1. Introduction
The 22q11.2 deletion syndrome (22q11DS) is a neurogenetic condition caused by hemizygous microdeletion of the long arm of chromosome 22. Approximately 90% of individuals with 22q11DS carry a 3Mb deletion, and the rest carry smaller (1.5Mb) and atypical deletions [Reference Michaelovsky, Frisch, Carmel, Patya, Zarchi and Green1]. The syndrome occurs in 1–2,000–4000 live births and is characterized by medical comorbidities (e.g., cardiovascular and cleft anomalies) and high rates of psychiatric disorders, developmental delays, and average IQ within the borderline range [Reference Bassett, McDonald-McGinn, Devriendt, Digilio, Goldenberg and Habel2–Reference Swillen, Vandeputte, Cracco, Maes, Ghesquière and Devriendt4].
Most studies to date have assessed the psychiatric phenotype of older children, adolescents and adults with 22q11DS (see Schneider et al., 2014 for review [Reference Schneider, Debbané, Bassett, Chow, Fung and van den Bree5]). Those studies found that ∼70% individuals with 22q11DS have at least one psychiatric disorder, including schizophrenia spectrum disorders evolving by early adulthood in about one-third of the patients, and high rates of attention deficit/hyperactivity disorder (ADHD), anxiety disorders, mood disorders and autism spectrum disorder (ASD) [Reference Schneider, Debbané, Bassett, Chow, Fung and van den Bree5, Reference Antshel, Aneja, Strunge, Peebles, Fremont and Stallone6].
There are, however, very few studies that assessed the severity of psychiatric symptoms in preschool and young children with 22q11DS, and none before the age of 6 years [Reference Antshel, Aneja, Strunge, Peebles, Fremont and Stallone6]. Two studies that examined behavior and cognition in preschool children with 22q11DS [Reference Briegel, Schneider and Schwab7, Reference Klaassen, Duijff, Swanenburg de Veye, Vorstman, Beemer and Sinnema8] reported psychiatric problems based on the child behavior checklist (CBCL), a parent-report questionnaire, and were not based on structured psychiatric diagnostic interviews, such as the Schedule for Affective Disorders and Schizophrenia (K-SADS), which is designed for diagnosing the presence or absence of psychiatric disorders. Based on the CBCL, children with 22q11DS had high mean scores on the CBCL scales of withdrawn behavior, attention, affective, and pervasive developmental problems [Reference Briegel, Schneider and Schwab7, Reference Klaassen, Duijff, Swanenburg de Veye, Vorstman, Beemer and Sinnema8].
While the rates of psychiatric disorders in young children with 22q11DS are unknown, the rates of ASD in individuals with 22q11DS are a matter of controversy. Several studies assessed the presence of ASD in individuals with 22q11DS and reported rates of ASD that varied greatly, i.e., from 7% to 50% [Reference Antshel, Aneja, Strunge, Peebles, Fremont and Stallone6, Reference Angkustsiri, Goodlin-Jones, Deprey, Brahmbhatt, Harris and Simon9–Reference Vorstman, Morcus, Duijff, Klaassen, Heineman-de Boer and Beemer15]. Possible explanations for such variability include differences in the age of the subjects and in the tools used to assess ASD. Of note, most studies on 22q11DS relied on interviews with the parents using the Autism Diagnostic Interview-Revised (ADI-R) [Reference Antshel, Aneja, Strunge, Peebles, Fremont and Stallone6, Reference Fine, Weissman, Gerdes, Pinto-Martin, Zackai and McDonald-McGinn10, Reference Kates, Antshel, Fremont, Shprintzen, Strunge and Burnette11, Reference Vorstman, Morcus, Duijff, Klaassen, Heineman-de Boer and Beemer15] while only a few of them used the Autism Diagnostic Observation Schedule (ADOS) [Reference Angkustsiri, Goodlin-Jones, Deprey, Brahmbhatt, Harris and Simon9, Reference Ousley, Evans, Fernandez-Carriba, Smearman, Rockers and Morrier14]. The ADOS relies on direct observation of the child's interaction abilities and behavior by a qualified examiner and is one of the gold-standard diagnostic instruments for the diagnosis of autism.
