Introduction
The exact contributions of explicit and implicit processes to motor adaptation are unknown. While the cerebellum is thought to have a major role in the implicit processes, it is thought that other brain areas contribute more explicit processes (Taylor et al., Reference Taylor, Krakauer and Ivry2014). In a seminal paper, Mazzoni and Krakauer (Reference Mazzoni and Krakauer2006) demonstrated that implicit adaptation overrides explicit strategy in a visuomotor-rotation task. Though the explicit strategy led to an instant correction of the error, participants started to drift away from the target ending close to their aiming location, i.e. implicit adaptation started to occur around the aiming location instead of the target and overrode the strategy – this rather counterintuitive behavioural finding has not been directly replicated. Interestingly, patients with cerebellar degeneration can better use strategy for adaptation compared to controls where performance deteriorates (Taylor et al., Reference Taylor, Klemfuss and Ivry2010), suggesting a primary role of the cerebellum in implicit, but not explicit motor adaptation.
Objective
To investigate the cerebellum’s role in implicit motor adaptation by inhibiting its function with 1 Hz rTMS before, participants performed a 45° counter-clockwise visuomotor-rotation task using an explicit strategy. rTMS has previously been used successfully to inhibit the cerebellum and produce behavioural changes (see Théoret et al., Reference Théoret, Haque and Pascual-Leone2001; Miall & Christensen, Reference Miall and Christensen2004; Jenkinson & Chris Miall, Reference Jenkinson and Chris Miall2010). If the cerebellum is responsible for the implicit motor adaptation that has been shown to drive the participant away from the target (Mazzoni & Krakauer, Reference Mazzoni and Krakauer2006) after initially correcting for the rotation using strategy, inhibition of the cerebellum should reduce the speed or size of the drift towards the aiming target and result in sustained explicit compensation of the rotation.
Methods
Stimulation (N = 12: 7 women, age 27 ± 2, range 18–36) was applied using a double-cone coil at 55% of maximum-stimulator output (MSO) (MagStim Super Rapid) (Jenkinson & Chris Miall, Reference Jenkinson and Chris Miall2010) over the right cerebellar hemisphere, 1 cm below and 3 cm lateral from the inion. Sham stimulation (N = 10: 8 women, age 24 ± 2, range 20–39) was performed with a flat figure-of-eight coil at 35% MSO, 4 cm below and 6 cm lateral from the inion, which induced muscle twitches around the neck that were similar to the active cerebellar stimulation without targeting the cerebellum (Hardwick et al., Reference Hardwick, Lesage and Chris Miall2014). All participants were blind to the effectiveness of stimulation and received 600 pulses of 1 Hz TMS before they performed a visuomotor-rotation task with a joystick similar to Mazzoni and Krakauer (Reference Mazzoni and Krakauer2006). See Figure 1 for details.
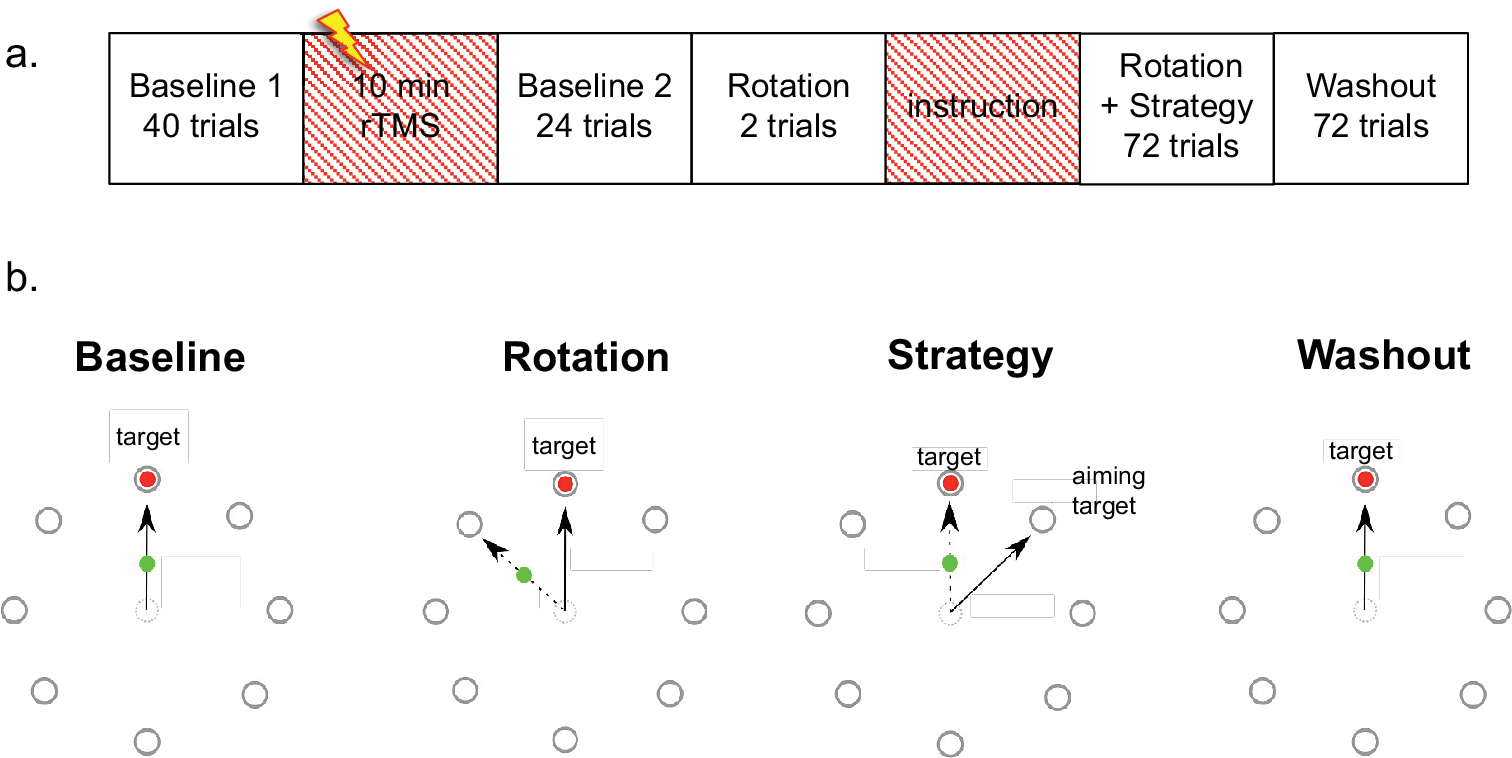
Figure 1. Experimental paradigm (a) and visuomotor-rotation task (b). Participants performed 40 trials without rotation (Baseline 1), before receiving 10 minutes of rTMS, followed by another 24 trials without rotation (Baseline 2; ~2.5 mins). They were then exposed to two trials where a 45° counter-clockwise rotation was imposed on the cursor output before being instructed to use strategy. The strategy entailed to aim for the clockwise neighbouring target next to where the red cursor would appear. After the instruction participants performed another 72 trials of rotation (Rotation + Strategy; ~7 mins) and then 72 trials where the rotation was removed and no strategy had to be used (Washout; ~7 mins).
