Introduction
Anoxygenic phototrophic Fe(II)-oxidising (pFeOx) bacteria, first reported by Widdel et al. (Reference Widdel, Schnell, Heising, Ehrenreich, Assmus and Schink1993), catalyse light-induced biological oxidation of Fe(II) coupled to the fixation of CO2 into biomass. They have been found in numerous aquatic and terrestrial environments, including lakes, streams and ditches (Ehrenreich et al., Reference Ehrenreich and Widdel1994; Jiao et al., Reference Jiao, Kappler, Croal and Newman2005; Bryce et al., Reference Bryce, Blackwell, Schmidt, Otte, Huang, Kleindienst, Tomaszewski, Schad, Warter and Peng2018). One such strain, Rhodopseudomonas palustris TIE-1, is a purple non-sulfur bacterium isolated from an iron-rich freshwater mat (Jiao et al., Reference Jiao, Kappler, Croal and Newman2005). Under dark conditions, R. palustris TIE-1 can grow chemoheterotrophically, whilst in light it can switch to a photoheterotrophic metabolism using a range of organic substrates as carbon source (Jiao et al., Reference Jiao, Kappler, Croal and Newman2005). Under illuminated, anoxic and organic-poor conditions, R. palustris TIE-1 has been shown to grow photoautotrophically using Fe(II), H2 or thiosulfate as electron donor for maintaining metabolic energy while turning CO2 (or dissolved HCO3–) into cell biomass (Larimer et al., Reference Larimer, Chain, Hauser, Lamerdin, Malfatti, Do, Land, Pelletier, Beatty and Lang2004; Hegler et al., Reference Hegler, Posth, Jiang and Kappler2008). Besides R. palustris TIE-1, a number of other pFeOx bacteria have been isolated from freshwater habitats, such as Chlorobium ferrooxidans strain KoFox (Heising et al., Reference Heising, Richter, Ludwig and Schink1999), Thiodictyon sp. strain F4 (Croal et al., Reference Croal, Johnson, Beard and Newman2004), Rhodomicrobium vannielii strain BS-1 (Heising et al., Reference Heising and Schink1998) and Rhodobacter ferrooxidans sp. strain SW2 (Ehrenreich et al., Reference Ehrenreich and Widdel1994). These pFeOx bacteria oxidise Fe(II) photoautotrophically according to the following stoichiometric equation (Eq. 1; Ehrenreich et al., Reference Ehrenreich and Widdel1994):
It has been suggested that Fe(II)-oxidising bacteria primarily oxidise dissolved, and thus easily bioavailable, Fe(II) (Fe2+aq) to form poorly soluble Fe(III) oxyhydroxides as reaction products (Kappler et al., Reference Kappler and Newman2004). Organically complexed forms of Fe(II) provide another bioavailable form of Fe(II) to these bacteria (Peng et al., Reference Peng, Bryce, Sundman and Kappler2019), increasing the known pool of Fe(II) sources for photoferrotrophic bacteria in the environment. However, it is still not fully resolved whether R. palustris TIE-1 can oxidise structural (solid-phase) Fe(II) bound in minerals or whether this process is controlled by the dissolution of the mineral, with bacteria instantly oxidising the released Fe2+aq, as was suggested previously (Kappler et al., Reference Kappler and Newman2004; Hegler et al., Reference Hegler, Posth, Jiang and Kappler2008; Lliros et al., Reference Lliros, Garcia-Armisen, Darchambeau, Morana, Triado-Margarit, Inceoglu, Borrego, Bouillon, Servais and Borges2015). Studies on biological phototrophic Fe(II) oxidation of naturally abundant mixed-valent Fe(II)/(III) minerals, such as magnetite and green rust, have provided evidence that R. palustris TIE-1 can gain metabolic energy by using Fe(II) present in Fe(II) minerals or released from solid iron minerals as source of electrons (Byrne et al., Reference Byrne, Klueglein, Pearce, Rosso, Appel and Kappler2015; Han et al., Reference Han, Tomaszewski, Sorwat, Pan, Kappler and Byrne2020).
Photoautotrophic growth by pFeOx bacteria on Fe(II) requires a carbon source for biomass formation. Commonly provided as an inorganic carbon species such as bicarbonate (HCO3–), it also acts as a pH buffer (Widdel et al., Reference Widdel, Schnell, Heising, Ehrenreich, Assmus and Schink1993), in equilibrium with an anoxic gas mixture containing CO2 in the gaseous phase (Jiao et al., Reference Jiao, Kappler, Croal and Newman2005; Camacho et al., Reference Camacho, Walter, Picazo and Zopfi2017). Recently, it was demonstrated that the extracellular electron uptake from solid phases, such as conductive electrodes, is linked to carbon fixation of dissolved HCO3– (CO2) in R. palustris TIE-1 (Guzman et al., Reference Guzman, Rengasamy, Binkley, Jones, Ranaivoarisoa, Singh, Fike, Meacham and Bose2019). This observation suggests that Fe(II) oxidation and CO2 fixation for building up biomass are enzymatically coupled within this microbial organism. However, the actual mechanism of extracellular electron transport (EET) in R. palustris TIE-1 has, so far, not been well documented in the context of structural imaging or specific pili-associated genes. However, results by Bose et al. (Reference Bose, Gardel, Vidoudez, Parra and Girguis2014) suggest that – even though pili were not directly visualised – structures facilitating direct electron transfer to and from solid phases are present.
Siderite represents a solid-phase resource for both ferrous iron and bicarbonate (FeIICO3). Following the chemical equilibrium for chemical partitioning in aqueous systems (Eq. 2; Bleam, Reference Bleam2017):
the dissolution of siderite in water provides ferrous iron (Fe(II)) as an electron source for energy generation and CO2 fixation via Fe(II) oxidation and inorganic carbon (as HCO3– or CO32–) for biomass formation in photoferrotrophic bacteria. Especially in oligotrophic, organic-poor environments, siderite could serve as the only substrate, enabling photolithoautotrophy by organisms such as R. palustris TIE-1. Such an environment has been recently described for a terrestrial hot spring where a thermophilic consortium of bacteria was found to grow by fully anaerobic autotrophic siderite oxidation with CO2/HCO3– as the only electron acceptor and only carbon source (Zavarzina et al., Reference Zavarzina, Kochetkova, Chistyakova, Gracheva, Antonova, Merkel, Perevalova, Chernov, Koksharov and Bonch-Osmolovskaya2020). Even in freshwater environments, such as artesian wells with siderite abundant as solid particles, in rivers (Ivanova et al., Reference Ivanova, Lepokurova, Pokrovskii and Shvartsev2014; Jakus et al., Reference Jakus, Mellage, Hoeschen, Maisch, Byrne, Mueller, Grathwohl and Kappler2021; Huang et al., Reference Huang, Gao, Liu, Mao, Kang, Geng, Song, Cai, Guo and Chen2024), in terrestrial wetlands such as intertidal marshes (Pye et al., Reference Pye, Dickson, Schiavon, Coleman and Cox1990; Lin et al., Reference Lin, Turchyn, Krylov and Antler2020) or even in extreme environments such as acidic rivers, as potential Mars analogues (Sanchez-Roman et al., Reference Sanchez-Roman, Fernandez-Remolar, Amils, Sanchez-Navas, Schmid, Martin-Uriz, Rodriguez, McKenzie and Vasconcelos2014; Silva et al., Reference Silva, Vincenzi, de Araujo, Venceslau and Rodrigues2024), the oxidation of siderite as a ferrous carbon source for pFeOx could considerably increase the pool of bioavailable substrates for these bacteria. Whether anoxygenic phototrophic Fe(II)-oxidising bacteria can grow and stay alive by utilising only siderite as the solely metabolic substrate, however, is unknown.