In addition to using gold standard measure tools, to better understand the autism phenotype in 22q11DS it is important to compare the social and repetitive symptoms observed in children with 22q11DS to those of children with idiopathic autism (iASD). In the only study to date that compared ASD symptoms exhibited by 22q11DS children aged 6–15 years with age- and sex-matched children with iASD [Reference Kates, Antshel, Fremont, Shprintzen, Strunge and Burnette11], the findings of the ADI-R revealed that both groups were equally affected in reciprocal social interaction and stereotyped behavior domains, while those with 22q11DS were less affected in the communication domains.
The overall aim of the present study was to identify the psychiatric disorders and rates and characteristics of autism in young children with 22q11DS, and to compare them to age and sex-matched young children with iASD. Specifically, we evaluated the prevalence of psychiatric disorders and ASD in young children with 22q11DS using comprehensive gold standard measures and compared them to the results of those assessments that were obtained from an age and sex-matched group of children with iASD. Lastly, we wished to compare the severity of psychiatric symptoms and the autistic phenotype of 22q11DS children diagnosed with autism compared to 22q11DS children without autism.
The study hypotheses were: 1. Children with 22q11DS will have lower percentages of ASD diagnosis than reported in past studies, which relied solely on parents’ interviews. 2. We expect to find high rates of psychiatric disorders in 22q11DS, especially ADHD and anxiety disorders. 3. Children with 22q11DS will show similar high scores to children with iASD on a behavioral checklist. 4. The social phenotype of 22q11DS children will be overall less impaired than that of iASD in most domains, except repetitive and restricted behaviors, which will be equally high in both populations.
2. Methods
2.1. Participants
Twenty-five children (18 males, 7 females, aged 3–8 years [mean ± SD 5.57 ± 1.55 years]) with a genetically confirmed diagnosis of 22q11DS using fluorescent in situ hybridization or chromosomal micro-array tests, were recruited from our Behavioral Neurogenetics Center at Sheba Medical Center. The Center coordinates research and comprehensive medical and psychiatric treatments of individuals with 22q11DS throughout the country, and children with 22q11DS are referred to the Center from genetic departments and parents’ associations nationwide. Note that our sample was not biased towards more affected children as none of the children recruited was referred for evaluation of suspected autism.
The control group consisted of 28 children with iASD who were recruited from the Preschool Day Care Program for Children with ASD at the same medical center. These children are referred to the program through the local municipality and represent preschoolers from the community with a range of ASD severity. The ASD group was evaluated by a clinical geneticist and developmental pediatricians, and their medical records were reviewed. Those children were referred for genetic testing when there was any suspicion of a genetic syndrome and were excluded from the study if that suspicion was confirmed. The matching procedure began with the ASD sample, which consisted of a 3.6:1 male to female ratio. The demographic characteristics of the study and control groups are presented in Table 1. The Sheba Medical Center Review Board approved the protocol of the study design, and informed consent was provided by the parents of all participants.
2.2. Measures
2.2.1. Psychiatric evaluations
The parents of all the study participants completed the CBCL preschool and school versions [Reference Achenbach and Rescorla16, Reference Achenbach and Rescorla17]. In addition, the parents of the children with 22q11DS were interviewed by a child psychiatrist using the semi-structured K-SADS to screen for the presence of DSM-5 psychiatric disorders in their children. To reliably assess the psychiatric diagnoses in our preschool sample, we adapted the K-SADS examinations to preschool children, as has done previously in studies that validated the use of the K-SADS in non-22q11DS preschool children [Reference Birmaher, Ehmann, Axelson, Goldstein, Monk and Kalas18].
2.2.2. Evaluation of ASD symptoms
All participants underwent the gold standard assessments of ASD [Reference Kim and Lord19] including the ADI-R [Reference Lord, Rutter and Le Couteur20] and the Autism Diagnostic Observation Schedule, second edition (ADOS-2) [Reference Lord, Rutter, DiLavore, Risi, Gotham and Bishop21]. They also underwent a clinical examination by a child psychiatrist in order to confirm the diagnosis and severity of autism. The ADI-R interview generates scores in three areas of content (communication and language, social interaction, and restricted, repetitive behaviors) and is based on DSM-IV and ICD-10 criteria for autism. A classification of autism was assigned when scores in all three areas met or exceeded the specified cutoffs. In line with the analysis of Bishop et al. [Reference Bishop, Farmer, Bal, Robinson, Willsey and Werling22] we used the nonverbal algorithm scores of the ADI-R to compare between groups because some of the children in our sample were not verbal.
