Introduction
Psychotic disorders such as schizophrenia are frequently severe and disabling and associated with poor functional outcomes [Reference Carrion, McLaughlin, Goldberg, Auther, Olsen and Olvet1, Reference Iasevoli, Giordano, Balletta, Latte, Formato and Prinzivalli2]. Given the sometimes-modest treatment response associated with antipsychotic agents, novel approaches including omega-3 polyunsaturated fatty acids (PUFAs) have been postulated as potential novel therapeutic options. Initial interest in omega-3 PUFAs derived from documented abnormalities of the phospholipid membrane in schizophrenia and other psychotic disorders, with Horrobin and colleagues, suggesting that dysfunctional fatty acid metabolism could be of aetiological significance [Reference Horrobin, Glen and Vaddadi3]. Considering the importance of phospholipids for neuronal functioning, alterations in phospholipid membrane may cause secondary abnormalities in various neurotransmitters, ion channels, and cell signalling systems due to changes in protein structures and cell signalling mechanisms [Reference Horrobin, Glen and Vaddadi3, Reference Horrobin4]. Correction of abnormal membrane structure by targeting modulatory activities involved in phospholipid metabolism using omega-3 PUFAs has therefore been suggested [Reference Nuss, Tessier, Ferreri, De Hert, Peuskens and Trugnan5]. Due to their role in phospholipid synthesis, enzyme regulation, and membrane modulation, omega-3 PUFAs may potentially prevent biochemical changes observed in psychotic disorders. For example, reduced levels of omega-3 PUFAs, in particular, docosahexaenoic acid (DHA) in peripheral blood plasma and erythrocyte membranes of schizophrenia patients at different development stages [Reference Yao, Leonard and Reddy6, Reference Rice, Schafer, Klier, Mossaheb, Vijayakumar and Amminger7] (individuals at ultra-high risk (UHR) for psychosis [Reference Amminger, Schafer, Klier, Slavik, Holzer and Holub8], unmedicated first-episode psychosis (FEP) [Reference Reddy, Keshavan and Yao9], and chronic patients [Reference Parletta, Zarnowiecki, Cho, Wilson, Procter and Gordon10]) have been reported. Moreover, the breakdown of phospholipids and reduction of DHA in the brain orbitofrontal cortex has been demonstrated in psychotic patients [Reference McNamara, Jandacek, Rider, Tso, Hahn and Richtand11], suggesting a potential association between a deficit of omega-3 PUFAs, including DHA deficit and the pathogenesis of psychotic disorders. Clinical trials examining the efficacy of omega-3 PUFAs in psychotic disorders have however provided variable results. Potential reasons for this variability include the utilization of different doses or formulations of omega-3 PUFAs (including either eicosapentaenoic acid (EPA) or DHA predominant formulations) and the conduct of trials in different subgroups of patients with psychosis (UHR, FEP, and chronic schizophrenia). Despite some initial studies demonstrating a reduction in transition to psychosis in individuals at UHR for psychosis [Reference Amminger, Schafer, Papageorgiou, Klier, Cotton and Harrigan12, Reference Mossaheb, Schafer, Schlogelhofer, Klier, Smesny and McGorry13], not all studies have subsequently replicated these findings [Reference McGorry, Nelson, Markulev, Yuen, Schafer and Mossaheb14]. For individuals experiencing a FEP, omega-3 PUFA supplementation has been associated with a reduction in psychotic symptoms [Reference Peet, Brind, Ramchand, Shah and Vankar15-Reference Pawelczyk, Grancow-Grabka, Zurner and Pawelczyk18], although again these findings have not been universally replicated [Reference Berger, Proffitt, McConchie, Yuen, Wood and Amminger19]. Among chronic schizophrenia patients, variable results have also been noted. For example, an amelioration of symptoms has been demonstrated in patients diagnosed with treatment-resistant schizophrenia on clozapine after omega-3 PUFA supplementation [Reference Peet, Horrobin and Group20]; however, a number of other studies have demonstrated no significant clinical improvement in chronic patients compared to healthy controls after omega-3 treatment [Reference Emsley, Niehaus, Koen, Oosthuizen, Turner and Carey21–Reference Bentsen, Osnes, Refsum, Solberg and Bohmer23]. A number of previous systematic reviews and meta-analyses have been conducted to ascertain the efficacy of omega-3 PUFA supplementation for psychotic symptoms [Reference Goh, Chen, Chen and Lu24–Reference Xu, Shao, Zhang, Hu, Huang and Su27]. Most consist of a modest number of studies and participants, with variable findings. No improvement with omega-3 PUFA supplementation has been noted with EPA and EPA/DHA supplementation in schizophrenia [Reference Chen, Chibnall and Nasrallah25–Reference Xu, Shao, Zhang, Hu, Huang and Su27] with the exception of the largest previous meta-analysis consisting of 13 studies that noted an improvement in overall symptoms (standard mean difference (SMD) = −0.27, 95% CI −0.41, −0.14, p < 0.001) [Reference Goh, Chen, Chen and Lu24]. Beneficial effects were also noted for individuals at UHR for psychosis or experiencing a FEP; however, only two studies were analyzed for both these groups [Reference Goh, Chen, Chen and Lu24] with other reviews noting no benefit of omega-3 PUFAs in individuals at UHR for psychosis albeit low numbers of participants were included in analyses [Reference Devoe, Farris, Townes and Addington28, Reference Devoe, Peterson and Addington29].