The aim of this study was to determine whether pFeOx bacteria can maintain growth and metabolic activity with siderite serving as the only electron donor and substrate for biomass fixation. We hypothesise that biological Fe(II) oxidation is mostly controlled by chemical siderite dissolution and thus by the chemical equilibrium between the solid and liquid phases of Fe(II) and HCO3–. The abundances of these species are in turn dictated by the geochemical constraints, including pH, ionic strength, geochemical composition of the medium and temperature (Roh et al., Reference Roh, Zhang, Vali, Lauf, Zhou and Phelps2003). However, we also anticipate that, in addition to using dissolved Fe2+aq released from the siderite by dissolution, R. palustris TIE-1 can harvest electrons from structurally bound Fe(II) in solid-phase siderite.
Materials and methods
Microbial strain, cultivation, medium
Rhodopseudomonas palustris strain TIE-1 was first isolated from a marsh in Woods Hole, MA, USA (Jiao et al., Reference Jiao, Kappler, Croal and Newman2005). For the current study, the culture was pre-cultured under anoxic conditions on a basal anoxic minimal salt medium (at pH 6.8) following Ehrenreich and Widdel (Reference Ehrenreich and Widdel1994) modified by Jiao et al. (Reference Jiao, Kappler, Croal and Newman2005) with 5 mM Fe2+aq (from FeCl2) as electron donor.
Siderite synthesis
Siderite was produced inside an anoxic glovebox (100% N2) by slowly adding 1.0 g of Na2CO3 to 400 mL of anoxic ultrapure water while stirring (E. Field, pers. comm.). Subsequently, 3.9 g of Fe(SO4)2NH4 was added while stirring the solution for another 3 min. Precipitates were allowed to settle before washing five times with anoxic ultrapure water. Siderite identity was confirmed by 57Fe Mössbauer spectroscopy (Fig. S5, supplementary material).
Unconstrained siderite dissolution
To determine whether R. palustris TIE-1 can grow on Fe(II) and HCO3– from siderite dissolution, we incubated TIE-1 on the culture medium at pH 6.8 at constant 27°C with siderite placed in dialysis bags (Spectra/Por7 Dialysis membrane, molecular weight cut-off (MWCO), 15 kD, 6.4 mL/cm; Spectrum Laboratories). These bags only allow diffusion of dissolved Fe2+aq and HCO3– out of the bag into the culture medium but prevent direct contact between bacteria and siderite particles.
Siderite precipitates were filled anoxically into sterile dialysis bags (3 mM final concentration). Bags were closed with plastic zip ties before being placed inside culture bottles containing 25 mL of medium (no HCO3–/CO2) and 100% N2 headspace. A 10% inoculum of R. palustris TIE-1, pre-grown on Fe2+aq (with 22 mM HCO3–), was added to each of the three biological replicates. No cells were added to abiotic controls. Incubations were kept under constant illumination (~700 lux, 60 W tungsten light bulb) at 27°C. Dissolved Fe(II) (Fe2+aq), total Fe(II) and Fe(III) were quantified as described below. At the end of the incubation (28 days), total Fe(II) and Fe(III) were quantified inside the dialysis bag and samples were collected from outside and inside the dialysis bag for imaging by scanning electron microscopy (SEM).
Constrained siderite dissolution
R. palustris TIE-1 was grown in medium at pH 6.8 amended with different HCO3– concentrations (0–300 mM) to vary and minimise siderite dissolution and to determine the extent of solid-phase siderite oxidation. The culture medium was prepared as detailed by Ehrenreich et al. (Reference Ehrenreich and Widdel1994) and HCO3– concentrations were adjusted to the desired concentrations (see the Geochemical model to predict the extent of siderite dissolution at various HCO3– concentrations in the supplementary material). A series of 10% cell inocula of R. palustris TIE-1, pre-grown on siderite and adapted to their respective HCO3– concentrations (i.e. 0, 22, 50, 100, 200 and 300 mM) were inoculated in 10 mL of medium at constant 27°C containing HCO3– concentrations of 0, 22, 50, 100, 200 and 300 mM and a 5 mL headspace of N2/CO2 (90/10 v/v) in triplicate.
Abiotic controls contained no cells. All setups were incubated for 35 days while samples for dissolved Fe(II) (Fe2+aq), as well as the precipitated Fe(II) and Fe(III) ratio (Fe(II)/(III)ppt) were collected over the course of the incubation. Samples for SEM were collected prior to incubation and at the end of the incubation. Samples for 57Fe Mössbauer spectroscopy were collected from one representative replicate of each treatment at the end of the incubation while two remaining replicates were sacrificed for a total iron mineral extraction to determine Fe2+aq and Fe(II)/(III)ppt.
Effect of elevated HCO3– concentrations on cell viability
To identify a potential inhibiting effect of elevated HCO3– concentrations on cell growth, R. palustris TIE-1 was grown under H2/CO2 (80/20 v/v) in triplicate with H2 as an electron donor in excess. HCO3– concentrations were adjusted to 0, 22, 50, 100, 200 and 300 mM and 10% inoculum (pre-grown on H2) was added. The cultures were incubated for 29 days under constant illumination at 27°C while optical density (600 nm) was measured to quantify cell growth (Specord 50 plus; Analytic Jena).
Quantification of iron concentration
Iron was quantified following the ferrozine assay (Stookey Reference Stookey1970). Samples (500–700 μL) were centrifuged anoxically (5 min, 13,600 rpm, 100% N2). Dissolved Fe(II) (Fe2+aq) was quantified in the supernatant (acidified in 1 M HCl [1:5]). The ratio of Fe(II) and Fe(III) in the precipitates (Fe(II)/(III)ppt) was quantified by dissolving the remaining pellet in 1 mL of HCl (2 M). Fe(II) and total iron (Fe(tot)) in all samples were quantified spectrophotometrically on a 96-well micro titer plate by measuring the absorbance at 562 nm (Multiskan GO; Thermo Fisher Scientific). Fe(III) was quantified as the difference between Fe(tot) and Fe(II).
