1. Introduction
High rates of premature mortality in patients with schizophrenia shorten their life expectancy by approximately 12–29 years, as compared to the general population [1–Reference Robinson3]. Higher death rates in schizophrenia have been associated with unhealthy lifestyle including smoking, poverty, comorbid physical diseases, adverse effects of treatments, suboptimal medical monitoring and care, accidents, and suicide [1–Reference Leucht, Burkard, Henderson, Maj and Sartorius4]. Life expectancy of the general population increased in the last few decades. This is not, however, the case for patients with schizophrenia, whose differential mortality worsened in recent decades [Reference Saha, Chant and McGrath5,Reference Hoang, Stewart and Goldacre6]. The generalizability of earlier findings has been limited by the fact that the vast majority of data on mortality and comorbidity in schizophrenia came from highly developed countries and very few studies included nationwide data [Reference Leucht, Burkard, Henderson, Maj and Sartorius4].
Mortality data of patients with schizophrenia are often compared to the mortality data of the general population [Reference Saha, Chant and McGrath5]. However, this approach has been questioned as it leads to a bias towards underestimating mortality risk, and it has been pointed out that “when using population based datasets, an internal control group drawn from the same dataset is preferable to the use of external rates for comparison” [Reference Card, Solaymani-Dodaran, Hubbard, Logan and West7]. In order to deal with this issue, our study was designed as a nationwide matched-cohort study. Matched-cohort studies, especially based on large databases may offer additional insight in the role of different variables associated with mortality (e.g., age, gender, place of residence, comorbid conditions).
Electronic reporting to the National Health Insurance Fund Administration (NHIFA) is mandatory in Hungary for all health care providers and pharmacies; the system exists in the recent form since 1998. NHIFA covers the vast majority of the population of approximately 10 million inhabitants. The NHIFA database captures all publicly funded healthcare events, including in- and outpatient care, and the drug reimbursement system is 100% covered by NHIFA. The private healthcare sector in Hungary covers not more than 5% of all healthcare events. Patients with the diagnosis of schizophrenia rarely use private services (no private inpatient psychiatric services are available in the country); however, most or possibly even all of these patients are included in the database since they may use publicly funded services (e.g., GP, laboratory, psychiatric hospitalization, fill prescriptions in the pharmacies).
2. Aims of the study
We conducted a study to investigate mortality in the population of patients with schizophrenia in Hungary. Our specific aims were:
to compare mortality in schizophrenia with individually matched controls in Hungary during a defined period of observation;
to describe comorbidities in patients and controls;
to delineate the relationship of mortality with suicide attempts and comorbid conditions of interest.
3. Methods
3.1. Data source
Subject-level data were selected both for patients with schizophrenia and controls from the national insurance database of Hungary, which includes records of all inpatient, outpatient, and other services (e.g. pharmacy, laboratory) occurring within and funded by the national health system. The database records include unique patient IDs and ICD-10 codes.
3.2. Study periods
This was a nationwide, register-based prospective matched-cohort study between 01/01/2005 and 31/12/2013, which comprised three periods: an inclusion period of three-years between 01/01/2006 and 31/12/2008; an observation period between the start of inclusion and 31/12/2013; and a retrospective period between 01/01/2005 and the start of the inclusion, and also a checking and confirmation period from 01/01/1998. The retrospective period was used to identify comorbidities that were present upon the Inclusion of a patient in the study. Based on the date of qualification for the study, the start of inclusion period could vary individually across patients; however, since the study's observation period lasted until 31/12/2013, all included subjects were observed for at least five years from Day 1 (start of the observation period). During the observation period, we identified all relevant events, including records of comorbidities, death and suicide attempts.
