0701, and 01ZZ0403). PSG assessment was in part supported by the German RLS organization (Deutsche Restless Legs Vereinigung). This study was further supported by the Deutsche Forschungsgemeinschaft (DFG, grant number 406711066).
Competing interests
HJG has received travel grants and speaker honoraria from Indorsia, Neuraxpharm, Servier, and Janssen-Cilag. HJG had personal contracts approved by the university administration for speaker honoraria. All other authors reported no financial interests or other potential conflicts of interest.








Comments
No Comments have been published for this article.