1. Introduction
The latest part of the Ediacaran System harbours signatures for some of the most important changes in the history of the biosphere. Multiple lines of fossil evidence indicate that many of the foundations of animal-dominated Phanerozoic-style ecosystems were assembled at this time, and include structurally complex Ediacaran macrofossils (e.g. Liu et al. Reference Liu, Li, Shao, Zhang, Wang and Qiao2014; Ivantsov et al. Reference Ivantsov, Zakrevskaya and Nagovitsin2019 a), animal-derived biomarkers (Bobrovskiy et al. Reference Bobrovskiy, Hope, Ivantsov, Nettersheim, Hallmann and Brocks2018 a), possible metazoan reefs (Grotzinger et al. Reference Grotzinger, Adams and Schroder2005; Penny et al. Reference Penny, Wood, Curtis, Bowyer, Tostevin and Hoffman2014) and the advent of macroscopic biomineralization (e.g. Cloudina (Grant, Reference Grant1990)). Perhaps most significantly, a trace fossil record of complex horizontal burrows and trails appears from ˜560 Ma, likely documenting the emergence of a bilaterian benthos (Martin et al. Reference Martin, Grazhdankin, Bowring, Evans, Fedonkin and Kirschvink2000; Jensen, Reference Jensen2003; Chen et al. Reference Chen, Zhou, Meyer, Xiang, Schiffbauer, Yuan and Xiao2013, Reference Chen, Zhou, Yuan and Xiao2019; Budd, Reference Budd2015; Budd & Jensen, Reference Budd and Jensen2017). These simple trace fossils are consistently found in association with bedding planes exhibiting microbial mat textures, and have been interpreted as representing a variety of mat-exploiting behaviours (Buatois et al. Reference Buatois, Narbonne, Mángano, Carmona and Myrow2014; Meyer et al. Reference Meyer, Xiao, Gill, Schiffbauer, Chen, Zhou and Yuan2014; Tarhan et al. Reference Tarhan, Droser, Gehling and Dzaugis2017; Ivantsov et al. Reference Ivantsov, Nagovitsyn and Zakrevskaya2019 b). Together, these lines of evidence point to a characteristic Ediacaran matground ecology which appears to have persisted into the early Cambrian Fortunian (Buatois et al. Reference Buatois, Narbonne, Mángano, Carmona and Myrow2014; Laing et al. Reference Laing, Mángano, Buatois, Narbonne and Gougeon2019). Despite the importance of these environments as cradles of early animal evolution (Budd & Jensen, Reference Budd and Jensen2017), there is currently little direct accounting of body fossils either from the biomat-forming organisms, or from the nascent bilaterian fauna themselves.
Organic walled microfossils (OWMs) are one source of direct body-fossil data that can be retrieved from siliciclastic rocks. Most studies of OWMs from the Ediacaran to date have focused on acritarchs (e.g. Moczydłowska, Reference Moczydłowska2005; Willman et al. Reference Willman, Moczydłowska and Grey2006). In studies of comparable OWM-bearing deposits from the Cambrian, there has recently been an increased awareness that a larger size class of organically preserved remains is accessible if a gentler processing procedure is applied. These larger, more delicate forms have been dubbed small carbonaceous fossils (SCFs), and encompass a polyphyletic mix of organic remains sourced from various organisms, including the fragmentary remains of metazoans (Butterfield & Harvey, Reference Butterfield and Harvey2012). Recently, several SCF biotas have been recovered from early Cambrian sediments in the Baltic region (Slater et al. Reference Slater, Harvey, Guilbaud and Butterfield2017; Guilbaud et al. Reference Guilbaud, Slater, Poulton, Harvey, Brocks, Nettersheim and Butterfield2018; Kesidis et al. Reference Kesidis, Slater, Jensen and Budd2019; Slater & Willman, Reference Slater and Willman2019), including from earliest Cambrian strata (Slater et al. Reference Slater, Harvey and Butterfield2018 a). Extending this record into the Ediacaran is crucial for capturing SCF diversity contemporaneous with the earliest stages of bilaterian evolution.
Finland is one region of Baltica that has been relatively under-explored in terms of its Ediacaran fossil record. Nevertheless, several localities preserving sediments of Ediacaran age are found in Finland, and crucially the thermal immaturity of these sediments makes them ideally suited for SCF preservation (Slater & Willman, Reference Slater and Willman2019). Here we report a rich record of organic microfossils from a late Ediacaran sequence in Finland, from Hailuoto Island and the Saarijärvi meteorite impact crater (Fig. 1).