Scanning electron microscopy
Samples for SEM were collected anoxically. A 1 mL sample was mixed with 100 μL of anoxic glutaraldehyde (GHA, 25%), 100 μL of anoxic paraformaldehyde (PFA, 20%) and 200 μL of anoxic PIPES buffer (100 mM) solution and stored at 4°C for 24 h. An aliquot was transferred onto a glass slide (diameter 0.5 cm) coated with poly-L-lysin (Sigma Aldrich) and allowed to rest for 10–15 min. The samples were dehydrated in anoxic ethanol series with concentrations of 15%, 30%, 50%, 70%, 80%, 90% and 96% for 10 min each and 2 × 10 min in 100% ethanol. The slide was then treated with hexamethyldisilazane (HMDS) for 10 s and air-dried. Slides were fixed on aluminium stubs with adhesive carbon tape and sputter-coated with platinum (8 nm). Image acquisition was performed on a Focused Ion Beam Scanning Electron Microscope (FIB-SEM) (Crossbeam 550L; Zeiss) in high-resolution imaging mode with electron high tension of 5 kV, working distance of 5 mm, probe current (I Probe) of 50 pA, dwell time of 50 ns and a secondary-electron secondary ion detector.
57Fe Mössbauer spectroscopy
Samples for 57Fe Mössbauer spectroscopy were collected from pristine siderite and from each biological treatment after 35 days. Slurry samples were filtered anoxically (0.45 μm MCE Membrane, 13 mm; Darmstadt), sealed between Kapton tape and frozen at –20°C until measurement. Samples were loaded inside a closed-cycle exchange gas cryostat (Janis cryogenics). Spectra were collected at 77 K with a constant acceleration drive system (WissEL) in transmission mode using a 57Co/Rh source that was calibrated against a 7 μm thick α-57Fe foil measured at room temperature. All spectra were analysed using Recoil (University of Ottawa) by applying a Voight-based fitting site analysis. The half width at half maximum was fixed to 0.132 mm/s for all samples.
Results and discussion
Photoautotrophy of R. palustris TIE-1 growing with siderite only
Experiments with siderite in dialysis bags, spatially separated from the R. palustris cells, were performed to investigate whether R. palustris TIE-1 can grow fully photoautotrophically with siderite as the only substrate undergoing chemical dissolution and providing Fe2+aq and HCO3–. Dissolved Fe2+aq concentrations increased rapidly over 7 days of incubation, reaching concentrations of >200 μM Fe2+aq in both biotic and abiotic control setups (Fig. 1a). This Fe2+aq accumulation outside the dialysis bag indicated both chemical siderite dissolution and diffusion of Fe2+aq from dialysis bags containing siderite into the surrounding liquid phase. In abiotic control setups, the accumulation of Fe2+aq continued until the end of the incubation (28 days), reaching concentrations of approximately 700 μM Fe2+aq, while in biotic setups, Fe2+aq concentrations rapidly decreased after 7 days, indicating microbial Fe(II) oxidation rates exceeding siderite dissolution and Fe2+aq diffusion rates out of the dialysis bags (Fig. 1a). Fe2+aq concentrations in biotic setups remained low (<100 μM) until the end of the incubation.
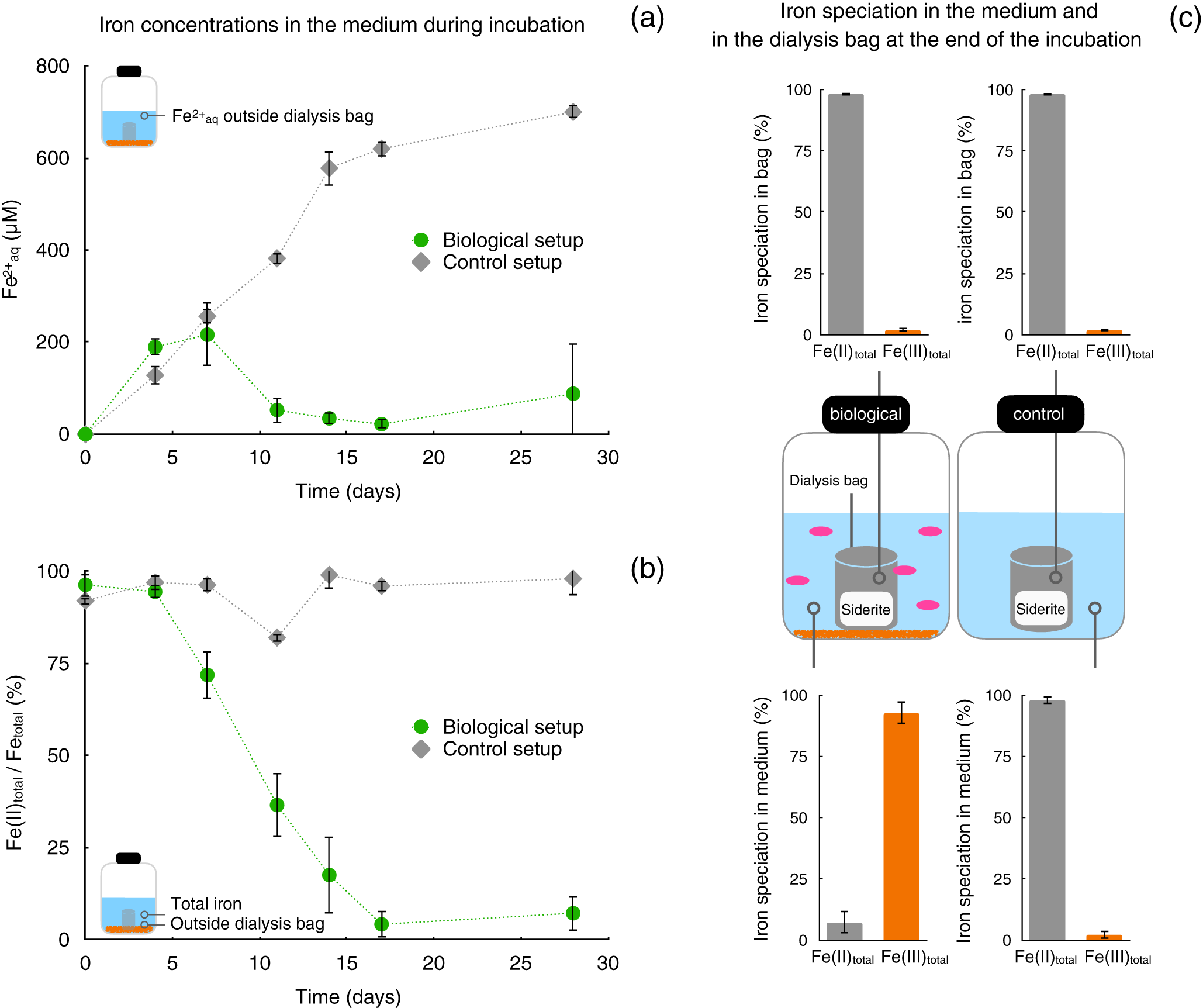
Figure 1. Mineral dissolution-controlled photoautotrophic siderite oxidation by Rhodopseudomonas palustris TIE-1. Dialysis bags filled with siderite incubated with anoxygenic phototrophic Fe(II)-oxidising bacteria, only allowing release of Fe2+aq and CO2/HCO3– into the medium while preventing direct contact of cells to the mineral surface. (a) Dissolved Fe(II) (Fe2+aq, μM) in the aqueous phase outside the dialysis bags and (b) Fe(II) ratios (Fe(II)total/(total) [%]) in the aqueous phase outside the dialysis bags over the course of incubations in setups without bacteria (control, grey) and with R. palustris TIE-1 (biological, green). (c) Total iron speciation at the end of the incubation (28 days) in aqueous phase and inside dialysis bags in biological setups (c, left) and abiotic control setups (c, right), respectively.