Table 1 Demographic characteristics of the study sample.

Table 2 Psychiatric disorders in children with 22q11DS.
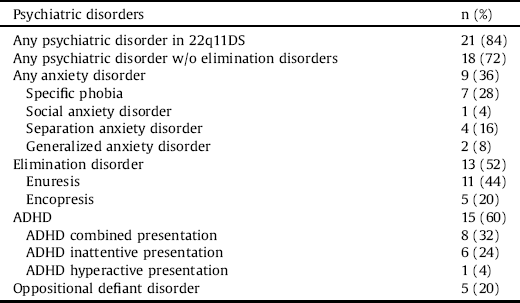
Table 3 Comparison of severity of autistic and psychiatric symptoms among study groups.
The ADOS-2 [Reference Lord, Risi, Lambrecht, Cook, Leventhal and DiLavore23] is a standardized observational assessment that consists of various activities aimed to elicit behaviors for evidencing the quality of social interactions and communicative abilities, as well as repetitive and restrictive behaviors associated with autism. We used three modules of the ADOS-2 in the current study: module 1 for participants with no speech and/or single-word vocabularies, module 2 for participants with phrase speech, and module 3 for participants with fluent speech (see Table 1 for the distribution of the modules). In addition to the ASD and autism cutoff scores, we used the diagnostic algorithms and calibrated scores for the overall severity score [Reference Gotham, Pickles and Lord24], as well as the calibrated severity scores for social affect and restricted and repetitive behaviors [Reference Hus, Gotham and Lord25]. These calibrated scores were standardized to allow comparison of symptom domains across modules of the ADOS-2.
2.2.3. Cognitive assessment
The general cognitive level was measured by the short version (vocabulary and block design tasks) [Reference Silverstein26] or the full version of the Wechsler Preschool and Primary Scale of Intelligence (WPPSI-III) for children aged 2–7 ½ years [Reference Wechsler27], or the Wechsler Intelligence Scale for children version IV (WISC-IV) for children older than 7 ½ years [Reference Wechsler28]. Some of the young children were assessed using the Mullen Scales of Early Learning [Reference Mullen29, Reference Swineford, Guthrie and Thurm30, see Table 1 for the distribution of the Wechsler versions].
2.2.4. Data analysis
Data were analyzed using SPSS software for Mac Version 25. Categorical variables were compared by Pearson's χ2 tests for proportions analysis. Continuous variables were compared by independent sample t-tests. Comparisons between the 22q11DS children with ASD to iASD and between 22q11DS with vs. without ASD were made using the Mann Whitney test.
3. Results
3.1. Rates of psychiatric disorders in 22q11DS
Of the 22q11DS group, 84% met DSM-5 diagnostic criteria for at least one psychiatric disorder, and the mean number of psychiatric diagnoses in that group was 2.04 ± 1.51. The prevalence of 22q11DS psychiatric diagnoses was: ADHD = 60%, elimination disorders = 52%, anxiety disorders = 36% and oppositional defiant disorder = 20%. None of the children fulfilled the criteria for a psychotic disorder (see Table 2).
3.2. Rates of ASD in 22q11DS
Based on the ADOS-2, the ADI-R and best clinical judgment, only four children with 22q11DS (16%) fulfilled the DSM-5 criteria for ASD. Four children (16%) were diagnosed as having autism on the ADI-R, and 12 children (48%) were diagnosed as being positive for any of the ADI-R domains. Within the different ADI-R domains, ten children (40%) were above the cutoff score of reciprocal social interaction, eight children (32%) above the communication cutoff score, and 12 children (48%) above the restricted, repetitive and stereotyped behavior cutoff score. Five children (20%) fulfilled the diagnostic criteria for ASD on the ADOS-2. One child that fulfilled the ADOS-2 but not the ADI-R criteria for ASD was judged clinically not to have ASD, but did fulfill the DSM-5 criteria for generalized anxiety disorder.