3.3. Subjects and data collection
We investigated mortality in schizophrenia based on data from patients who had at least two records of schizophrenia diagnosis (F20.0–F20.9 according to the International Classification of Diseases, 10th revision ICD-10) [8] between 01/01/2006 and 31/12/2008; and from control subjects who had no diagnosis of schizophrenia. The requirement for a second record of F20.0–F20.9 diagnosis was used in the study in order to improve case ascertainment for patients with the diagnosis of schizophrenia. Control subjects were individually matched to schizophrenia patients at a 5:1 ratio on the basis of age, gender, and neighborhood (geographic area), during the inclusion period of 01/06/2006 and 31/12/2008. Control subjects had to be alive at the second F20 diagnosis of his/her respective pair with schizophrenia in order to be selected as a matched-pair control for the investigation. No further criteria were applied; patients of any age were eligible for the investigation. The broad inclusion criteria aimed to increase the generalizability of the findings using the database of the NHIFM. Information on patient-related events including comorbidity and mortalities are recorded in the system both for inpatient and outpatient care.
3.4. Statistical analyses
The principal outcome measure was mortality, defined as death due to any reason. The main independent variable of interest was the study group (schizophrenia and control).
For descriptive statistical purposes, we applied a non-parametric approach, the Kaplan-Meier model for survival analysis to investigate the five-year mortality based on the longitudinal follow-up data from the study's observation period. Subjects with no event (death) at the end of the observation period were considered as censored. For the Kaplan-Meier based survival analysis, group (schizophrenia vs. control) served as an independent variable. The analyses were performed for the full group of study subjects, and for subgroups stratified by gender and age. In particular, in these analyses age at inclusion was used as a categorical variable in the analyses, classified into five categories (< 20, = 20– < 40, = 40– < 60, = 60– < 80, and = 80 years).
For inferential statistical analysis, we used the Cox proportional-hazards regression model [Reference Cox9]. To eliminate basic demographic differences between the study groups, we conducted matched-pair [Reference Sekhon10] analyses using the Cox proportional-hazards regression model for clustered data based on the matched pairs. For the Cox-regression approach based on the full set of data (patients with schizophrenia and controls combined), study group (patients with schizophrenia vs. controls) served as a principal independent variable of interest. Gender, age category (5 categories as described above), and comorbidities of interest were used as covariates in the analyses. An interaction term between study group and covariates was included in the analyses. Since the interaction terms reached statistical significance in the pooled analysis, we conducted additional analyses to further delineate the associations in each of the two study groups.
Comorbidities were used as dichotomous variables (‘present’ or ‘absent’) in the analyses. They were applied either as time-invariant (considered as ‘present’ from the time of the onset of comorbidity but ‘absent’ before, e.g., cerebro- and cardiovascular diseases) or time-variant (considered as ‘present’ concurrently with the database records of comorbidity). The following list provides the comorbidities defined for the investigation, and describes their usage (as time-invariant [INV] or time-variant [VAR]) in the Cox-regression model:
cerebro- and cardiovascular diseases (INV);
neoplasm, without benign neoplasms (INV);
diseases of liver (INV); diabetes mellitus (INV);
emphysema, or other chronic obstructive pulmonary disease, or asthma (INV);
acute lower respiratory infections (VAR);
suicide attempts (INV);
viral hepatitis and HIV virus disease (INV);
epilepsy (INV);
other infections (VAR);
respiratory tuberculosis (VAR);
other diseases of the nervous system (without epilepsy and transient cerebral ischemic attacks) (INV);
external causes of morbidity and mortality (VAR).
A detailed list of comorbidities included in the various comorbidity categories used in the statistical analyses is presented in Supplementary Table 1.
We used the risk ratio (RR, or hazard ratio [HR]) statistics in order to assess the strength of the association between mortality and each of the independent variables in the analyses. For the computation of risk ratios from the Cox-regression model, the mortality in the age category of > 40–60 years was used for reference. We report the resulting risk ratios with 95% confidence intervals (CIs). Based on the baseline hazard function estimated by the Cox-regression analyses, we determined the five-year baseline mortality both in patients and controls, stratified by gender (i.e., estimated mortality in the absence of covariates, e.g., in the absence of comorbid conditions). Furthermore, we examined the impact of the covariates on mortality in both groups by computing the 5-year mortality in the presence of each of the covariates in the statistical model.