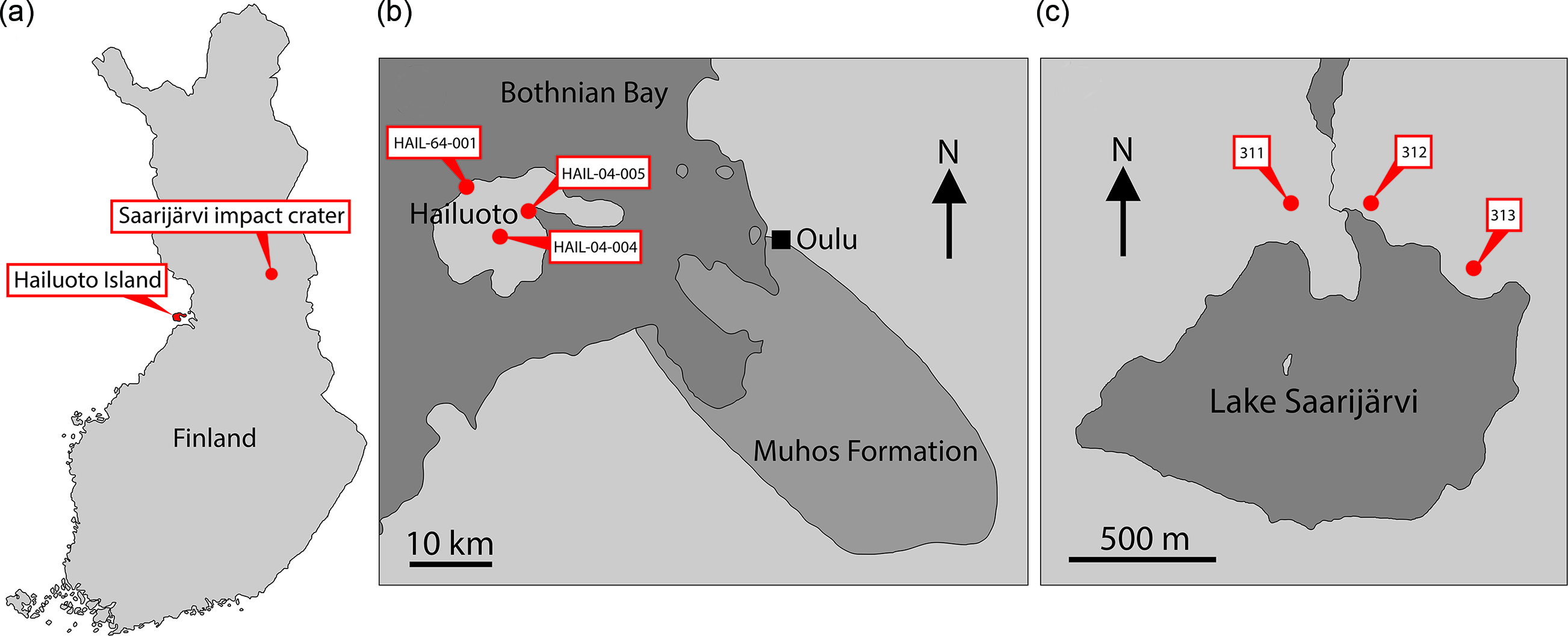
Fig. 1. (a) Map of Finland showing location of Hailuoto Island and the Saarijärvi impact structure. (b) Map showing Hailuoto Island in relation to the onshore Muhos basin, and core localities. (c) Map showing Lake Saarijärvi within the Saarijärvi impact structure and position of core localities. (Based on Klein et al. Reference Klein, Salminen and Mertanen2015, fig. 1; Solismaa, Reference Solismaa2008, fig. 4; Öhman & Preeden, Reference Öhman and Preeden2013, fig. 3.)
2. Geological setting
An extended episode of erosion during the early- to mid-Neoproterozoic era left Baltica as a peneplained continent of exceptionally low relief (Lidmar-Bergström, Reference Lidmar-Bergström1993, Reference Lidmar-Bergström1995). Subsequent transgressions during the late Neoproterozoic and early Phanerozoic flooded large regions of this topographically flat landscape, resulting in extensive shallow marine deposition (Nielsen & Schovsbo, Reference Nielsen and Schovsbo2011). Siliciclastic sediments deposited in these epeiric seaways extend over large regions of the Bothnian Sea and the Baltic states. Based on the thickening trend of these sediments toward the Finnish coastline, equivalent deposits are thought to have once covered much of Finland (Puura et al. Reference Puura, Amantov, Tikhomirov and Laitakari1996; Kohonen & Rämö, Reference Kohonen, Rämö, Lehtinen, Nurmi and Rämö2005; Bogdanova et al. Reference Bogdanova, Bingen, Gorbatschev, Kheraskova, Kozlov, Puchkov and YA2008; Klein et al. Reference Klein, Salminen and Mertanen2015; Slater & Willman, Reference Slater and Willman2019). In Finland, even more than elsewhere in Baltica, the vast majority of these sediments have subsequently been eroded and now remain only as relatively small and geographically scattered outliers.
One region where a substantial portion of late Neoproterozoic sediments has survived in Finland is the island of Hailuoto (Fig. 1). Hailuoto is situated in the Bothnian Bay off the northwest coast of the Finnish mainland, west of the coastal city of Oulu (Fig. 1). Beneath a covering of Quaternary sediments lies the subsurface Hailuoto Formation (known entirely from drillcore), a package of sandstones, mudstones, siltstones, clays and conglomerates which varies in thickness across the island, reaching a maximum thickness of ˜560 m (Solismaa, Reference Solismaa2008; Klein et al. Reference Klein, Salminen and Mertanen2015). The upper ˜55–65 m of the formation consists of greenish-grey fine-grained sandstones, shales and siltstones. Below this are deposited red-coloured arkosic sandstones and shales, which rest unconformably on the crystalline basement or on sediments of the Mesoproterozoic–Neoproterozoic Muhos Formation which underlies much of the Bothnian Bay and outcrops adjacent to Hailuoto on the Finnish mainland (Fig. 1). The Muhos Formation consists of red-green-grey siltstones and shales (Kohonen & Rämö, Reference Kohonen, Rämö, Lehtinen, Nurmi and Rämö2005), similar in lithology to the Hailuoto Formation (Tynni & Siivola, Reference Tynni and Siivola1966; Tynni & Donner, Reference Tynni and Donner1980; Kohonen & Rämö, Reference Kohonen, Rämö, Lehtinen, Nurmi and Rämö2005; Solismaa, Reference Solismaa2008; Klein et al. Reference Klein, Salminen and Mertanen2015).