3.3. Comparison of autistic and psychiatric symptoms severity
3.3.1. 22q11DS versus iASD
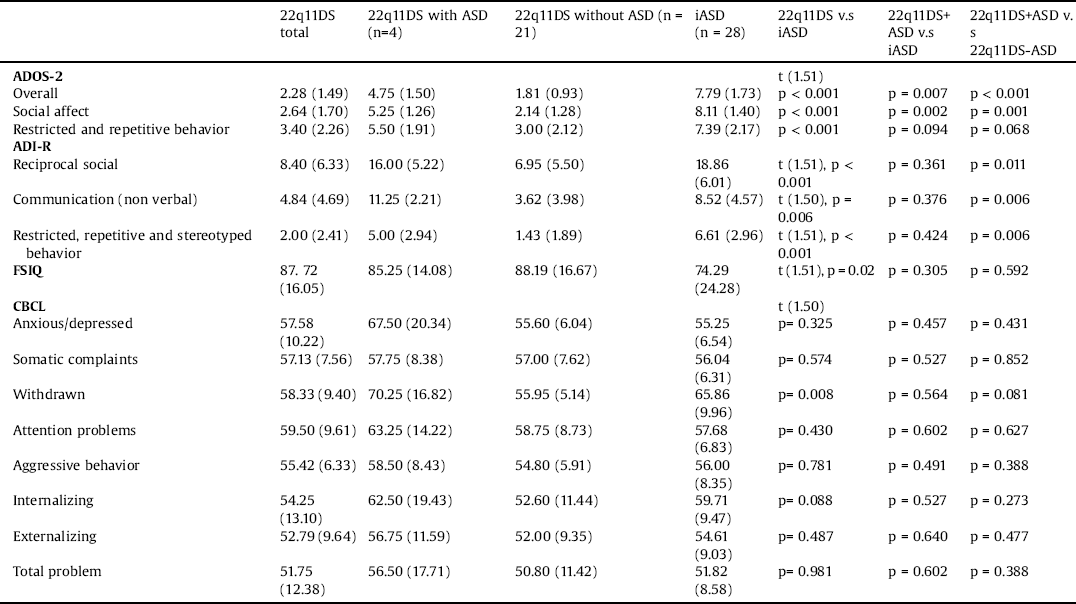
Children with idiopathic autism had significantly higher overall and subscale scores on the ADOS-2 and the ADI-R compared to children with 22q11DS (p < 0.050 for all, see Table 3). Severity of psychiatric symptoms, as measured by the CBCL, was similar for the children with 22q11DS and those with iASD for all domains except for the scores for withdrawal, which were significantly higher in the iASD group (p = 0.008, Table 3).
3.3.2. 22q11DS with ASD versus iASD
The overall calibrated scores of the ADOS-2 were significantly higher in children with iASD compared to 22q11DS with ASD (p = 0.007 Mann Whitney). Within the ADOS-2 subscales, the social affect calibrated scores were higher in the iASD group than in the 22q11DS with ASD group (p = 0.002), while the repetitive restricted behavior calibrated scores did not differ significantly between groups (p = 0.094). Comparison of the specific items within the ADOS-2 revealed that children with 22q11DS and ASD were less impaired than children with iASD in nonverbal communication items, including gestures (p = 0.028) and facial expressions (p = 0.017). Within the ADI-R domains, there were no significant differences between 22q11DS with ASD and the iASD group.
There were no significant differences in the severity of psychiatric symptoms measured by the CBCL for the children with 22q11DS with ASD and those with idiopathic autism for all domains (see Table 3).
3.3.3. 22q11DS with ASD versus 22q11DS without ASD
Children with 22q11DS with ASD scored significantly higher in the overall calibrated scores of the ADOS-2 compared to 22q11DS without ASD (p < 0.001). Within the ADOS-2 subscales, the social affect calibrated scores were also higher in the 22q11DS with ASD group than in the 22q11DS without ASD (p = 0.001). Conversely, the repetitive restricted behavior calibrated scores did not differ significantly between groups (p = 0.068). When comparing the severity of psychiatric symptoms on the CBCL, there were no significant differences between groups for all domains. There were also no differences in cognitive abilities between 22q11DS groups as indicated by FSIQ scores (see Table 3).
4. Discussion
To our knowledge, this is the first study to assess psychiatric disorders in young children with 22q11DS using the approach of a structured psychiatric diagnostic interview. It is also one of a few studies to use the complete gold standard diagnostic evaluation (i.e., ADI-R + ADOS + clinical evaluation) to examine the prevalence of ASD in young children with 22q11DS and to compare it to a matched control group of children with iASD.