Microbial activity and phototrophic Fe(II) oxidation are also reflected in the Fe oxidation state of the total iron outside the dialysis bags. In control setups, Fe(II)/Fe(total) ratios remained high (>80%), indicating that Fe(II) oxidation was not catalysed by abiotic processes such as photooxidation (Fig. 1b). The Fe(II)/Fe(total) ratio in biological setups, however, gradually decreased following 4 days of incubation, demonstrating the occurrence of microbial Fe(II) oxidation outside the dialysis bag (Fig. 1b). After 17 days until the end of the incubation (28 days), Fe(III) made up more than 90% of the total iron fraction present outside of the dialysis bag. This was also indicated by the accumulation of Fe(III) precipitates (Fig. 1c), which were visually observed by orange precipitates on the outside surface of the bag. Samples collected from the outside of the dialysis bag compared to pristine siderite particles inside the bag using SEM (Fig. S2) further demonstrated the difference in the mineral shape. Iron precipitates inside the dialysis bags were largely dominated by Fe(II) (>98%) at the end of the incubation in both control and biotic setups (Fig. 1c).
Evidently, the accumulation of Fe(III) precipitates only appeared in biotic setups and only outside the dialysis bags. This not only suggests that (1) cells were kept spatially separated from the solid siderite substrate inside the dialysis bag over the entire course of the incubation but also that (2) biological phototrophic Fe(II) oxidation can be sustained by siderite alone, fully controlled by the chemical dissolution of the iron mineral and Fe2+aq (and HCO3–) diffusion out of the dialysis bag into the liquid culture medium in this setup.
These findings showed that fully photoautotrophic growth of R. palustris TIE-1 was possible with siderite serving as the only substrate. Under these experimental conditions, however, both microbial activity and Fe(II) oxidation kinetics were limited by the chemical dissolution of the pristine siderite mineral (Fig. S2a) and Fe2+aq (and HCO3–/CO2) diffusion into the culture medium. Chemical dissolution was previously speculated to be the main parameter controlling microbial oxidation of Fe(II)-bearing minerals. Several studies have demonstrated that phototrophic Fe(II)-oxidising bacteria can oxidise Fe(II)-bearing minerals (Kappler et al., Reference Kappler and Newman2004; Byrne et al., Reference Byrne, Klueglein, Pearce, Rosso, Appel and Kappler2015; Han et al., Reference Han, Tomaszewski, Sorwat, Pan, Kappler and Byrne2020). The largest extent in oxidation, however, was suggested to be attributed to iron mineral dissolution processes and free Fe2+aq becoming bioavailable to bacteria which oxidise the dissolved Fe2+aq to Fe(III).
Fully photoautotrophic growth on siderite could also affect the role of these bacteria in the environment. Especially in sunlit aquatic environments, which are dominated by fluctuating geochemical conditions (i.e. periodically poor in organics, Fe(II) and CO2), the availability of siderite as a source of Fe(II) and CO2/HCO3– might compensate for a temporary lack in metabolic substrates enabling phototrophic Fe(II)-oxidising bacteria to overcome harsh conditions to survive. Such environments were recently reported for tidal wetlands rich in Fe(II) and poor in organics, where siderite was found to be an abundant Fe(II)-bearing mineral phase that can potentially serve as an electron and carbon source to phototrophic Fe(II) oxidisers in the sunlit surface waters or the upper few millimetres of the sediments (Yu et al., Reference Yu, Xie, Song, Xia and Astrom2021). It remains unknown whether the growth and activity of phototrophic Fe(II)-oxidising bacteria is limited to chemical constraints that determine the dissolution and bioavailability of Fe(II) from siderite. Fully autotrophic activity of these bacteria based on siderite only can, however, affect other biogeochemical parameters, such as the oxidation state of dissolved iron, secondary iron mineral precipitation or the (im)mobilisation of other elements (Hegler et al., Reference Hegler, Posth, Jiang and Kappler2008; Lliros et al., Reference Lliros, Garcia-Armisen, Darchambeau, Morana, Triado-Margarit, Inceoglu, Borrego, Bouillon, Servais and Borges2015; Camacho et al., Reference Camacho, Walter, Picazo and Zopfi2017). In particular, the poorly soluble Fe(III) oxyhydroxides formed, as a biological Fe(II) oxidation product, can act as an efficient adsorbent for contaminants, such as arsenic, but also for numerous other elements such as phosphorous or potassium, which are essential for a large variety of organisms inhabiting the same environmental niche (Richardson Reference Richardson1985; Bjerrum et al., Reference Bjerrum and Canfield2002; Hohmann et al., Reference Hohmann, Winkler, Morin and Kappler2010). The immobilisation of these essential elements by freshly precipitated Fe(III) minerals can in turn favour the geochemical conditions for other flanking Fe(II) oxidisers by concentrating essential elements on the surface of Fe(III) oxides (Otte et al., Reference Otte, Harter, Laufer, Blackwell, Straub, Kappler and Kleindienst2018).
Phototrophic siderite oxidation by R. palustris TIE-1
To determine whether the phototrophic Fe(II) oxidiser R. palustris TIE-1 can directly oxidise Fe(II) minerals, and not just via oxidation of dissolved Fe2+aq, chemical siderite dissolution was minimised by increasing HCO3– concentrations in the liquid phase (following Eq. 2). Cultures of R. palustris TIE-1 were incubated in setups with elevated HCO3– concentrations of up to 300 mM, while Fe2+aq was quantified and the Fe(II)/Fe(total) ratio was determined for the precipitates over the course of the incubation. Control experiments with H2 as electron donor, rather than Fe(II), demonstrated such high HCO3– concentrations did not negatively affect the growth of the bacteria (Fig. S1). In the actual experimental setups using siderite, Fe2+aq accumulated rapidly in the dissolved phase, both in the control and in the biotic setup, within the initial 2 days of incubation (Fig. 2a,b). Dissolved Fe2+aq concentrations reached up to 100–150 μM, with the highest concentrations measured in the low (0–22 mM HCO3–) bicarbonate setup. In the abiotic control setups, Fe2+aq concentrations gradually increased during the incubation of 35 days. After approximately 27 days of incubation, accumulated Fe2+aq concentrations remained stable in control setups. With increasing bicarbonate concentrations of 0, 22, 50, 100, 200 and 300 mM, the final Fe2+aq concentrations decreased from 1.1, 0.9, 0.4, 0.2 to 0.1 mM with gradually less Fe2+aq in solution in setups with increasing HCO3– concentrations (Fig. 2b). These data confirmed that the elevated HCO3– concentrations successfully reduced the chemical dissolution of siderite, consequently leading to less Fe2+aq in the dissolved phase at higher HCO3– concentrations.