Consequently, there is a lack of clarity in relation to the potential therapeutic benefit of omega-3 PUFAs across the spectrum of psychotic disorders. Thus, this systematic review and meta-analysis including all previously published RCTs explores if omega-3 PUFAs exhibit (1) a therapeutic benefit in psychotic disorders, (2) greater efficacy at different stages of psychosis (UHR v. FEP v. chronic schizophrenia), and (3) a differential impact on positive compared to negative symptoms.
Method
Data sources
The systematic review was conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann and Mulrow30]. The PRISMA checklist is presented in Supplementary Table S1. The protocol for the systematic review was registered on PROSPERO, the National Institute of Health Research Database (Registration Number: CRD42023438350).
A manual systematic electronic search of studies utilizing omega-3 PUFAs in psychotic disorders was conducted through the following databases: Medline, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL). The search included all relevant articles published until November 2024, without language restrictions. The following subject heading keywords were used to find all relevant articles: psychosis OR psychotic disorder(s) OR schizophrenia OR schizophrenia spectrum OR non-affective disorder(s) OR FEP OR UHR for psychosis OR at-risk mental state (ARMS) AND omega-3 (n-3/ω-3) fatty acids OR essential fatty acids (EFAs) OR PUFAs OR EPA OR DHA OR fish oil OR nutritional supplement. A manual search was further performed for the above references from the papers identified, relevant reviews, Trials Central (http://www.trialscentral.org), the ISRCTN (http://controlled-trials.com), and Clinical Trials (http://clinicaltrials.gov) registries.
Study selection
Double-blind placebo-controlled studies examining the therapeutic effect of omega-3 PUFAs on psychotic symptoms either as a monotherapy or adjunctive therapy in adults and children with psychosis (UHR for psychosis, FEP, or established schizophrenia) either as a primary or secondary outcome were included. Unblinded, single-blind, open-label, and pilot studies were excluded. Studies examining the effect of omega-3 PUFAs on various neurochemical, biochemical, and biological compounds were excluded. Substance and/or medication-induced psychotic disorders and disorders where psychotic symptoms were a consequence of a mood disorder (i.e. affective psychotic disorders: bipolar disorder, major depressive disorder) were additionally excluded. A diagnosis of a psychotic illness required the utilization of operational criteria including the Diagnostic and Statistical Manual of Mental Disorders (DSM) – IV [31] or the International Classification of Disease (ICD) – 10 [32] for FEP and schizophrenia, or the Comprehensive Assessment of At-Risk Mental States (CAARMS) [Reference Yung, Yuen, McGorry, Phillips, Kelly and Dell’Olio33], or Structured Interview for Psychosis Risk Syndromes (SIPS) [Reference Miller, McGlashan, Rosen, Cadenhead, Cannon and Ventura34], for individuals with UHR for psychosis. For all studies, symptomatic assessment was either considered a primary or secondary outcome measure.