Life expectancies were computed on the basis of life tables presenting age-specific observed mortality rates, grouped into age categories of 5-years up to the age of 90 years, and for a 10-year category for ages > 90 to 100 years. The mortality of 100% was assumed for the ages of > 100 years. For the purpose of the computations, we assumed that that there is a constant mortality within the age intervals. We performed a separate computation for males and females. We calculated the life expectancy for patients with schizophrenia and control subjects at an age of transition to young adulthood (20 years) and at adulthood (45 years), respectively.
All statistical tests were two-sided; significance level was set at a = .05. Statistical analyses were performed with R-software version 2.9.1 (R Development Core Team, 2009) and by the Statistical Analysis System (SAS) version 9.4 (SAS Institute, Cary, NC). Based on data privacy regulations, all statistical analyses were run on the servers of NHIFM and outputs were eligible for further investigations, without any access to patient-level data outside of NHIFM.
4. Results
4.1. Patient disposition
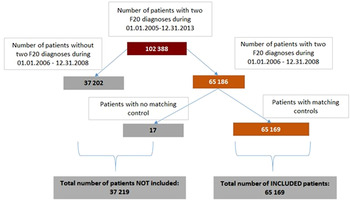
As shown in Fig. 1, 102388 patients received the diagnosis of schizophrenia (i.e., code F20.0–F.20.9 according to ICD-10) at least twice during the entire study period of 01/01/2005–31/12/2013, either as an in- or outpatient.

Fig. 1. Study flowchart for patient selection and eligibility for the study.
Out of these 102,388 patients, a total of 37,202 patients had only one schizophrenia diagnosis during the inclusion period, therefore they were not included in the study. As shown by the figure, a total of 65,186 of the patients had two records for the diagnosis of schizophrenia during the inclusion period of 01/01/2006–31/12/2008. Of these patients, a total of 17 had no matching controls in the database, and therefore could not be used in the matched-pair analyses. Thus, 65,169 patients with schizophrenia were included in the current investigation. Matching of the included patients to control subjects at the planned 5:1 ratio could be accomplished in the overwhelming majority of cases. Specifically, out of the 325,845 control subjects that were expected based on the 5:1 ratio, a total of 32,543, 99.87% of the expected total were available for analyses (i.e., in some cases, the matched-pair clusters contained fewer controls than 5).
4.2. Demographic and basic descriptive characteristics
Table 1 displays basic descriptive demographic data on the patient population in terms of gender and age (broken down by age groups of 20 years), and pooled across the entire patient population. We note that control subjects had the same age distribution as they were matched individually to patients based on age (within 1-year of age) and gender in the study. As shown in Table 1, approximately 41.8% of the total sample of patients with schizophrenia was male. The gender composition varied substantially across age groups. The proportion of males showed a steady increase with younger age; in particular, the proportion of males reached 64% in the youngest age group as compared to 19% in the oldest). Mean age at enrollment was approximately 8 years lower in males than in females (mean [SD] age male = 45.7 years ([SD = 15.5], female 53.3 years [SD = 16.0]). Both in males and females, the 40–59 years age group comprised the highest proportion of all subjects enrolled for the study.
Table 1 Demographic and basic descriptive data of the patient population.

4.3. Distribution of comorbidities in the study population
Table 2 displays the distribution of comorbidities in the study population. The rightmost column of the table provides summary data for all subjects in both study groups (‘Total group’) while additional columns of the table display the proportion (%) of comorbidities broken down by individual age categories. The first row provides the proportion of subjects who presented with any comorbid condition of interest, while the subsequent rows display the comorbidity distribution in terms of individual comorbidity groups.
Table 2 Distribution of comorbidities and suicide attempts: percentage (%) of patients in the schizophrenia and control populations.