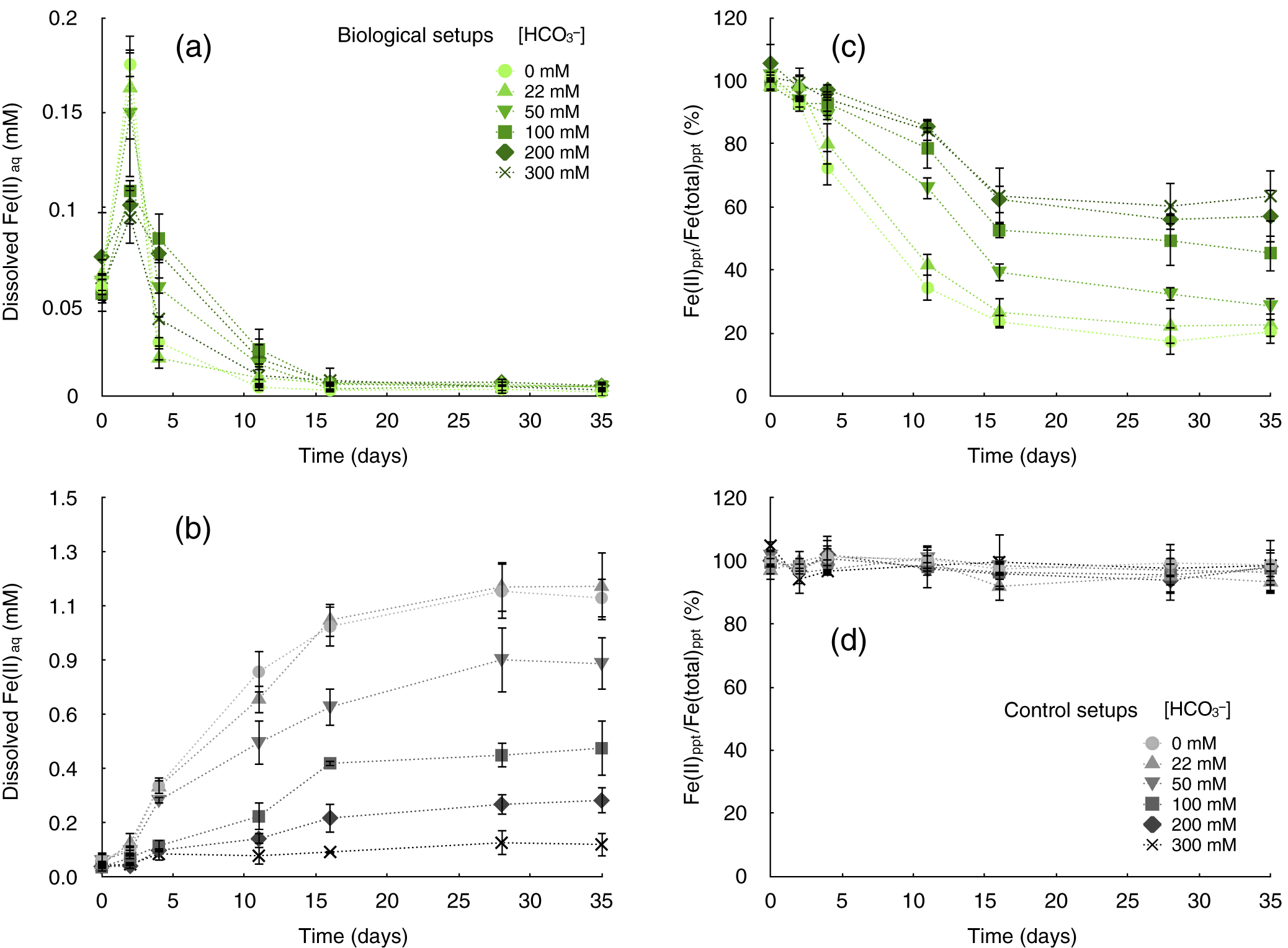
Figure 2. Microbial siderite oxidation by Rhodopseudomonas palustris TIE-1 in setups with suppressed siderite dissolution. Chemical siderite dissolution was suppressed by a gradual increase of HCO3– concentrations from 0 to 300 mM in setups with 3 mM siderite incubated with a 10% inoculum of R. palustris TIE-1 (a and c, biological setups, green symbols) and without cells (b and d, control setups, grey symbols). (a) Dissolved iron(II) concentrations (Fe2+aq, [mM]) in biological setups containing 0–300 mM HCO3–. (b) Concentrations of Fe2+aq (mM) in abiotic control setups containing 0–300 mM HCO3–. (c) Iron speciation in precipitates (Fe(II)/(total)ppt [%]) in biological setups containing 0–300 mM HCO3–. (D) Iron speciation in precipitates (Fe(II)/(total)ppt [%]) in abiotic control setups containing 0–300 mM HCO3–.
In biological incubations with TIE-1, however, the Fe2+aq concentrations rapidly decreased at all HCO3– concentrations within 4 days of incubation, indicating microbial Fe(II) oxidation of dissolved Fe2+aq (Table S2). Over 16 days of incubation, Fe2+aq remained constantly low until the end of the incubation in all biological treatments, which suggests that R. palustris TIE-1 instantly oxidised all Fe2+aq when it was released into solution from dissolving siderite particles. The microbial Fe(II) oxidation was also reflected in the iron speciation of the remaining solid mineral particles. Within the initial 16 days of incubation, the Fe(II) content in the solid mineral phase rapidly decreased to less than 25% in setups containing low (0 and 22 mM) HCO3– concentrations and to ~75% in setups with higher (200 and 300 mM) HCO3– concentrations (Fig. 2c). These Fe(II)/Fe(total) ratios remained constant after 16 days until the end of the incubation. In contrast, abiotic control setups showed no significant change in the Fe(II)/Fe(total) ratio in the solids over the course of the incubation independent from HCO3– concentrations, demonstrating that photocatalytic reactions did not impact the iron oxidation state of the siderite (Fig. 2d).
The microbial impact on the overall iron speciation and oxidation of Fe(II) in siderite becomes evident when considering the extent of oxidation and siderite dissolution in each HCO3– treatment, in both control and biotic setups, at the end of the incubation (after 35 days). While up to 30% of the Fe(II) from siderite was found as Fe2+aq in the dissolved phase in abiotic controls, no considerable Fe2+aq was measured in any biotic setup independently from HCO3– concentrations (Fig. 3). However, the extent of siderite dissolution (i.e. the relative concentration of Fe2+aq that accumulated in abiotic control setups) largely varied between the different HCO3– treatments. The accumulated Fe2+aq sharply decreased from >30% of the total iron for the low (0 and 22 mM) HCO3– treatments to <10% in setups with high (200 and 300 mM) HCO3– concentrations. In all biological setups, the relative abundance of Fe(III) that accumulated in the solid mineral fraction was high. The accumulated iron minerals were largely, by >80%, dominated by Fe(III) oxyhydroxides in setups containing 0–50 mM HCO3–. However, also in microbial setups containing high HCO3– concentrations (100–300 mM) (i.e. with less chemical siderite dissolution, thus less Fe2+aq in solution) more than 25% Fe(III) was found in the mineral precipitates (Fig. 3). As stated above, both the extent of siderite dissolution in control setups and the production of Fe(III) negatively correlated with increasing HCO3– concentrations. Most importantly, the amount of Fe(III) that was formed in microbial setups exceeded by far the amount of Fe2+aq that was considered to be available from siderite dissolution in abiotic controls.