Data extraction
Two reviewers (A.R., B.H.) independently assessed and extracted relevant data including participants’ age, diagnosis, and type of antipsychotic treatment, trial duration, baseline psychometric scores and/or difference in scores between baseline and follow-up review(s) along with type, dosage, and prescription schedule of intervention. Corresponding authors of eligible studies were contacted by email in the event of incomplete or partly unavailable results. The following data were extracted by reviewers from July 8, 2023, and manually entered into a Microsoft Excel worksheet, later adapted into a table to visually display the results of individual studies (Table 1). Our primary analysis selected the following hierarchy of psychometric instruments: the Positive and Negative Syndrome Scale (PANSS; n = 21), the Brief Psychiatric Rating Scale (BPRS; n = 3), and the CAARMS (n = 1).
Table 1. Characteristics of randomized placebo-controlled trials of omega-3 polyunsaturated fatty acids on symptom severity of psychosis in UHR population and schizophrenia patients.
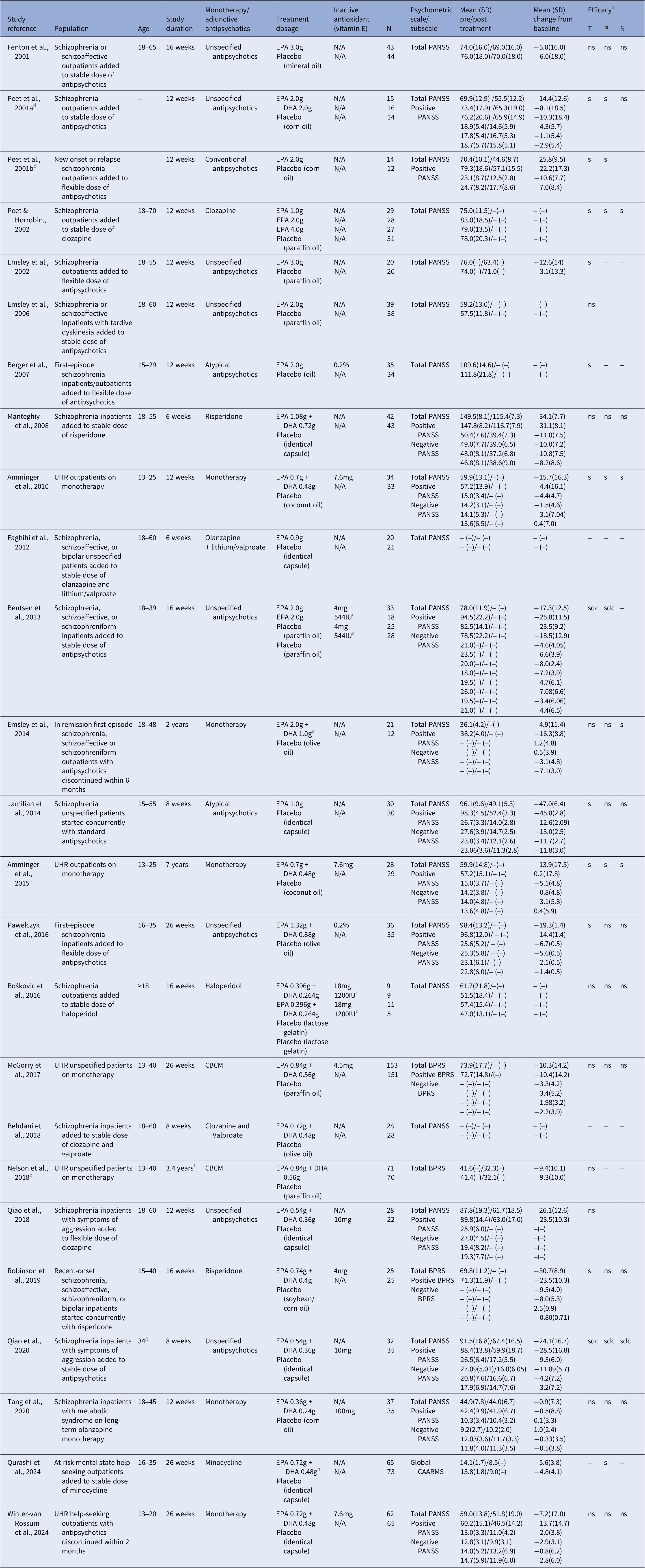
Abbreviations: BPRS, brief psychiatric rating scale; CAARMS, comprehensive assessment of at-risk mental state; CBCM, cognitive behavioural case management; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; N/A indicates data not applicable; PANSS, positive and negative syndrome scale. - indicates data unavailable; SD, standard deviation.