Based on correlation with adjacent strata and its microfossil contents, the Hailuoto Formation is considered to be late Neoproterozoic in age, with estimates in the range of 600–570 Ma (Veltheim, Reference Veltheim1969; Tynni & Donner, Reference Tynni and Donner1980; Paulamäki & Kuivamäki, Reference Paulamäki and Kuivamäki2006; Klein et al. Reference Klein, Salminen and Mertanen2015; Luukas et al. Reference Luukas, Kousa, Nironen and Vuollo2017). This would place the deposition of the Hailuoto Formation in approximately the middle of the Ediacaran Period. A microfossil analysis of sediments from the upper parts of the Hailuoto Formation by Tynni & Donner (Reference Tynni and Donner1980) drew comparisons with the uppermost parts of the Visingsö Formation of Sweden. Detrital zircon U–Pb ages have subsequently constrained the Visingsö Formation to a maximum depositional age of ≤886 ± 9 Ma (Moczydłowska et al. Reference Moczydłowska, Pease, Willman, Wickström and Agić2017), and recent microfossil studies also suggest this formation was deposited during the Tonian (Loron & Moczydłowska, Reference Loron and Moczydłowska2018). Klein et al. (Reference Klein, Salminen and Mertanen2015), however, point out that many of the form-taxa reported from the Hailuoto Formation by Tynni & Donner (Reference Tynni and Donner1980) are actually found in much younger sediments elsewhere in the Baltic region and East European Platform, for example, in late Ediacaran strata from the Kotlin Formation of Estonia (Mens & Pirrus, Reference Mens and Pirrus1997; Meidla, Reference Meidla2017; Arvestål & Willman, Reference Arvestål and Willman2020; Slater et al. Reference Slater, Harvey, Bekker and Butterfield2020). A particularly close comparison can also be drawn with OWM assemblages from the late Ediacaran Redkino and Kotlin regional stages of the Lyamtsa, Verkhovka, Zimnie Gory and Yorga formations of the White Sea region in Russia (e.g. Leonov & Ragozina, Reference Leonov and Ragozina2007). These similarities to assemblages from comparatively well-constrained late Ediacaran strata (e.g. in the White Sea region) would suggest a substantially younger age for the Hailuoto Formation than suggested in previous studies, closer to the Ediacaran–Cambrian boundary. Indeed, U–Pb zircon dating of volcanic tuffs has indicated an age of 551–548 Ma for the lowermost Kotlin in the White Sea area of northern Russia (Grazhdankin et al. Reference Grazhdankin, Marusin, Meert, Krupenin and Maslov2011). With this advancement in understanding of the local and regional stratigraphy we favour a younger, latest Ediacaran age for the upper part of the Hailuoto Formation here.
Another subsurface remnant of comparable Neoproterozoic sediments is preserved within the Saarijärvi impact structure in central Finland (Fig. 1). The Saarijärvi impact crater is situated 30 km south of Taivalkoski near the border between the Finnish regions of northern Ostrobothnia and Kainuu, and is largely covered by a lake (Saarijärvi) that has formed in the crater depression (to avoid confusion, it is worth noting that in Finnish ‘Saarijärvi’ is a very common lake name – there are at least 198 lakes named Saarijärvi in Finland). The local geology has largely been reconstructed based on drillcore material. As with many impact structures, the precise geological history has been problematic to disentangle. Up to 156 m thickness of sediments is preserved within the ˜1.5 km diameter crater. These packages of sediment are difficult to correlate even between closely spaced cores, likely as a result of separate cores intersecting different coherent megablocks of sediment arranged in a chaotic way (Hyyppä & Pekkala, Reference Hyyppä and Pekkala1987; Öhman & Preeden, Reference Öhman and Preeden2013). The signatures of impact-disruption are evident throughout the cores: Sediments display significant changes in dip direction and angle over relatively short intervals of core depth. Further, fractured angular clasts of basement rock (granite) are found within the sediments at several depths in different cores. Another notable feature is that even among soft lithologies the core material tends to break along shiny, polished surfaces which cut across the bedding; such features may represent subsequent fracturing of the sediments related to tectonism (Öhman & Preeden, Reference Öhman and Preeden2013). Precise dating of the impact event has been difficult, and there are competing scenarios between a Proterozoic and an early Cambrian age impact hypothesis (see Öhman & Preeden, Reference Öhman and Preeden2013). Shales and siltstones in the upper parts of the Saarijärvi impact structure are reminiscent of those in the Hailuoto Formation, and have produced comparable OWM assemblages both in terms of taxonomic composition and preservation (Tynni & Donner, Reference Tynni and Donner1980; Tynni & Uutela, Reference Tynni and Uutela1984, Reference Tynni and Uutela1985; Paulamäki & Kuivamäki, Reference Paulamäki and Kuivamäki2006), suggesting that sediments of the Hailuoto Formation originally extended north and east to cover a substantial portion of central Finland.
3. Materials and methods
Sampling targeted three cores intersecting the Neoproterozoic sediments of Hailuoto Island: M52-Hail-04-004 (drilled at an angle of 70° towards 226° direction), M52-Hail-04-005 and M52-Hail-64-001 (Fig. 1). In all cases our samples are derived from the green-grey and grey-brown mudstones which make up the upper parts of Hailuoto cores from 64–77 m depth of M52-Hail-04-004 (28 samples), 60–67 m depth of M52-Hail-04-005 (21 samples) and 75–80 m depth of M52-Hail-64-001 (5 samples) (Fig. 2; for additional details of cores see Solismaa, Reference Solismaa2008). In addition, three cores intersecting the Saarijärvi impact crater were sampled: M52-3533-81-311, M52-3533-81-312 and M52-3533-84-313 (Figs 1, 2). These samples were also selected from green-grey mudrocks, which at Saarijärvi are distributed chaotically even among closely spaced cores, due to the displacement of megablocks associated with crater formation (see Öhman & Preeden, Reference Öhman and Preeden2013). Processing followed the techniques outlined in Butterfield & Harvey (Reference Butterfield and Harvey2012). Cores are housed at the Geological Survey of Finland national drillcore archive in Loppi. All imaged fossil material is deposited in the Palaeontological collections of the Museum of Evolution (PMU), Uppsala University, Sweden.