4.1. Rates of ASD in 22q11DS
In accordance with our hypothesis, young children with 22q11DS showed lower rates of ASD diagnosis (16%) using the comprehensive gold standard diagnostic evaluation for ASD that included ADOS evaluations of the children in contrast to most previous studies in 22q11DS that relied solely on interviews with the parents as a single diagnostic tool [Reference Antshel, Aneja, Strunge, Peebles, Fremont and Stallone6, Reference Fine, Weissman, Gerdes, Pinto-Martin, Zackai and McDonald-McGinn10, Reference Kates, Antshel, Fremont, Shprintzen, Strunge and Burnette11, Reference Vorstman, Morcus, Duijff, Klaassen, Heineman-de Boer and Beemer15].
The 16% rate of ASD found in our study is in line with the 14% rate reported in a study by Fine et al. [Reference Fine, Weissman, Gerdes, Pinto-Martin, Zackai and McDonald-McGinn10] on younger children (2–12 years) with 22q11DS. Of note, most studies that reported higher rates of ASD in individuals with 22q11DS (20%–50%) were conducted on older children and adults [Reference Antshel, Aneja, Strunge, Peebles, Fremont and Stallone6, Reference Kates, Antshel, Fremont, Shprintzen, Strunge and Burnette11, Reference Niklasson, Rasmussen, Oskarsdóttir and Gillberg12, Reference Niklasson, Rasmussen, Oskarsdóttir and Gillberg13, Reference Vorstman, Morcus, Duijff, Klaassen, Heineman-de Boer and Beemer15]. Three of the four children in our study with 22q11DS who were diagnosed with ASD met the criteria for ASD on the ADOS-2, but did not meet the ADOS-2 criteria for Autism, in line with previous studies identifying that a less severe form of ASD is common in 22q11DS [Reference Niklasson, Rasmussen, Oskarsdóttir and Gillberg12, Reference Niklasson, Rasmussen, Oskarsdóttir and Gillberg13].
As previously mentioned, one potential explanation for the lower rate of ASD in our study compared to some previous studies [Reference Antshel, Aneja, Strunge, Peebles, Fremont and Stallone6, Reference Vorstman, Morcus, Duijff, Klaassen, Heineman-de Boer and Beemer15] could be related to differences in methods of assessment of autism between our research and others. Previous studies on ASD and 22q11DS that relied only on parents’ interviews using the ADI-R [Reference Antshel, Aneja, Strunge, Peebles, Fremont and Stallone6, Reference Kates, Antshel, Fremont, Shprintzen, Strunge and Burnette11, Reference Vorstman, Morcus, Duijff, Klaassen, Heineman-de Boer and Beemer15] reported higher ASD rates in 22q11DS (20–50%), while the studies that used an observational diagnostic instrument like the ADOS [Reference Angkustsiri, Goodlin-Jones, Deprey, Brahmbhatt, Harris and Simon9, Reference Ousley, Evans, Fernandez-Carriba, Smearman, Rockers and Morrier14] reported lower rates of ASD (0–18%), suggesting that adding observational measures of ASD to parent-report measures provide lower rates of ASD diagnosis in 22q11DS.
Another potential explanation for the relatively low ASD rates is the high rates of ADHD found in our young sample, which may suggest that some individuals diagnosed with ADHD could switch diagnosis to ASD later during childhood and adolescence. This has been reported in non-22q11DS preschool children with ADHD: about one-third of those with remitted ADHD were diagnosed with ASD at age 12 years [Reference Law, Sideridis, Prock and Sheridan31]. Longitudinal studies beginning in preschool years and focused on ADHD and ASD in 22q11DS are needed to verify our assumption that some young children with ADHD might switch to ASD later in childhood and adolescence.
A third potential explanation for the low ASD rates found may be that children with high comorbid psychiatric or neurologic disorders, like in 22q11DS, were found to be diagnosed with ASD later than children without such comorbidities [Reference Daniels and Mandell32]. This example, along with the diagnostic overlap between ADHD and ASD, raises the possibility that ADHD and neurological conditions may overshadow the diagnosis of ASD in 22q11DS. It is important to note that since there is a high proportion of males in our 22q11DS sample, the rates of ASD found in our study are most likely to be higher than if our 22q11DS sample was balanced in terms of the sex distribution.
4.2. Psychiatric diagnoses in 22q11DS
As hypothesized, we found high rates of psychiatric comorbidities in our cohort of young 22q11DS children, including ADHD (60%) and anxiety disorders (36%).