a Efficacy of omega-3 treatment on symptom improvement for each individual study. T = PANSS total scores; P = PANSS positive subscale; N = PANSS negative subscale; s = significant improvement of symptoms; sdc = significant decline of symptoms; ns = no significant change of symptoms.
b Medium-term/long-term follow-up outcome study from a previous randomized placebo-controlled trial.
c Vitamin E was treated as an active comparison in the study.
d Two different studies were published in a single article.
e Patients received daily dosages of 2 g EPA, 1 g DHA, and 0.3 g alpha-lipoic acid.
f Mean time to follow-up with a range of 1.5–5.7 years.
g Mean age of participants.
h Intervention groups included minocycline, omega-3, combined minocycline, and omega-3 or double placebo. Data presented are for omega-3 and double placebo only.
Statistical analysis
Mean change in psychometric data was calculated by subtracting post-intervention scores with baseline scores while SD change from baseline was calculated using the following equation:
![]() $ {\mathrm{SD}}_{\mathrm{Change}}=\sqrt{\left({\mathrm{SD}}_{\mathrm{B}\mathrm{aseline}}^2+{\mathrm{SD}}_{\mathrm{Final}}^2-2{\rho}_{\mathrm{B},\mathrm{F}}{\mathrm{SD}}_{\mathrm{B}\mathrm{aseline}}{\mathrm{SD}}_{\mathrm{Final}}\right)} $
. Correlation coefficient was estimated at 0.5 in the case of an unknown value. Results of mean difference were presented as negative values representing a reduction in psychotic symptoms.
$ {\mathrm{SD}}_{\mathrm{Change}}=\sqrt{\left({\mathrm{SD}}_{\mathrm{B}\mathrm{aseline}}^2+{\mathrm{SD}}_{\mathrm{Final}}^2-2{\rho}_{\mathrm{B},\mathrm{F}}{\mathrm{SD}}_{\mathrm{B}\mathrm{aseline}}{\mathrm{SD}}_{\mathrm{Final}}\right)} $
. Correlation coefficient was estimated at 0.5 in the case of an unknown value. Results of mean difference were presented as negative values representing a reduction in psychotic symptoms.
The Cochrane Review Manager version 5.4 was used to evaluate any treatment effect between the omega-3 and control groups. The effect sizes and covariate effects were combined across studies using random-effects meta-analysis models with inverse variance weighting used to summarize the effects across studies and estimate the SMDs and their corresponding 95% confidence intervals (CIs) for continuous outcomes. Finally, the heterogeneity of studies was assessed using the I 2 statistic before evaluating any publication bias using a funnel plot asymmetry.
Results
Literature search
A copy of the PRISMA flow diagram, outlining the search strategy of the literature is presented in Figure 1. The literature search yielded a total of 517 potentially relevant articles. Titles and abstracts were reviewed, and irrelevant articles were discarded. The use of automation filter tools when available were used to refine the literature search to RCTs only. Consequently, 48 full-text articles were examined, with 25 RCTs selected after meeting the inclusion and exclusion criteria. Eight studies were subsequently excluded from the meta-analysis due to insufficient baseline and/or follow-up psychometric scores [Reference Berger, Proffitt, McConchie, Yuen, Wood and Amminger19–Reference Emsley, Niehaus, Koen, Oosthuizen, Turner and Carey21, Reference Faghihi, Jahed, Mahmoudi-Gharaei, Sharifi, Akhondzadeh and Ghaeli35–Reference Behdani, Roudbaraki, Saberi-Karimian, Tayefi, Hebrani and Akhavanrezayat37], and for being duplicate samples of medium or long-term follow-up RCTs [Reference Nelson, Amminger, Yuen, Markulev, Lavoie and Schafer38, Reference Amminger, Schafer, Schlogelhofer, Klier and McGorry39].

Figure 1. PRISMA 2020 flow diagram. Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flowchart. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. Doi: 10.1136/bmj.n71. For more information, visit: https://www.prisma-statement.org.