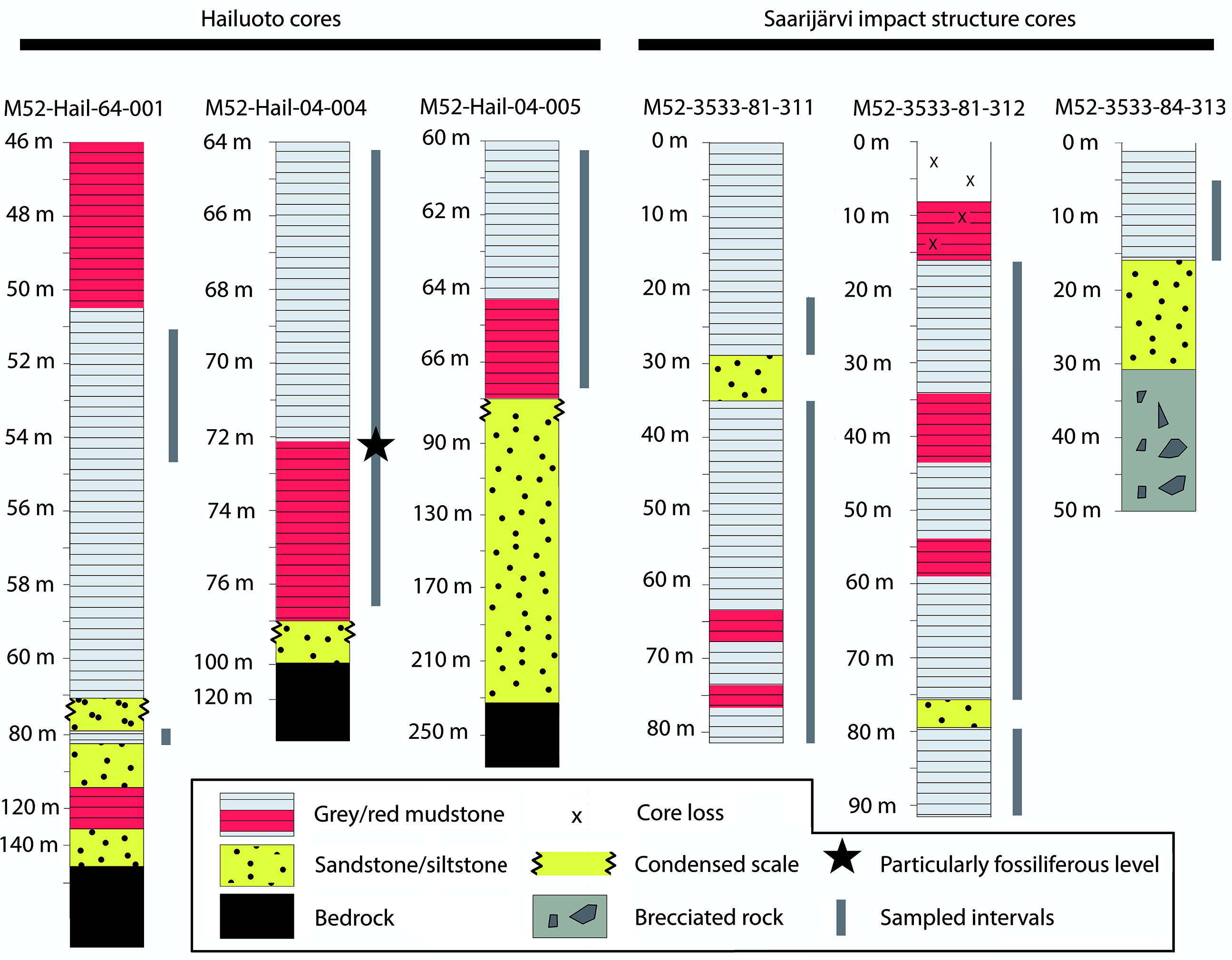
Fig. 2. Simplified stratigraphic sections of cores highlighting the major lithological changes and units. Grey bars represent sampled horizons, with sampling spaced at c. 1 m intervals, with denser sampling among finer-grained mudstones. Hailuoto sections are described in further detail in figures 10, 25 and 36 in Solismaa (Reference Solismaa2008).
4. Organic-walled fossils
Of the 64 processed samples, all were productive for microfossils, although with significant variation in contents and abundance. A particularly productive section was identified in mudstones of the M52-Hail-04-004 drillcore, spanning ˜71–73 m depth. The majority of recovered fossils fall into the broad form-taxonomic distinctions of acritarchs (vesicular organic-walled microfossils of unknown biological affinity; Evitt, Reference Evitt1963) or filamentous forms. Specimens of metazoan origin were also recovered from Hailuoto. Assemblages from Hailuoto and Saarijärvi contained the same acritarchs and filamentous form taxa, supporting previous hypotheses that these strata are remnants of once widespread late Ediacaran deposits in Finland (Tynni & Donner, Reference Tynni and Donner1980; Tynni & Uutela, Reference Tynni and Uutela1985; Paulamäki & Kuivamäki Reference Paulamäki and Kuivamäki2006).
4.a. Metazoan remains
An individual triangular structure of ˜800 µm length and ˜450 µm width at the base was recovered from a particularly fossil-rich sample at 72.7 m depth in the M52-Hail-04-004 drillcore. This spine-shaped fossil possesses a thin-walled flared basal region exhibiting rhombus-shaped surficial scaly ornamentation, which tapers to a darkened, presumably sclerotized tip (Fig. 3a). The same sample also produced a broadly blade-shaped sclerotized element edged with crenate serrations that are densely encrusted with pyrite euhedra (Fig. 3b).