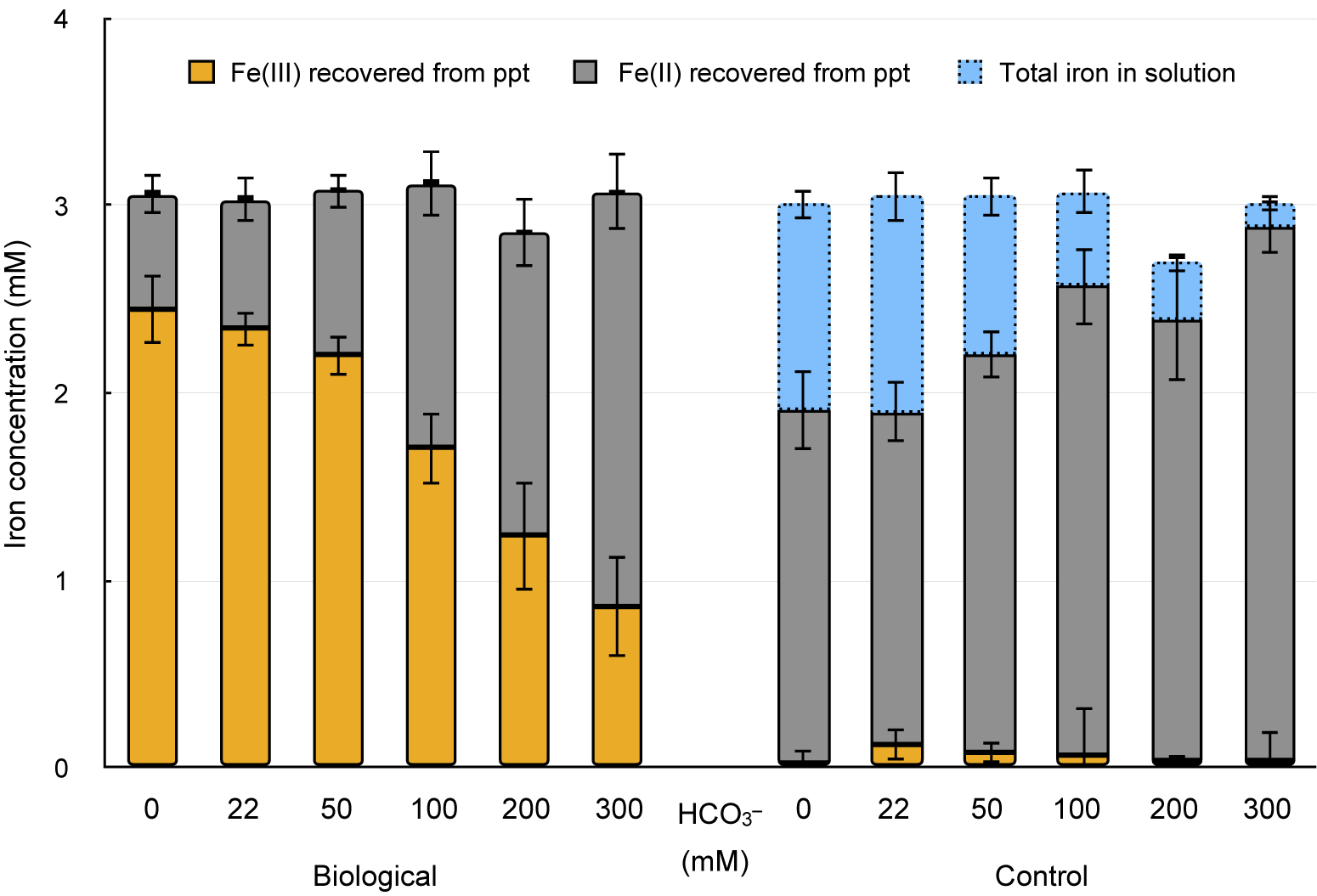
Figure 3. Iron quantification and speciation recovered from precipitates at the end of the incubation (after 35 days) (orange, Fe(III)ppt; grey, Fe(II)ppt) and total iron from solution (blue, Fe(total)aq) in setups containing 3 mM from siderite and different HCO3– concentrations (0–300 mM) in microbial (left set of columns) and abiotic control setups (right set of columns).
Evidence for solid-phase oxidation of siderite?
Measured Fe2+aq concentrations in abiotic control setups exceeded the expected Fe2+aq concentrations calculated from geochemical modelling by a factor of approximately 4 in all setups containing HCO3– (Fig. S4). Similar observations have been made in previous studies comparing theoretical dissolution kinetics of Fe2+aq from iron minerals to experimentally measured concentrations (French, Reference French1971; Lasaga, Reference Lasaga1984; Schwertmann, Reference Schwertmann1991; Kappler et al., Reference Kappler and Newman2004). This suggests that freshly precipitated mineral particles with a large surface-to-volume area often exceed the relative equilibrium solubilities for Fe2+aq calculated for these different minerals.
Moreover, in all biological setups Fe(III) accumulation was observed to be considerably higher compared to dissolved Fe2+aq available from initial siderite dissolution. The amount of Fe(III) that accumulated in biological setups exceeded Fe2+aq concentrations in abiotic controls by a factor of 2 for the low HCO3– treatments (0–50 mM) and up to a factor of 7 for the high HCO3– treatments (100–200 mM). In other words, cells of R. palustris TIE-1 oxidised up to 7-fold more Fe(II) than what would be considered bioavailable (i.e. as dissolved Fe2+aq) from the initial siderite dissolution alone (Fig. 4). Following Fe(II) dissolution kinetics from siderite also illustrates that most Fe(II) was dissolving within the initial 16 days of incubation, especially in the low HCO3– treatments (Fig. 2). Within these 16 days, Fe2+aq dissolution rates from siderite were significantly slower compared to biological Fe(II) oxidation rates in all setups (Table S2). Especially in the high HCO3– treatments, biological Fe(II) oxidation rates exceeded dissolution rates in the control setups by more than a factor of 6 (Fig. S4). In cases where only dissolved Fe2+aq was bioavailable to phototrophic Fe(II)-oxidising bacteria, the rate of Fe(III) formation should be within a similar range as Fe2+aq dissolution rates from siderite. However, biological Fe(II) oxidation rates greatly exceeded Fe2+aq dissolution rates and concentrations that accumulated in abiotic control setups. We therefore concluded that the cell activity and Fe(II)aq oxidation must either shift the chemical equilibrium and enhance siderite dissolution kinetics and oxidation or involve a non-dissolved pool of Fe(II), potentially solid-phase siderite, being directly oxidised by the bacteria. However, we acknowledge that our data cannot fully resolve whether direct oxidation occurs or if enhanced dissolution alone can explain the observations, and further studies are required to distinguish these mechanisms conclusively.