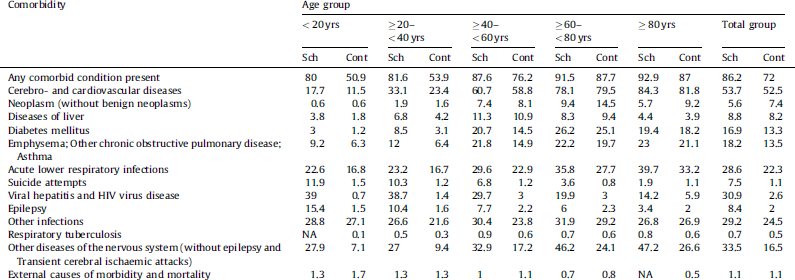
Table 2 (first row) indicates that, while in both study groups the proportion of comorbid conditions was high and increased with age, it was markedly higher in the schizophrenia group than in the control in all age categories. The group difference was the most pronounced at the two younger age groups, with slightly more than half of the control subjects having a comorbid condition, as opposed to the patients with schizophrenia who presented with at least one comorbid condition in the majority of cases [> 80%]). In order to identify differences in terms of individual comorbidities pooled across all age groups in the schizophrenia group as compared to controls, we summarized any deviation from the control group that exceeded 3% in terms of absolute % comorbidities. We found an increase in:
diabetes mellitus;
emphysema & other chronic obstructive pulmonary diseases and asthma;
acute lower respiratory infections;
suicide attempts;
viral hepatitis and HIV virus disease;
epilepsy;
other infections;
other diseases of the nervous system (without epilepsy and transient cerebral ischemic attacks).
While an increase in the prevalence of comorbid conditions were generally observed across all age categories, age-specific increases in the prevalence were found in the schizophrenia group as compared to controls with regard to suicide attempts, diabetes, and epilepsy. These differences were highly pronounced at younger ages, and decreased with increasing age.
4.4. Mortality in patients with schizophrenia compared to controls
There was a higher proportion of mortality in the schizophrenia group as compared to controls. Specifically, 22.2% (14,481 of 65,169) of the patients with schizophrenia died during the observation period of the study, whereas in the control group 10.8% (35,176 of the 325,435) of the subjects died. The analogous values were the following for males: 21.5% (5843 of 27,221) for patients with schizophrenia and 10.7% (14,483 of 35,952) controls; and for females 22.8% (8638 of 37,948) for patients and 10.9% (20,693 of 189,483) for controls, respectively. The mean age at death in each of the two groups was the following:
schizophrenia = 66.34 (SD = 14.74) years;
controls = 70.63 (SD = 13.62) years.
The mean age at death in males and females in the schizophrenia and control group, respectively, was the following: males with schizophrenia = 61.37 (SD = 14.51) years; females with schizophrenia = 69.69 (SD = 13.92) years. The analogous numbers for the control group were the following: males = 65.21 (SD = 13.12) years; females = 74.42 (SD = 12.64) years.
Observed crude data on mortality for patients with schizophrenia and matched controls broken down by gender and age categories at enrollment in 20-year age groups are provided in Supplementary Table 2.
Based on the non-parametric Kaplan Meier survival analysis, Table 3 provides 5-year mortality estimates and their 95% confidence limits for both study groups broken down by gender and pooled across all subjects. Columns of the table display the mortality estimates broken down by categories of age at inclusion in the investigation. The rightmost column shows the mortality estimates summarized for all age groups. Risk ratios for mortality for patients with schizophrenia versus controls, pooled for all subjects and broken down by gender and age category, are also provided in the table. As shown by the table, the risk ratios indicate a pronounced elevation in the mortality risk of patients with schizophrenia at lower ages, especially under 40 years of age. Additionally, as indicated by the risk ratios, the increased mortality risk at younger ages in patients with schizophrenia vs. controls, is substantially greater in females than in males.
Table 3 Kaplan–Meier based 5-year mortality (%) estimates in the two study groups and their 95% confidence limits broken down by gender, and pooled across all subjects.