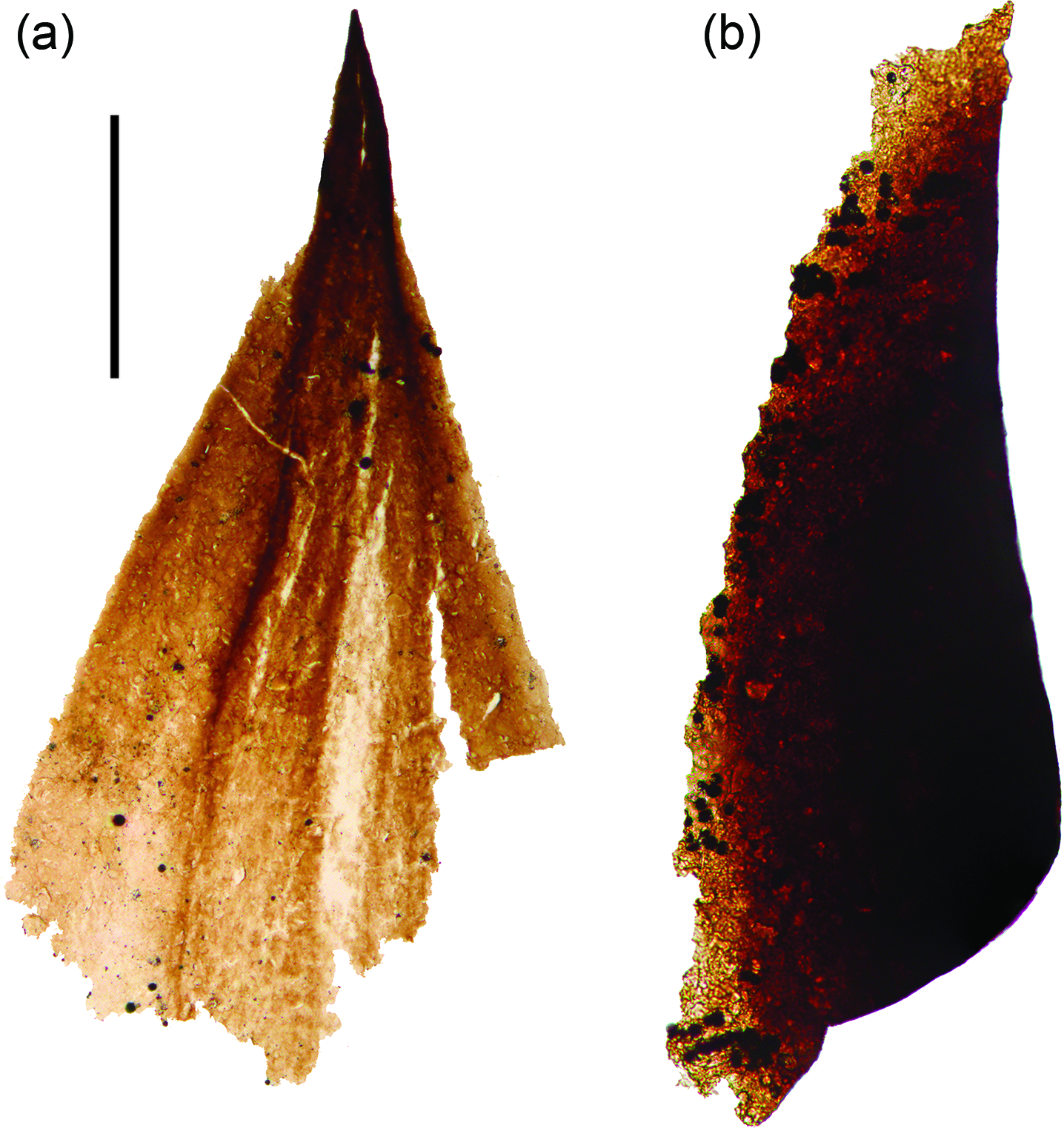
Fig. 3. Metazoan remains. (a) Sclerite likely derived from a bilaterian-grade metazoan. (b) Possible metazoan-derived serrated structure. Scale bar represents 200 µm. (a) 72.70 m M52-Hail-04-004 core; (b) 72.2 m M52-Hail-04-004 core. Specimen numbers: (a) PMU 38-156/1; (b) 38-157/1.
Close comparisons can be drawn between the spine-shaped element (Fig. 3a) and early Cambrian carbonaceous ‘protoconodont’ spines (see Protohertzina compressa, figs 3, 4 of Slater et al. Reference Slater, Harvey and Butterfield2018 a; fig. 3 of Slater & Willman, Reference Slater and Willman2019). In particular, the tip closely resembles known early Cambrian spines of this type (compare to holotype specimen of P. compressa, fig. 3BA of Slater et al. Reference Slater, Harvey and Butterfield2018 a). The basal portion of the Hailuoto spine, however, differs from these protoconodont-type spines: P. compressa exhibit a dense fibrous microstructure, whereas the Hailuoto spine displays a faint scaly ornament on an otherwise smooth basal portion. Broadly comparable ornamentation occurs on the basal pad of cuticular sclerites of scalidophoran worms (notably the triangular ‘teeth’ borne on the pharynx of such worms; see fig. 9K of Smith et al. Reference Smith, Harvey and Butterfield2015; fig. 3B of Slater et al. Reference Slater2018 b; fig. 2R of Wallet et al. Reference Wallet, Slater, Willman and Peel2021).
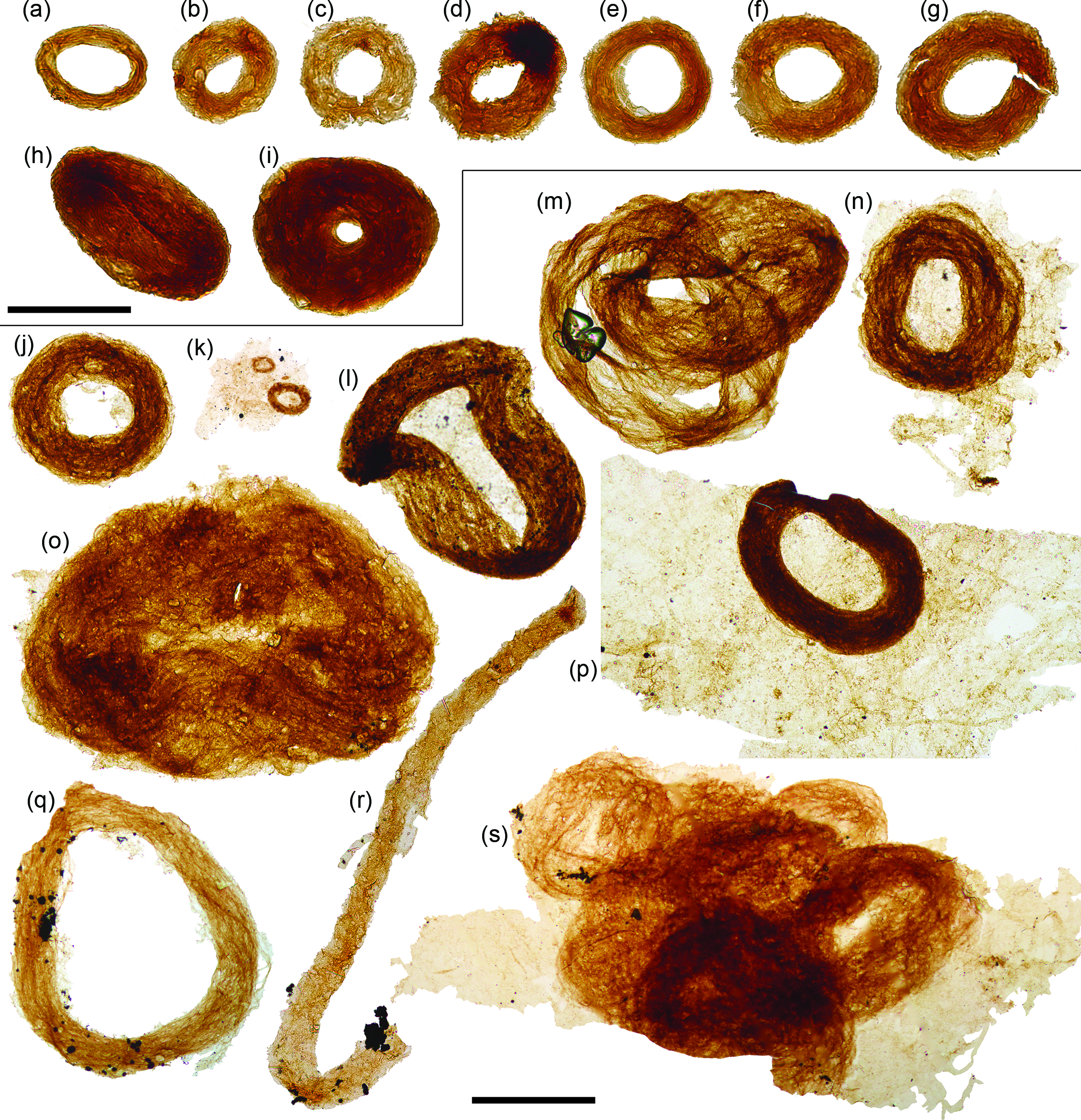
Fig. 4. Siphonophycus ‘donuts’. (a–r) Bundled filaments of the form-taxon Siphonophycus forming ‘donut-shaped’ masses, possibly resulting from flattened Nostoc-ball-like masses of filaments. (k, m, s) Clusters of donut-shaped colonies. (k, n, p, s) Filament bundles adhered to broader, background mass of mat-like filamentous remains. (r) Broken loop. Scale bars represent 100 µm (a–i)); 200 µm (j–s). (a, c, d) 7.53 m Saarijärvi M52/3533/84/313 core; (b, e, i) 20.20 m Saarijärvi M52/3533/81/312 core; (j, l–p, s) 72.2 m M52-Hail-04-004 core; (k, q, r) 72.70 m M52-Hail-04-004 core. All specimen numbers have the prefix PMU 38: (a) 165/6; (b) 166/6; (c) 165/7; (d) 165/8; (e) 166/1; (f) 166/2; (g) 166/3; (h) 166/4; (i) 166/5; (j) 158/1; (k) 159/1; (l) 157/2; (m) 157/3; (n) 158/2; (o) 158/3; (p) 158/4; (q) 160/1; (r) 161/1; (s) 162/1.
This spine is perhaps the most unexpected find from the Hailuoto assemblage, and appears to derive from a metazoan. Several Ediacaran metazoans (e.g. Dickinsonia, Yorgia and Kimberella) are known to have produced dorsal integumentary shields that were covered with various tubercles and spines (see Ivantsov et al. Reference Ivantsov, Zakrevskaya and Nagovitsin2019 a, figs 2, 3). The fine-scale structures of these integuments are unclear however, since they are known solely from preservation as casts and moulds. Cnidarians can possess a