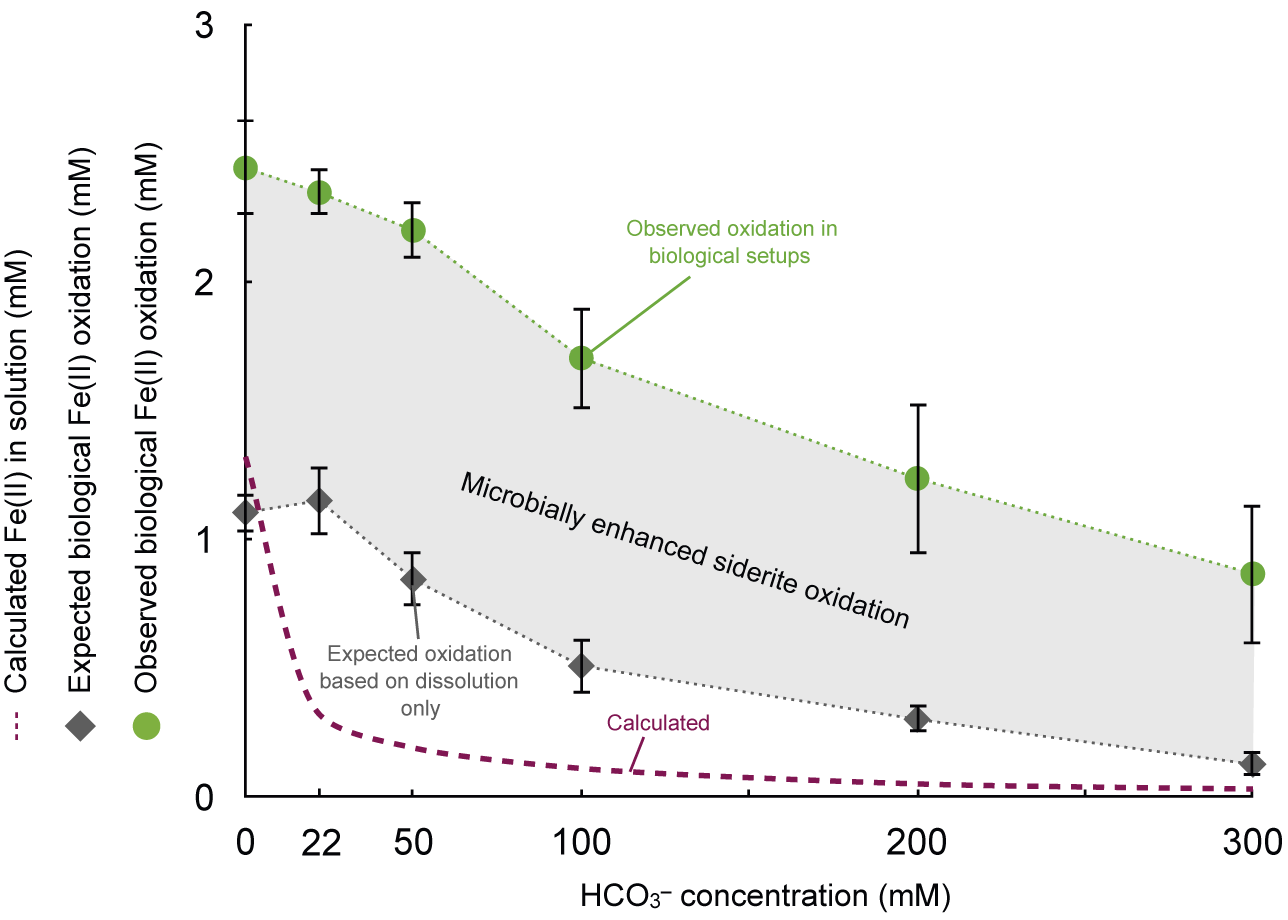
Figure 4. Biological iron(II) oxidation exceeds aqueous Fe(II) availability from siderite dissolution. Y axis: Calculated dissolved Fe2+aq concentration from siderite dissolution based on PhreeqC modelling (mM, ‘calculated’, dashed violet line); expected Fe(II) oxidation possible from siderite dissolution measured in abiotic control setups (mM, ‘expected’, grey diamonds) and observed Fe(II) oxidation in biological setups containing siderite and Rhodopseudomonas palustris TIE-1 after 35 days. The difference between Fe(II) oxidised in biological setups and Fe(II) in solution in control setups can be attributed to microbial activity enhancing siderite oxidation.
Following the evidence obtained from our geochemical data for a potential direct microbial siderite oxidation, we used SEM to visualise the association of the cells with mineral particles. We found that cells were often found in close association with mineral particles. In most cases, cells of R. palustris TIE-1 were found between minerals shaped as platelets or globular structures (Fig. 5). Comparison with SEM micrographs from pristine siderite particles indicated platelets to be siderite (Fig. S2A, S3) whereas the globular mineral structure can likely be attributed to the oxidised counterpart, for example short-range ordered Fe(III) oxyhydroxides (Fig. S2B, S5; Table S3). These globular structures were absent in all non-oxidised abiotic control setups (Fig. S3).

Figure 5. Representative scanning electron microscopy (SEM) images from biological setups amended with Rhodopseudomonas palustris TIE-1, siderite and gradually increased concentrations of HCO3–. Samples for SEM imaging were collected at the end of the incubation corresponding to 35 days. All samples show platelet structures likely to represent siderite mineral particles (arrow S) with cells (arrow C) closely associated to and globular particles potentially representing an iron(III) oxyhydroxide such as ferrihydrite (arrow F) as siderite (Fe(II)) oxidation product.
Earlier studies on Fe(II)-oxidising bacteria, using SEM, often found close associations of cells to Fe(III) minerals as their Fe(II) oxidation product. In some cases, cells were reported to be even fully encrusted in (Kappler et al., Reference Kappler, Schink and Newman2005; Miot et al., Reference Miot, Maclellan, Benzerara and Boisset2011), or in direct contact with (Chan et al., Reference Chan, Fakra, Emerson, Fleming and Edwards2011; Byrne et al., Reference Byrne, Schmidt, Gauger, Bryce and Kappler2018) Fe(III) minerals. However, cells of R. palustris TIE-1 were often observed to be closely associated with Fe(III) minerals but not encrusted (Jiao et al., Reference Jiao, Kappler, Croal and Newman2005; Han et al., Reference Han, Tomaszewski, Sorwat, Pan, Kappler and Byrne2020).
The microbial oxidation of structurally bound Fe(II) on the surface of siderite particles, however, would be a plausible explanation as the cells are located in the direct vicinity of (or even in contact with) the siderite particles. Moreover, the end of Fe(II) oxidation (i.e. the stable Fe(II)/Fe(total) ratio in biotic setups from day 16 onwards; Fig. 2C) might be attributed to the fact that the bulk of the Fe(II) minerals were no longer accessible to the microorganisms via direct contact. In particular, if microbial Fe(II) oxidation only oxidised the few nanometres of surface atoms of each siderite particle, then the inner bulk of the siderite particles might be preserved from Fe(II) oxidation by an oxidised ‘shell’ of Fe(III) oxyhydroxides covering the particles, as was previously observed in the presence of, for example, organic acids (Rothwell et al., Reference Rothwell, ThomasArrigo, Kaegi and Kretzschmar2025).