Selected studies
Included studies evaluated UHR (n = 6) [Reference Amminger, Schafer, Papageorgiou, Klier, Cotton and Harrigan12, Reference McGorry, Nelson, Markulev, Yuen, Schafer and Mossaheb14, Reference Nelson, Amminger, Yuen, Markulev, Lavoie and Schafer38–Reference Winter-van Rossum, Slot, van Hell, Bossong, Berger and Aschauer41], FEP (n = 3) [Reference Pawelczyk, Grancow-Grabka, Kotlicka-Antczak, Trafalska and Pawelczyk17, Reference Berger, Proffitt, McConchie, Yuen, Wood and Amminger19, Reference Emsley, Chiliza, Asmal, du Plessis, Phahladira and van Niekerk42], and chronic schizophrenia (n = 16) [Reference Peet, Brind, Ramchand, Shah and Vankar15, Reference Peet, Horrobin and Group20–Reference Bentsen, Osnes, Refsum, Solberg and Bohmer23, Reference Faghihi, Jahed, Mahmoudi-Gharaei, Sharifi, Akhondzadeh and Ghaeli35–Reference Behdani, Roudbaraki, Saberi-Karimian, Tayefi, Hebrani and Akhavanrezayat37, Reference Fenton, Dickerson, Boronow, Hibbeln and Knable43–Reference Qiao, Liu, Han, Liu, Shao and Xie48, Reference Hartmann, Yuen, McGorry, Yung, Lin and Wood50] (Table 1). Of the 16 studies examining individuals with schizophrenia, five included individuals either with schizoaffective or schizophreniform disorder [Reference Emsley, Niehaus, Koen, Oosthuizen, Turner and Carey21,Reference Bentsen, Osnes, Refsum, Solberg and Bohmer23,Reference Emsley, Chiliza, Asmal, du Plessis, Phahladira and van Niekerk42,Reference Fenton, Dickerson, Boronow, Hibbeln and Knable43]. In addition, two studies included participants with bipolar disorder [Reference Faghihi, Jahed, Mahmoudi-Gharaei, Sharifi, Akhondzadeh and Ghaeli35, Reference Robinson, Gallego, John, Hanna, Zhang and Birnbaum47] and were deemed eligible for inclusion considering the small number of participants from this cohort [Reference Faghihi, Jahed, Mahmoudi-Gharaei, Sharifi, Akhondzadeh and Ghaeli35, Reference Robinson, Gallego, John, Hanna, Zhang and Birnbaum47]. Omega-3 supplementation was administered as an adjunct to stable or flexible doses of antipsychotics with the exception of 6 RCTs using monotherapy only [Reference Amminger, Schafer, Papageorgiou, Klier, Cotton and Harrigan12, Reference McGorry, Nelson, Markulev, Yuen, Schafer and Mossaheb14, Reference Nelson, Amminger, Yuen, Markulev, Lavoie and Schafer38, Reference Amminger, Schafer, Schlogelhofer, Klier and McGorry39, Reference Emsley, Chiliza, Asmal, du Plessis, Phahladira and van Niekerk42, Reference Hartmann, Yuen, McGorry, Yung, Lin and Wood50]. Clozapine was used as an adjunctive treatment in 6 RCTs [Reference Peet, Horrobin and Group20, Reference Bentsen, Osnes, Refsum, Solberg and Bohmer23, Reference Fenton, Dickerson, Boronow, Hibbeln and Knable43–Reference Jamilian, Solhi and Jamilian45, Reference Qiao, Liu, Han, Liu, Shao and Xie48]. However, only a single study provided separate outcome data for this group [Reference Peet, Horrobin and Group20], preventing subgroup analyses of differential treatment response between clozapine and first or second-generation antipsychotics.
The 17 identified studies for the meta-analysis included 1440 participants between 13 and 70 years of age. All studies used either mixed EPA and DHA formulations or EPA-predominant formulations, with only one study including a stratum with a DHA formulation [Reference Peet, Brind, Ramchand, Shah and Vankar15]. Consequently, no analysis comparing EPA versus DHA predominant formulations was undertaken.