4.5. Mortality, comorbidities and suicide attempts in patients with schizophrenia as compared to controls
As described in the statistical analyses, we performed a Cox-regression analysis based on the full set of data (patients with schizophrenia and controls combined), applying study group (patients with schizophrenia vs. controls) as the main independent variable of interest, and gender, age category and comorbidity group as covariates in the analyses. Our results indicated that patients with schizophrenia had a substantially higher risk of mortality than the control subjects (RR = 2.4; P < 0.0001). Furthermore, with the exception of respiratory tuberculosis, we found statistically significant interactions (P < 0.0001) between study group and the covariates in the Cox-regression model, including comorbidity categories and gender and age, which indicated significant group differences with regard to these covariates. In order to delineate the associations further, we conducted a separate Cox-regression analysis in each of the two study groups (Table 4).
Table 4 Results of survival analysis in patients with schizophrenia and controls to examine the impact of covariates on mortality in the Cox-regression model.

a 5-year mortality estimated from the Cox-regression model. Mortality value in each row was estimated on the basis of the assumption that the given condition depicted in the row is met in the group (e.g., diabetes mellitus) while no other conditions (e.g., comorbidities) are met.
b Reference (control) condition for the Cox-regression analysis (e.g., RRs for age groups were derived on the basis of comparisons with the reference age group [age 40–59 years]).
c “External causes of morbidity and mortality” are defined according to Chapter XX of the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10)-WHO Version, 2016. It permits the classification of environmental events and circumstances such as the cause of injury, poisoning and other adverse effects. The chapter includes various types of accidents, including: falls, exposure to electric current, fire, intentional self-harm, complications of medical and surgical care, etc.
As shown by Table 4, results indicate increased mortality in patients with schizophrenia in the younger age groups as compared to controls. In both study groups, the mortality was lower in females than in males as indicated by the values of relative risk for females (schizophrenia = 0.68, control = 0.63). These values are consistent with the Kaplan-Meier estimates, which show lower mortality in females than in males with schizophrenia, especially in the younger age groups.
Additionally, comorbidities were associated with a significantly increased mortality in both groups. In the control group, the largest increases in mortality occurred in association with:
acute lower respiratory infections (RR = 14.22);
external causes of morbidity and mortality (RR = 8.83);
other infections (RR = 4.03);
neoplasms (RR = 3.14).
Overall, the relative risks for mortality with regard to comorbidities were lower in the schizophrenia groups as compared to controls, with one exception (Acute lower respiratory infections). In order to examine baseline mortality across age categories without presence of covariates, we estimated the baseline mortality using the Cox-regression model for both males and females, and for the total groups of subjects. Results of this analysis are depicted in Supplementary Table 3.
As shown by Table 4, estimates of baseline mortality are somewhat lower than the Kaplan-Meier based estimates since no comorbidities are assumed for the baseline model. As shown by the prevalence estimates, the relative increase in mortality was somewhat lower in females than in males.
In addition to the examination of the association of mortality with comorbidities and suicide attempts, we computed life expectancy for 20- and 45 year-olds, respectively. As described in the statistical analyses, life expectancies were computed on the basis of life tables presenting the actual age-specific observed mortality rates in the patient and control groups, respectively. Our results indicated that that 20 years of age, males with schizophrenia had a life expectancy of 36.5 years in 2006 and 2007, while the controls had 48.0 years; this resulted in a mortality gap of 11.5 years for patients with schizophrenia. The analogous life expectancy numbers for females were the following: schizophrenia = 43.2 years, control = 56.9 years; mortality gap = 13.7 years. At the age of 45 years, males with schizophrenia had a life expectancy of 17.9 years, compared with 26.0 years in the control group, with a mortality gap of 8.1 years; the life expectancy for females at this age was the following: schizophrenia = 23.2 years, control = 32.8 years; mortality gap = 9.6 years.
5 Discussion
To our knowledge, this is the second study – after the Swedish National Cohort Study [Reference Crump, Winkleby, Sundquist and Sundquist1] – that comprehensively examines the somatic health effects of schizophrenia using both outpatient and inpatient diagnoses from all health care settings for a national population and the first nationwide mortality study in schizophrenia using matched-cohort design.
All investigated comorbidities but neoplasms were more frequent in the group of patients with schizophrenia. The difference between the cumulative frequencies of comorbid conditions of the two groups was largest in the youngest age group and the difference between the groups decreased with increasing age.