Although a high prevalence of ADHD and anxiety disorders has been reported in previous 22q11DS studies [Reference Schneider, Debbané, Bassett, Chow, Fung and van den Bree5], none of them assessed children under the age of six years [Reference Schneider, Debbané, Bassett, Chow, Fung and van den Bree5], and none examined young children with 22q11DS using a structured psychiatric diagnostic interview.
The diagnosis of psychiatric disorders in preschool children with developmental disorders can be a complex endeavor and can probably explain why this is the first study that conducted a structured psychiatric evaluation of 22q11DS preschool children. Psychiatric disorders can be reliably diagnosed already in preschool age. For example, the Diagnostic Classification of Mental Health and Developmental Disorders of infancy and Early Childhood (DC:0-5) points out that ADHD can be diagnosed from age 36 months and provides the same specifiers- inattentive and hyperactive-impulsive - similarly to the ADHD specifiers in older children [33].
While the 36% rate of anxiety disorders found in our sample of young children with 22q11DS is similar to that reported in older children and adolescents with 22q11DS [Reference Schneider, Debbané, Bassett, Chow, Fung and van den Bree5], the 60% rate of ADHD in our sample was much higher than the 37% that had been reported in previous studies on school-age children with 22q11DS [Reference Schneider, Debbané, Bassett, Chow, Fung and van den Bree5]. A recent longitudinal study demonstrated that the rate of ADHD in 22q11DS declines from 37% in children age 6–12 years to 16% in adults [Reference Schneider, Debbané, Bassett, Chow, Fung and van den Bree5]. Taken together with the findings from our study, it seems that there is a decline in ADHD rates from preschool years to childhood to adulthood. The higher rates of ADHD in preschoolers with 22q11DS is also in line with findings from longitudinal studies of non-22q11DS children showing that 30% of preschoolers diagnosed with ADHD at the age 5 no longer met ADHD criteria at age 12, and several of them are diagnosed instead with different psychiatric disorders including anxiety disorders, ASD and learning disorders [Reference Law, Sideridis, Prock and Sheridan31]. We believe that some cases of ADHD in 22q11DS preschoolers can be a nonspecific 'prodrome' presentation for variety of psychiatric disorders that will later 'crystalize' to other psychiatric diagnoses such as ASD and schizophrenia. This is supported by findings of a recent study showing that ADHD is a risk factor for the later development of psychotic disorders in 22q11DS [Reference Niarchou, Chawner, Fiksinski, Vorstman, Maeder and Schneider34].
4.3. Comparison of psychiatric symptoms severity between 22q11DS and iASD
The children in our study with 22q11DS and ASD had similar scores on all CBCL scales besides withdrawal scores, on which children with iASD scored higher than the 22q11DS children (Table 3). These findings indicate that even though children with 22q11DS present with fewer impairments in the social-communication profile than children with ASD, they have the same severity of psychiatric symptomatology as children with iASD, emphasizing the disability of young children with 22q11DS.
4.4. Comparison of the autism phenotype between 22q11DS and iASD
To our knowledge, the current study is the second report that compared individuals with 22q11DS with individuals with idiopathic autism, and the first to compare the autism phenotype of 22q11DS to idiopathic autism in younger ages. As predicted, we found that children with 22q11DS diagnosed with ASD are less impaired than children with iASD in measures of ASD core symptoms, expressed by lower overall calibrated scores of the ADOS-2 (p = 0.007). In line with these findings, children with de novo mutations diagnosed with ASD showed less impairment in measures of ASD core symptoms in comparison to children with iASD [Reference Bishop, Farmer, Bal, Robinson, Willsey and Werling22].
Within the ADOS-2 subscales, we found less impairment in the social affect domain and similar severity in the restricted and repetitive behavior domain of the ADOS-2 in children with 22q11DS with autism compared to those with iASD. Within the social affect domain, nonverbal communication symptoms were less severe in our children with 22q11DS autism, in line with Kates et al. [Reference Kates, Antshel, Fremont, Shprintzen, Strunge and Burnette11] who also observed that communication was less impaired in 22q11DS than in iASD and that repetitive behaviors were equally severe in both groups. The relatively high scores found in our 22q11DS group for the restricted and repetitive behavior domain is also in line with previous research led by members of the current study found that repetitive and restrictive behaviors, such as perseveration in speech and repetitive questions, were very common in 22q11DS, occurring in 53% of individuals with 22q11DS [Reference Gothelf, Presburger, Zohar, Burg, Nahmani and Frydman35].