Therapeutic efficacy
Omega-3 PUFAs did not demonstrate any significant reduction in psychotic symptoms compared to placebo on psychometric total scales (G = −0.26, 95% CI −0.55 to 0.03, p = 0.08) (Figure 2), positive subscales (G = −0.12, 95% CI −0.42 to 0.19, p = 0.45) (Figure 3), and negative subscales (G = 0.12 95% CI −0.30 to 0.53, p = 0.58) (Figure 4). Subgroup analyses did not demonstrate significant omega-3 treatment efficacy for total symptom scores in UHR (G = −0.09, 95% CI −0.45 to 0.27, p = 0.63), FEP (G = −1.20, 95% CI −5.63 to 3.22, p = 0.59), and schizophrenia (G = −0.17, 95% CI −0.38 to 0.03, p = 0.10) (Figure 2). Similarly, no improvement was found for positive symptom scores across all groups (UHR: G = −0.26, 95% CI −0.88 to 0.36, p = 0.41), (FEP: G = −1.02, 95% CI −3.30 to 1.26, p = 0.38) and (schizophrenia: G = 0.09, 95% CI −0.14 to 0.32, p = 0.47) (Figure 3) nor did for negative symptoms scores (UHR: G = −0.17, 95% CI −0.71 to 0.36, p = 0.53), (FEP: G = −0.25, 95% CI −2.51 to 2.01, p = 0.83) and (schizophrenia: G = 0.32, 95% CI −0.30 to 0.53, p = 0.26) (Figure 4). Subgroup analyses of omega-3 PUFA dosages did not demonstrate any statistical overall effect between improvement in psychopathology and dosages administered across all stages of psychosis (Figure 5).

Figure 2. Standardized mean difference for changes in psychopathology from scores on selected psychometric total scale. UHR: ultra-high risk for psychosis; FEP: first-episode psychosis; CI: confidence interval; SD: standard deviation.

Figure 3. Standardized mean difference for changes in psychopathology from scores on selected psychometric positive subscale. UHR: ultra-high risk for psychosis; FEP: first-episode psychosis; CI: confidence interval; SD: standard deviation.

Figure 4. Standardized mean difference for changes in psychopathology from scores on selected psychometric negative subscale. UHR: ultra-high risk for psychosis; FEP: first-episode psychosis; CI: confidence interval; SD: standard deviation.

Figure 5. Standardized mean difference for changes in psychopathology from scores on selected psychometric total scale by dosage of omega-3 supplementation. UHR: ultra-high risk for psychosis; FEP: first-episode psychosis; CI: confidence interval; SD: standard deviation.
Quality assessment
The quality assessment for all the RCTs is presented in Table 2. The overall risk of bias in the individual studies was low as assessed by the Cochrane Risk of Bias Assessment Tool with the exception of 4 studies [Reference Manteghiy, Shakeri, Koohestani and Salari22, Reference Boskovic, Vovk, Koprivsek, Plesnicar and Grabnar36, Reference Jamilian, Solhi and Jamilian45, Reference Qiao, Mei, Han, Liu, Yang and Shao46]. Some concerns in the randomization process, specifically regarding knowledge of the forthcoming interventions allocation by investigators and/or participants, were revealed for nine studies [Reference McGorry, Nelson, Markulev, Yuen, Schafer and Mossaheb14,Reference Manteghiy, Shakeri, Koohestani and Salari22,Reference Boskovic, Vovk, Koprivsek, Plesnicar and Grabnar36,Reference Fenton, Dickerson, Boronow, Hibbeln and Knable43–Reference Robinson, Gallego, John, Hanna, Zhang and Birnbaum47] and for missing outcome measures for two studies [Reference Boskovic, Vovk, Koprivsek, Plesnicar and Grabnar36, Reference Fenton, Dickerson, Boronow, Hibbeln and Knable43]. High risk of bias due to missing outcome data was also noted for four studies [Reference Manteghiy, Shakeri, Koohestani and Salari22, Reference Boskovic, Vovk, Koprivsek, Plesnicar and Grabnar36, Reference Jamilian, Solhi and Jamilian45, Reference Qiao, Mei, Han, Liu, Yang and Shao46]. Publication bias for the meta-analysis RCTs was assessed qualitatively by funnel plot asymmetry (Figure 6). It was estimated that a number of studies with negative effect sizes might never have been published; however, this plot evaluated all studies, with the three studies demonstrating a more positive therapeutic effect noted to include individuals at UHR for psychosis or with a FEP.