Results
No group differences in directional error (DE) were found for baseline 1 (average last 2 trials); t(20) = .944, p = .357 and baseline 2; t(20) = .582, p = .567, confirming similar behaviour before adaptation. DE during the initial two trials of rotation did not differ between the groups; t(15.7) = .161, p = .874. The use of strategy initially helped overcome the rotation, but implicit adaptation overrode this explicit strategy around the aiming target in both groups (see Figure 2). TMS did not have an effect on DE during early; t(20) = .920, p = .368 and late adaptation; t(20) = .093, p = .927, nor during early; t(20) = .150, p = .882 or late washout; t(20) = 1.761, p = .093 (first and last 8 trials).

Figure 2. Average directional error across all trials during the five phases (Baseline 1, Baseline 2, 2 trials Rotation, Rotation + Strategy, and Washout) for both the Sham (blue) and TMS (red) group.
Discussion
We found that rTMS of the cerebellum had no effect on implicit adaptation; in both groups implicit adaptation overrode explicit strategy. The stimulation site used is thought to correspond to cerebellar lobule VIII (Manni & Petrosini, Reference Manni and Petrosini2004), and has been used for cerebellar studies investigating hand use (Théoret et al., Reference Théoret, Haque and Pascual-Leone2001). Though hand representations are mainly thought to be in lobule VIII, there are representations in lobule V (Manni & Petrosini, Reference Manni and Petrosini2004; Wiestler et al., Reference Wiestler, McGonigle and Diedrichsen2011). However, this may be an underestimate of the distribution (see Mottolese et al., Reference Mottolese, Richard, Harquel, Szathmari, Sirigu and Desmurget2012) which may have prevented us from inhibiting all the representations. Regardless of the negative finding, this interesting behavioural paradigm demonstrating the strength of implicit motor adaptation appears robust and replicable.
Conclusions
Contrary to our hypothesis, 1Hz TMS of the cerebellum did not result in a reduced implicit drift. Therefore, we are left with two likely conclusions:
1. The cerebellum was inhibited, but inhibition of the cerebellum had no influence on implicit motor adaption.
2. The stimulation failed to inhibit the cerebellum, or the area of the cerebellum was not the area (or areas) of the cerebellum controlling adaptation of the hand.
Our results represent an important replication of a rather counterintuitive behavioural finding, and emphasizes the strength of the implicit processes that take place during motor adaptation (wherever these processes takes place) and the lack of influence that explicit strategy has upon these processes.
Author contribution
NJ & SV conceived and designed the study. SV, MP & NJ conducted data gathering. SV performed statistical analyses. SV & NJ wrote the article.
Funding information
This work was supported by funding from both Parkinson’s UK (H-1402 & G-1108) and the Medical Research Council (MR//J004588/1).
Conflict of interest
The authors NJ, MP & SV declare none.
Ethics statement.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008
Data Availability Statement
The data that support the findings of this study are openly available upon request to the corresponding author.





Comments
Comments to the Author: The negative result is interesting but requires commenting on whether the protocol was adequate to achieve cerebellar inhibition. I suggest pointing out that the protocol appropriately reproduced elements of two previous studies that achieved motor task disruption through cerebellar rTMS (Theoret et al. 2001, and Jenkinson and Miall 2010) but pulse intensity (35% of max. stimulator output) was lower than in Jenkinson and Miall 2010 (who used 45%, 55% of MSO and the same coil type). It could be argued that 35% could have been adequate because Jenkinson & Miall targeted the oculomotor vermis, and if this region is deeper than the region targeted here then 35% could have been adequate. The present study was well designed, with attention to details of rTMS protocol that make the study of interest even if the result is negative. Indeed, the authors should point out more explicitly that their protocol reproduced the crucial features of protocols that previously showed inhibitory effect on cerebellar tissue. Then they should acknowledge that the pulse intensity they chose may have been inadequate to inhibit cerebellar circuits responsible for hand visuomotor adaptation, and that this may be an alternative explanation for the negative result.The language has minor errors including some syntax that makes a few sentences unclear. I suggest that the authors have the manuscript edited to smooth out these minor unclear spots. Examples: lines 31, 33, 37, 46, 77.