chitinous exoskeleton (Mendoza-Becerril et al. Reference Mendoza-Becerril, Maronna, Pacheco, Simões, Leme, Miranda, Morandini and Marques2016), which conceivably could produce sclerite-like elements preservable as carbonaceous fossil remains. Fossil examples of early cnidarians with a chitinous exoskeleton include early Cambrian (Fortunian) coronate scyphozoans such as Olivooides (Dong et al. Reference Dong, Cunningham, Bengtson, Thomas, Liu, Stampanoni and Donoghue2013), Quadrapyrgites (Y Liu et al. Reference Liu, Li, Shao, Zhang, Wang and Qiao2014) and Qinscyphus (Liu et al. Reference Liu, Shao, Zhang, Wang, Zhang, Chen, Liang and Xue2017), as well as possible Ediacaran cnidarians such as the tubular Corumbella (Warren et al. Reference Warren, Pacheco, Fairchild, Simões, Riccomini, Boggiani and Cáceres2012). None of these, nor any other cnidarians we are aware of, produce spine-like sclerites, however, and we deem it likely the sclerite from Hailuoto is sourced from a bilaterian-grade animal. Among the bilateria, protoconodonts and scalidophorans both have fossil records extending to almost the base of the Cambrian (Kouchinsky et al. Reference Kouchinsky, Bengtson, Runnegar, Skovsted, Steiner and Vendrasco2012; Slater et al. Reference Slater, Harvey and Butterfield2018 a). Some of the earliest Cambrian, and even latest Ediacaran, burrows may have been produced by scalidophoran or cycloneuralian worms (see Kesidis et al. Reference Kesidis, Slater, Jensen and Budd2019). Based on the simple set of characters, however, this spine cannot confidently be attributed to either a protoconodont, or a cuticular sclerite of a scalidophoran worm. Indeed, spines of this type could conceivably be sourced from a much broader array of bilaterians, including various ecdysozoans, gastrotrichs (Rieger & Rieger, Reference Rieger and Rieger1977), gnathiferans (Marlétaz et al. Reference Marlétaz, Peijnenburg, Goto, Satoh and Rokhsar2019), as well as stem-protostomes or even stem-bilaterians.
4.b. Filamentous microfossils
Both the Hailuoto and Saarijärvi material produced a rich assortment of microfossils with a broadly filamentous construction, some of which may represent different life-cycle stages, or taphomorphs of the same organism. Structures such as simple multicellular filaments or coccoid cells have been repeatedly convergent in some clades over geological time (e.g. cyanobacteria: Dvořák et al. Reference Dvořák, Casamatta, Poulíčková, Hašler, Ondřej and Sanges2014; Butterfield, Reference Butterfield2015 b), meaning that assigning these fossils to a specific group is fraught with difficulty. To circumvent some of this phylogenetic ambiguity, we discuss these forms in terms of distinctive morphogroups and, where possible, use established form-taxonomic terminology, whilst drawing comparisons to useful fossil and extant analogues.