Table 2. ROB2 risk of bias assessment for individual randomized placebo-controlled trials.
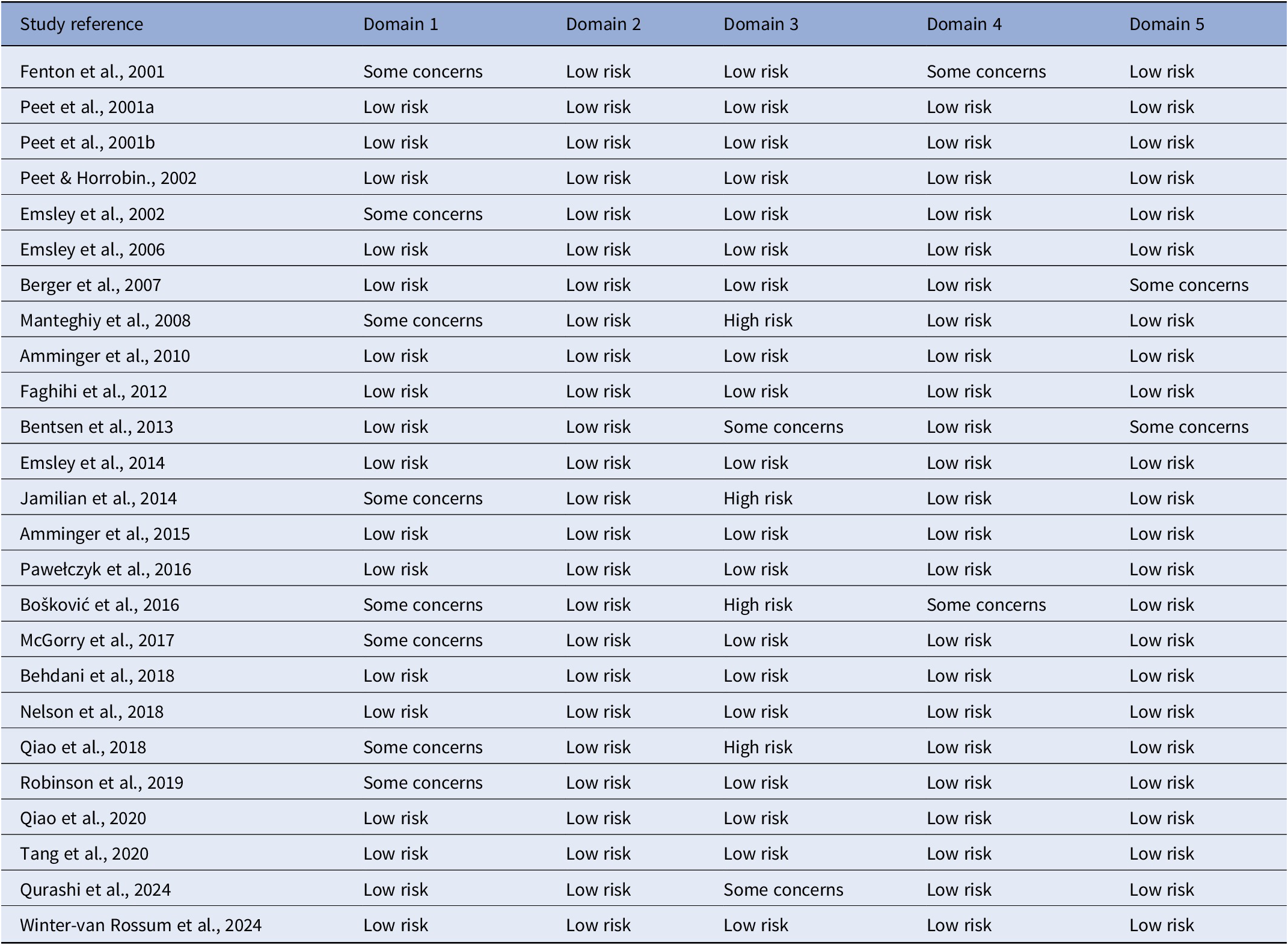
Note: Domain 1: Risk of bias arising from the randomization process; Domain 2: Risk of bias due to deviations from the intended interventions; Domain 3: Risk of bias due to missing outcome data; Domain 4: Risk of bias in measuring of the outcome; Domain 5: Risk of bias in selection of the reported results.