The results of this study concerning mortality rates in schizophrenia (22.2%) are consistent with earlier data: a 2.4 times increase with significant sex difference was observed in patients with schizophrenia compared to individually matched (age, gender and neighborhood) control subjects [Reference Crump, Winkleby, Sundquist and Sundquist1,Reference Saha, Chant and McGrath5]. As expected, mortality increased with age and it was increased in males compared to females. Data about sex differences in the mortality of schizophrenia have been rather heterogeneous, and many studies having found no sex differences [Reference Saha, Chant and McGrath5].
Our results indicate that the relative risks for mortality with regard to comorbidities were generally lower in the schizophrenia group as compared to controls. This needs to be interpreted in view of the findings that the proportion of comorbidities showed an overall elevation in the schizophrenia group, and that the diagnosis of schizophrenia in itself was associated with an elevated relative risk of mortality (i.e., RR = 2.4, see above) as compared to controls. Nonetheless, these findings call for further studies with the aim of identifying potential hidden covariates of the schizophrenia × comorbidities interactions.
The limitations of the study include its register-based observational nature, which does not permit to establish causality based on the observed associations. Some important data (e.g. smoking status of the patients) are not available in the database, the authors have no control over the quality of the data in the database, and truncation at the beginning of follow-up makes it difficult to differentiate between prevalent and incident cases.
The markedly higher rate of mortality associated with comorbidtities, and the large increase of comorbidities in schizophrenia identifies a need for the improvement of graduate and postgraduate medical education and training in general and specifically in psychiatry with more focus on somatic diseases including neurological diseases, diabetes mellitus, and diseases of the respiratory tract. Many other factors deserving attention include lack of resources, access to and model of medical care, lifestyle, medication side effects, smoking, stigma, need for early intervention and adequate health care organization could help to better address the physical health needs of patients with schizophrenia. [Reference Leucht, Burkard, Henderson, Maj and Sartorius4].
Role of the sponsor
The study was conducted through a collaboration of academia (Department of Psychiatry and Psychotherapy, Semmelweis University, Budapest, Hungary), a governmental organization (NHIFM, Hungary), the pharmaceutical industry (Janssen-Cilag Hungary and EMEA) and an independent consulting company (Healthware Consulting Ltd, Hungary). Five employees of the Sponsor participated in the study (they are all authors). The statistical analysis plan was outlined by P. Czobor. Data selection and anonymization of the data were performed by the NHIFM staff, and NHIFM gave Healthware Consulting Ltd access to the data. The statistical analyses were performed by a team of Healthware Consulting Ltd. (M. Bacskai, P. Rakonczai, R. Hegyi and T. Németh, who are authors) under the supervision of P. Czobor. The original data set used for this analysis is available on a claim basis from the Hungarian National Health Insurance Fund Manager (http://www.oep.hu/).
Although staff at Janssen – in addition to those authors, who are employees of Janssen – reviewed the manuscript, final approval for the decision to submit the manuscript was the sole decision of the authors, as agreed between the collaborating partners prior to the study.
Disclosure of interest
Five authors (P. Takács, A. Borsi, B.Z. Nagy, L. Fehér and J. Sermon) are employees of Janssen. M. Bacskai, P. Rakonczai, R. Hegyi and T. Németh are employees of an independent company (Healthware Consulting Ltd) which received funding for contribution to the study design and data analyses. I. Bitter has been an advisory board member/consultant/lecturer for or received research support from Eli Lilly, EGIS, EGRIS, Janssen, Lundbeck, Medavante, Gedeon Richter, Pierre Fabré and Servier.
P. Czobor, P. Varga, J. Gimesi-Országh, P. Fadgyas-Freyler, P. Takács declare that they have no competing interest.
Acknowledgements
Janssen-Cilag Hungary Ltd., Budapest, Hungary supported the study, which is part of a larger Janssen sponsored Real World Evidence study, called “ATTILA, the HUN” (Antipsychotic Treatment with Injectable Long Actings in Hungary).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.eurpsy.2017.05.022.







Comments
No Comments have been published for this article.