4.5. Comparison of the autism phenotype between 22q11DS with vs. without ASD
When comparing children with 22q11DS with ASD versus 22q11DS without ASD, we found that children with 22q11DS and ASD had more severe ASD symptoms in all ADI-R and ADOS-2 domains besides the repetitive restricted behavior calibrated scores on the ADOS-2. As stated previously, high repetitive and restricted behaviors are common and debilitating in young children with 22q11DS [Reference Gothelf, Presburger, Zohar, Burg, Nahmani and Frydman35], and as our study shows, this is common in 22q11DS both with and without autism. From our experience, when a child with 22q11DS is informed about exciting events (e.g, a gift he or she is expecting to receive) within a short notice rather than far ahead of time, it reduces his or her repetitive questions reflecting inability to delay gratification. Furthermore, when parents are guided how to better regulate the anxieties and phobias of their child with 22q11DS the repetitive questions related to future events they are anxious about are lessened.
4.6. Limitations
The main limitation of this study is the relatively small sample size, particularly the small number of children with 22q11DS and coexisting ASD. In addition, there were multiple comparisons conducted. Yet, most of the results of the analysis showed similar directionality and mostly in line with our hypotheses. The sample was composed mainly of males in order to match the children in the idiopathic autism group, thus precluding our ability to analyze potential sex effects. To overcome the small sample size, we chose to use non-parametrical analysis when comparing ASD symptomatology and psychiatric symptom severity between the 22q11DS subgroups and iASD group. It is noteworthy that because children with 22q11DS have a distinctive facial phenotype, we were not able to make this a blind study. The CBCL means in our study sample were relatively low- in the non-clinical range. Although the CBCL has been used in several studies of children with intellectual disabilities including in 22q11DS [Reference Briegel, Schneider and Schwab7, Reference Klaassen, Duijff, Swanenburg de Veye, Vorstman, Beemer and Sinnema8], to our knowledge there are no studies that provide norm-referenced data in youth with 22q11DS or other developmental disabilities. Possibly, the relatively lower than expected CBCL scores found in our study sample suggest that the thresholds for abnormal CBCL scale may be lower than those established for typically developing children.
The mean FSIQ in our cohort was higher than the typical FSIQ reported for 22q11DS [Reference Swillen36]. It is assumed that FSIQ scores are somewhat higher in younger children than in older ones with 22q11DS, but there is limited empirical data on FSIQ in preschoolers with 22q11DS [Reference Swillen36]. Most of the children with 22q11DS in our study were assessed with the short versions of the Wechsler test, which can lead to higher IQ estimates compared to full version assessments [Reference Hurks, Hendriksen, Dek and Kooij37]. The fact that the iASD group was not available for the K-SADS evaluation, limited our ability to assess any differences in the prevalence of comorbid psychiatric disorders between the 22q11DS and iASD groups.
5. Conclusion
In conclusion, this is among the first studies to comprehensively assess the psychiatric phenotype in young children with 22q11DS. Using a rigorous clinical assessment, we found that 16% of young children with 22q11DS met DSM-5 criteria for ASD. We also found high rates of psychiatric disorders, specifically ADHD and anxiety, already apparent at a very young age. These findings stress the need for an early psychiatric assessment in this population. To our knowledge, there are overall limited data on the effectiveness of pharmacological and non-pharmacological treatments for individuals with 22q11DS, and none for preschoolers with 22q11DS. Because anxiety disorders and ADHD are risk factors for present and future maladjustment in 22q11DS, we believe study treatments targeting these disorders should be conducted with 22q11DS preschoolers.
Ethics
The work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Informed consent was obtained from all the subjects included in the study.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
This study was funded by the Binational Science Foundation, grant number 2017369; The National Institute of Mental Health of the National Institutes of Health under Award Number U01MH101722.
Authors’ contribution
GD, SY and SC designed the study. SY, SFD, DK, SHD, WR and SC collected the original data. SY, GD and SFD managed and analyzed the data. SY, GD and SC wrote the first draft of the manuscript. All the authors contributed to and approved the final manuscript.
Acknowledgments
The authors thank all individuals and their parents who participated in the study.






Comments
No Comments have been published for this article.