4.b.1. Siphonophycus ‘donuts’
A ubiquitous constituent of fossiliferous samples is bundled filaments of the form-taxon Siphonophycus (Fig. 4). Siphonophycus are smooth-walled, unbranched, tubular filaments that lack septa or preserved cellular remains (Knoll et al. Reference Knoll, Swett and Mark1991). The Siphonophycus filaments from Finland are a few microns in thickness, and are often found bundled up into tight rings varying in size between 50 and 650 µm in maximum diameter, often with a central hollow (ranging between absent and up to ˜300 µm diameter). These structures likely represent flattened Nostoc-ball-like masses of interwoven filaments (see Butterfield et al. Reference Butterfield, Knoll and Swett1994; Mollenhauer et al. Reference Mollenhauer, Bengtsson and Lindstrøm1999; Guiry & Guiry, Reference Guiry and Guiry2008). The ‘donut-shaped’ (Fig. 4a–k, n, p, q) and more irregular agglomerations (Fig. 4l, m, o, s) possibly formed via the collapse of originally torus-shaped colonies, or alternatively the central hollow may represent the void left by a mucilage-filled interior space within an originally spherical colony. Though lacking an overall ring shape, bundled filaments described as Polytrichoides lineatus Hermann Reference Hermann1974 emend. (Hermann in Timofeev et al. Reference Timofeev, Hermann and Mikhailova1976) exhibit similarities to the bundles of Siphonophycus described here, and are frequently recovered from Proterozoic assemblages (Li et al. Reference Li, Pang, Chen, Zhou, Han, Yang, Wang, Yang and Yin2019). A closer comparison comes from comparable tightly wound rings of filaments recorded from the Neoproterozoic Svanbergfjellet Formation of Spitsbergen (fig. 26G of Butterfield et al. Reference Butterfield, Knoll and Swett1994), suggesting this growth habit is widespread among Neoproterozoic cyanobacterial mats.
4.b.2. Palaeolyngbya
The majority of samples produced smooth, strap-shaped filamentous sheaths, ranging between ˜50 and 110 µm in diameter, and of various lengths up to ˜3 mm (Fig. 5). In a subset of these filaments, the outer envelopes enclose optically darker, unbranched, uniseriate, multicellular trichomes, composed of shrunken and degraded cells (Fig. 5m–p, x, ac, an). The trichome is c. 30–40 % of the outer envelope diameter in most specimens, though this may represent taphonomic shrinkage since a few specimens preserve much broader trichomes (e.g. Fig. 5x). The internal trichome may run the entire filament length (e.g. Fig. 5o); however, in some specimens it is fragmented or incomplete (e.g. Fig. 5p). The cells of the trichome are occasionally separated and displaced obliquely within the sheath, appearing as a series of discoidal cells (e.g. Fig. 5ac). These filaments can be ascribed to the form-taxon Palaeolyngbya Schopf Reference Schopf1968 emend. Butterfield, Knoll and Swett Reference Butterfield, Knoll and Swett1994 (see pl. 2, fig. 1 of Yun, Reference Yun1981; fig. 2.1 of Vidal & Moczydłowska, Reference Vidal and Moczydłowska1992; fig. 25E–G of Butterfield et al. Reference Butterfield, Knoll and Swett1994; figs 4–5 of Moczydłowska, Reference Moczydłowska2008; fig. 3J of Loron et al. Reference Loron, Rainbird, Turner, Greenman and Javaux2019; fig. 10O–X of Arvestål & Willman, Reference Arvestål and Willman2020). These filaments are distinguishable from other fossil multicellular trichomes such as Oscillatoriopsis from the manner in which specimens break (across pseudosepta vs between true cells in Oscillatoriopsis). Occasional specimens display a pseudoseptate sheath comparable to some Tortunema (e.g. fig. 7.5 of Sergeev et al. Reference Sergeev, Knoll, Vorob’eva and Sergeeva2016), likely reflecting taphomorphic and/or ontogenetic variation.

Fig. 5. Filamentous microfossils. (a–l, r–w, y–ab, af–am) Rugosoopsis. (m–p, x, ac, an) Palaeolyngbya. (q, ad, ae) Exhibit mixed morphology of Rugosoopsis-like filaments with occasional lengths of Palaeolyngbya-like trichome. (an) Palaeolyngbya filaments adhered to matted sheet of filamentous remains. Arrow indicates shrivelled cell (necridia) in specimen (i). Note the discoidal cells of the trichome in (r), (ac) and (ae). Scale bars represent 200 µm. (a–l, n, r–t, w), y–ab, ad, af, ag, ai–am) 72.70 m M52-Hail-04-004 core; (m, o–q, u, v, x, ac, ae, ah, an) 72.20 m M52-Hail-04-004 core. All specimen numbers have the prefix PMU 38: (a) 161/2; (b) 161/3; (c) 161/4; (d) 163/1; (e) 161/5; (f) 156/2; (g) 161/6; (h) 160/2; (i) 160/3; (j) 159/2; (k) 161/7; (l) 161/12; (m) 162/2; (n) 159/3; (o) 162/3; (p) 162/4; (q) 162/5; (r) 159/4; (s) 160/4; (t) 161/8; (u) 158/5; (v) 157/4; (w) 156/3; (x) 157/5; (y) 160/5; (z) 164/4; (aa) 160/6; (ab) 161/9; (ac) 157/6; (ad) 160/7; (ae) 158/6; (af) 161/10; (ag) 161/11; (ah) 162/6; (ai) 163/2; (aj) 163/3; (ak) 159/5; (al) 163/4; (am) 163/5; (an) 157/7.