Figure 6. Funnel plot of standard error (SE) by SMD.
Discussion
The meta-analysis evaluated the efficacy of omega-3 supplementation in UHR population and schizophrenia patients from all previously published RCTs. Omega-3 PUFAs supplementation did not reveal clinical efficacy for psychosis when compared to placebo. A trend towards improvement in total psychotic symptoms was however noted (p = 0.08). Subgroup analyses did not demonstrate any beneficial effect on both positive and negative symptoms across all groups.
In line with previous research, our findings demonstrate that omega-3 PUFAs supplementation is not recommended as a treatment for acute exacerbation of chronic schizophrenia nor for prevention of relapse [Reference Chen, Chibnall and Nasrallah25, Reference Boskovic, Vovk, Koprivsek, Plesnicar and Grabnar36, Reference Fenton, Dickerson, Boronow, Hibbeln and Knable43, Reference Qiao, Liu, Han, Liu, Shao and Xie48]. Additionally, our findings are in line with recent reports that found no beneficial effect of omega-3 supplements in UHR population [Reference McGorry, Nelson, Markulev, Yuen, Schafer and Mossaheb14, Reference Qurashi, Chaudhry, Khoso, Omair Husain, Hafeez and Kiran40, Reference Winter-van Rossum, Slot, van Hell, Bossong, Berger and Aschauer41] although in contradiction with the results from Amminger and colleagues [Reference Amminger, Schafer, Papageorgiou, Klier, Cotton and Harrigan12]. In light with the accumulating evidence of negative findings, these past positive findings are complex to understand. It is suggested that factors such as improvement in nonpharmacological treatments, comedication, illness severity, and a recent decline in transition rates may contribute to this discrepancy [Reference Winter-van Rossum, Slot, van Hell, Bossong, Berger and Aschauer41, Reference Hartmann, Yuen, McGorry, Yung, Lin and Wood50]. The most effective dosage of omega-3 PUFAs was 1 g when compared to the other dosages. No optimal omega-3 PUFAs dosage could however be established as no superior statistical effect was found between administration of <1 g, 1g, 2 g, >2 g across all groups. The EPA/DHA dosage and content needed for treatment efficacy has yet to be confirmed and dose-ranging studies are currently limited.
Our findings should be considered in light of some limitations. First, the sample sizes and patient populations in the included RCTs were not consistent, particularly for RCTs of FEP which may have affected the validity and generalizability of the outcomes. Furthermore, baseline characteristics varied across all studies when controlling for diagnostic tools, severity of illness and omega-3 formulations. Publication bias was also found for a number of studies and included non-reporting of outcome measures and/or psychopathology domains. In addition, the effect of adjunctive antipsychotics or nonpharmacological treatments such as CBCM could not be excluded. Because no specification of adjunctive antipsychotics was provided on a number of RCTs, this prevented further analyses for such effect. The outcome measures in this study had relatively small to medium effect sizes, suggesting limited practical significance of the results. Finally, significant heterogeneity in findings was noted. This is suggested, however, to be related to heterogeneous sample populations and variable omega-3 PUFA formulations utilized.
Conclusion
The current evidence supports initially reported results on the use of omega-3 PUFAs in the treatment of symptom severity in prodromal/chronic schizophrenia patients and more recently in populations at high-risk states for psychosis. Omega-3 supplementation is not considered to be a suitable early treatment strategy for psychotic disorders and future studies in this line of research are not suggested.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1192/j.eurpsy.2024.1804.
Data availability
All data supporting the findings of this study are available within the article.
Acknowledgements
The authors have no acknowledgements to make.
Author contribution
Conceptualization, A.R., and B.H.; study design, A.R., and B.H.; data collection and analysis, A.R., and B.H.; writing – original draft preparation, A.R.; writing – review and editing, A.R., and B.H.; supervision, B.H. All authors have read and agreed to the published version of the manuscript.
Competing interest
The authors declare none.











Comments
No Comments have been published for this article.