Several mechanisms could possibly be involved in the enhanced oxidation of Fe(II) in siderite. It has been shown for a variety of phototrophic Fe(II)-oxidising bacteria that cells can metabolically acidify their local environment surrounding the cell wall (Kappler et al., Reference Kappler and Newman2004; Hegler et al., Reference Hegler, Posth, Jiang and Kappler2008). Dissolution of siderite might then be enhanced by the microbial activity acidifying the environment as siderite was shown to dissolve faster at pH values <5.5 (Duckworth et al., Reference Duckworth and Martin2004). The siderite dissolution rates measured for abiotic setups were two to six times lower than the dissolution rates necessary to account for the Fe(III) which formed in all biotic setups (Table S2). These higher dissolution rates, which would be necessary to account for the Fe(III) that was precipitated biologically, could potentially be caused by the described pH effect and enhanced siderite dissolution. Dissolved Fe(II)aq was thus not detectable due to the instantaneous microbial oxidation. Additionally, it was shown that the metabolically versatile photoferrotroph R. palustris TIE-1 can harvest electrons from solid surfaces by extracellular electron uptake (Bose et al., Reference Bose, Gardel, Vidoudez, Parra and Girguis2014). Phototrophic extracellular electron uptake was recently observed in multiple phototrophic bacteria, also capable of oxidising Fe(II) in solid phases (Gupta et al., Reference Gupta, Guzman, Rengasamy, Stoica, Singh, Ranaivoarisoa, Davenport, Bai, McGinley and Meacham2021). Evidence in support of this comes from a study by Guzman et al. (Reference Guzman, Rengasamy, Binkley, Jones, Ranaivoarisoa, Singh, Fike, Meacham and Bose2019). They found the pioABC operon in R. palustris TIE-1, which we investigated as a model photoferrotrophic bacteria in our study, which encodes a protein system essential for Fe(II) oxidation and extracellular electron uptake (i.e. oxidation of solid surfaces) (Gupta et al., Reference Gupta, Sutherland, Rengasamy, Meacham, Kranz and Bose2019; Guzman et al., Reference Guzman, Rengasamy, Binkley, Jones, Ranaivoarisoa, Singh, Fike, Meacham and Bose2019). While direct evidence of pili in R. palustris TIE-1 could also not be identified in the current study, its ability to perform extracellular electron transfer (EET) via the pioABC operon suggests the potential for pili or similar conductive appendages. Studies such as Guzman et al. (Reference Guzman, Rengasamy, Binkley, Jones, Ranaivoarisoa, Singh, Fike, Meacham and Bose2019) and Bose et al. (Reference Bose, Gardel, Vidoudez, Parra and Girguis2014) highlight EET mechanisms in R. palustris TIE-1 but do not confirm the structural presence of pili. Further research, such as high-resolution imaging or genetic studies, is needed to verify whether pili play a role in its interactions with solid-phase minerals such as siderite. Given HCO3– and light availability in excess, R. palustris TIE-1 could not only conceptually but enzymatically be able to oxidise Fe(II) even in solid-state siderite. However, it needs to be noted that the potential for siderite oxidation is more positive than the potentials that the electrodes were poised at in the previous bioelectrochemical system studies (Gupta et al., Reference Gupta, Sutherland, Rengasamy, Meacham, Kranz and Bose2019; Guzman et al., Reference Guzman, Rengasamy, Binkley, Jones, Ranaivoarisoa, Singh, Fike, Meacham and Bose2019). Summarising our observations considering (1) the close association of cells with siderite as the Fe(II) source, (2) the considerable Fe(III) formation in setups where siderite dissolution should be suppressed to a minimum and (3) the fact that R. palustris TIE-1 was recently demonstrated to be genetically and enzymatically able to perform extracellular electron uptake from solid (poised electron) surfaces (Bose et al., Reference Bose, Gardel, Vidoudez, Parra and Girguis2014; Gupta et al., Reference Gupta, Sutherland, Rengasamy, Meacham, Kranz and Bose2019; Guzman et al., Reference Guzman, Rengasamy, Binkley, Jones, Ranaivoarisoa, Singh, Fike, Meacham and Bose2019) led us to the conclusion that the activity of R. palustris TIE-1 and subsequent oxidation of Fe(II)aq from siderite dissolution enhances the overall Fe(II) oxidation in siderite-doped environments. Even though we did not find direct evidence for solid-state Fe(II) oxidation in siderite particles, our observations can fuel future research to investigate whether anoxygenic phototrophic Fe(II)-oxidising bacteria have the capability to access and oxidise solid-phase Fe(II) in minerals such as siderite particles.
Conclusions
The capability of R. palustris TIE-1 to grow with siderite as the only Fe(II) substrate increases our understanding of the significance of phototrophic Fe(II) oxidisers to iron cycling in the environment. Besides their metabolic versatility to use a large variety of organic substrates (Ehrenreich et al., Reference Ehrenreich and Widdel1994), numerous studies have shown that the reduced forms of iron, such as in mixed-valent Fe(II)/(III) minerals, in Fe(II)–S solids and as dissolved Fe2+aq can serve as metabolic substrates for photoautotrophic growth (Bryce et al., Reference Bryce, Blackwell, Schmidt, Otte, Huang, Kleindienst, Tomaszewski, Schad, Warter and Peng2018). Knowing that siderite can serve as the only substrate for both energy generation and biomass fixation increases the niches that these bacteria can inhabit in the environment. Moreover, our observations led to the conclusion that cells of R. palustris TIE-1 not only oxidise dissolved Fe2+aq but can also enhance the dissolution of iron-rich minerals and therefore the release of structural Fe(II) from solid-state Fe(II) carbonates, such as siderite. By doing so, the microbial activity of these bacteria significantly impacts the abundance and redox speciation of iron in the environment by enhancing Fe(II) dissolution and oxidation beyond chemical constraints. Further studies should look more closely into the biological oxidation mechanisms and the genetic operons involved in solid-state electron transfer mechanisms during microbial siderite oxidation. The limitation in the extent of oxidation also deserves more attention to identify microbially induced shells of Fe(III) oxyhydroxides that can preserve the integrity of otherwise readily oxidisable Fe(II) minerals. Our findings and future studies can then help to fully elucidate the role of phototrophic Fe(II)-oxidising bacteria in the iron cycle of both modern and ancient environments.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1180/gbi.2025.4.
Acknowledgements
The authors acknowledge infrastructural support by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy, cluster of Excellence EXC2124, project ID 390838134. This study was funded by the DFG to J.M.B. (grant BY 82/4-1) and C.B. (grant BR 5927/2-1). J.M.B is supported by a UKRI Future Leaders Fellowship (MR/V023918/1). The authors gratefully acknowledge the Tübingen Structural Microscopy Core Facility (funded by the Federal Ministry of Education and Research and the Baden-Württemberg Ministry of Science as part of the Excellence Strategy of the German Federal and State Governments) for their support and assistance in this work, and thank the DFG (INST 37/1027-1 FUGG) for financial support provided for the acquisition of the cryogenic focused ion beam scanning electron microscope.
Competing interests
The authors declare no competing financial interest.