4.b.3. Rugosoopsis
Another common filament type found in most samples from Hailuoto and Saarijärvi consists of a bi-layered form with a dense, smooth-walled inner sheath (similar to Siphonophycus or Palaeolyngbya), enclosed by a thinner-walled outer sheath with a pronounced series of transverse ridges (or rugose ornamentation) on the surface (Figs 5a–l, r–w, y–ab, af–am, 6d). The broader outer sheath is often damaged or torn, but where intact it appears as a faint outline extending beyond the margins of the inner sheath (Fig. v, y, z). Occasional atrophied cells (probable necridia) are visible within the inner sheath (e.g. Fig. 5i). These filaments are assignable to the form-taxon Rugosoopsis Timofeev and Hermann Reference Timofeev and Hermann1979, which has been recovered from Proterozoic shales (Pjatiletov, Reference Pjatiletov1988; Jankauskas et al. Reference Jankauskas, Mikhailova and Hermann1989; fig. 25A–D of Butterfield et al. Reference Butterfield, Knoll and Swett1994; fig. 3 of Samuelsson & Butterfield Reference Butterfield2001; fig. 10.1 of Sergeev et al. Reference Sergeev, Knoll and Vorob’eva2011; fig. 2J, T of Riedman et al. Reference Riedman, Porter, Halverson, Hurtgen and Junium2014; fig. 15.13 of Riedman & Porter, Reference Riedman and Porter2016) and as silicified three-dimensional fossils within Proterozoic shallow water carbonates (fig. 9F–I of Butterfield, Reference Butterfield2001). In both the Hailuoto and Saarijärvi samples, these Rugosoopsis filaments co-occur with and may grade into other filament types, including Palaeolyngbya, Siphonophycus and occasional pseudoseptate Tortunema-like sheaths (Fig. 4q, ad, ae), underscoring the substantial taphomorphic and ontogenetic overlap among filamentous microfossils.
4.b.4. Obruchevella
Abundant coiled, thin-walled filaments (˜10–20 µm in diameter) occasionally with apparent septa were recovered in all samples from Hailuoto and Saarijärvi (Figs 7f, 8h–n). The majority of specimens are compressed into a coiled ring, but occasional specimens show laterally displaced coils (Fig. 7f, 8i, l), revealing a Spirulina-like helical habit (see Sili et al. Reference Sili, Torzillo and Vonshak2012). Compressed cylindrical forms were recovered in previous investigations by Tynni & Donner (Reference Tynni and Donner1980), who assigned these to the form-taxon Volyniella cylindrica (compare also with fig. 1F of Leonov & Ragozina, Reference Leonov and Ragozina2007; figs 3, 5 of Sharma & Shukla, Reference Sharma and Shukla2012). Jankauskas et al. (Reference Jankauskas, Mikhailova and Hermann1989) point out that the distinction between the flattened, two-dimensional Volyniella (Shepeleva, Reference Shepeleva1973) and typically three-dimensionally preserved Obruchevella (e.g. Anderson et al. Reference Anderson, McMahon, Macdonald, Jones and Briggs2018; Willman et al. Reference Willman, Peel, Ineson, Schovsbo, Rugen and Frei2020) is essentially artificial, and based on taphomorphs. Noting that there are several possible names for spiralled filaments (in addition to Volyniella, also Glomovertella and Circumiella), we prefer to follow the recommendations of Jankauskas et al. (Reference Jankauskas, Mikhailova and Hermann1989) and term these coiled filaments Obruchevella.
4.b.5. Matted filaments
The majority of samples produced abundant entangled mats of Siphonophycus, Palaeolyngbya and Rugosoopsis exhibiting a mesh-like growth habit (Figs 6, 7), where filaments are densely interwoven (Figs 6a–d, 7a). Frequently the density of overlapping filaments is such that they form a sheet-like layer that has been compressed into a single carbonaceous film (see Martí Mus, Reference Martí Mus2014; Slater et al. Reference Slater, Harvey, Bekker and Butterfield2020). Some of these mats are of mixed composition (e.g. Fig. 6a); however, others are composed entirely from a single filament morphology (e.g. Figs 6c, d, 7a). In this latter category, the filaments are all of similar dimensions, suggesting that they are at the same ontogenetic stage (e.g. Fig. 7a). Within these mats, individual filamentous structures may be encrusted with pyrite euhedra (Figs 6a, b, 7c, e). Pyrite also occurs as circular or more irregular patches within the sheet, possibly representing voids within the mat created by gas bubbles. Together, these agglomerations are reminiscent of various acid-isolated mat-forming filaments from Proterozoic shales (compare Fig. 6c with fig. 8.5 of Sergeev et al. Reference Sergeev, Knoll, Vorob’eva and Sergeeva2016; fig. 2 of Samuelsson & Butterfield, Reference Butterfield2001; fig. 2G of Butterfield, Reference Butterfield2015 a; fig. 4D of Butterfield, Reference Butterfield2015 b) and carbonates (Knoll et al. Reference Knoll, Wörndle and Kah2013).

Fig. 6. Matted filaments. (a, b) Large sheets of matted filaments where many of the broader filaments are outlined in dense encrustations of pyrite euhedra. (c) Interwoven Siphonophycus filaments. (d) Tangled mass of Rugosoopsis filaments. (e, f) Interwoven masses of fine ‘hyphae-like’ filaments. (g) Enlargement of area inside dashed box in specimen (a) showing filament outlined by pyrite euhedra. (h) Enlargement of fine filament in specimen (a) indicated by arrow 1. (i) Enlargement of filaments in (d) showing prominent shrivelled necridia. Pyrite outlining apparent cells or septa (arrow 2). A variety of organic walled microfossils can be found among these matted filaments (e.g. leiosphaerid acritarch entangled with filaments; arrow 3). Scale bars represent 0.5 mm (a–f), 100 µm (g, h), 200 µm (i). (a, b, g, h) 72.20 m M52-Hail-04-004 core; (c–f, i) 72.70 m M52-Hail-04-004 core. All specimen numbers have the prefix PMU 38: (a, g, h) 162/7; (b) 162/8; (c) 164/1; (d) I, 163/6; (e) 160/8; (f) 159/6.
Fig. 7. Matted filaments. (a) Interwoven filamentous mat. (b) Sinuous ribbon-like filaments on surface of mat, alongside chain of pyritized trichome. (c) Mixture of Siphonophycus, Rugosoopsis and pyrite-encrusted filaments (arrow points to necridia within Rugosoopsis-type filament). (d) Dense mat of Rugosoopsis exhibiting pyrite encrustation (arrow points to prominent necridia). (e) Pyrite-encrusted filaments. (f) Coiled Obruchevella-type filament within mat. (g) Degraded mat with prominent Rugosoopsis filament (white arrow points to necridia). Scale bars represent 200 µm (a, b, d); 100 µm (c, e–g). (a–g) 72.70 m M52-Hail-04-004 core. All specimen numbers have the prefix PMU 38: (a) 164/5; (b) 163/7; (c) 163/8; (d) 163/9; (e) 164/2; (f) 163/10; (g) 164/3.
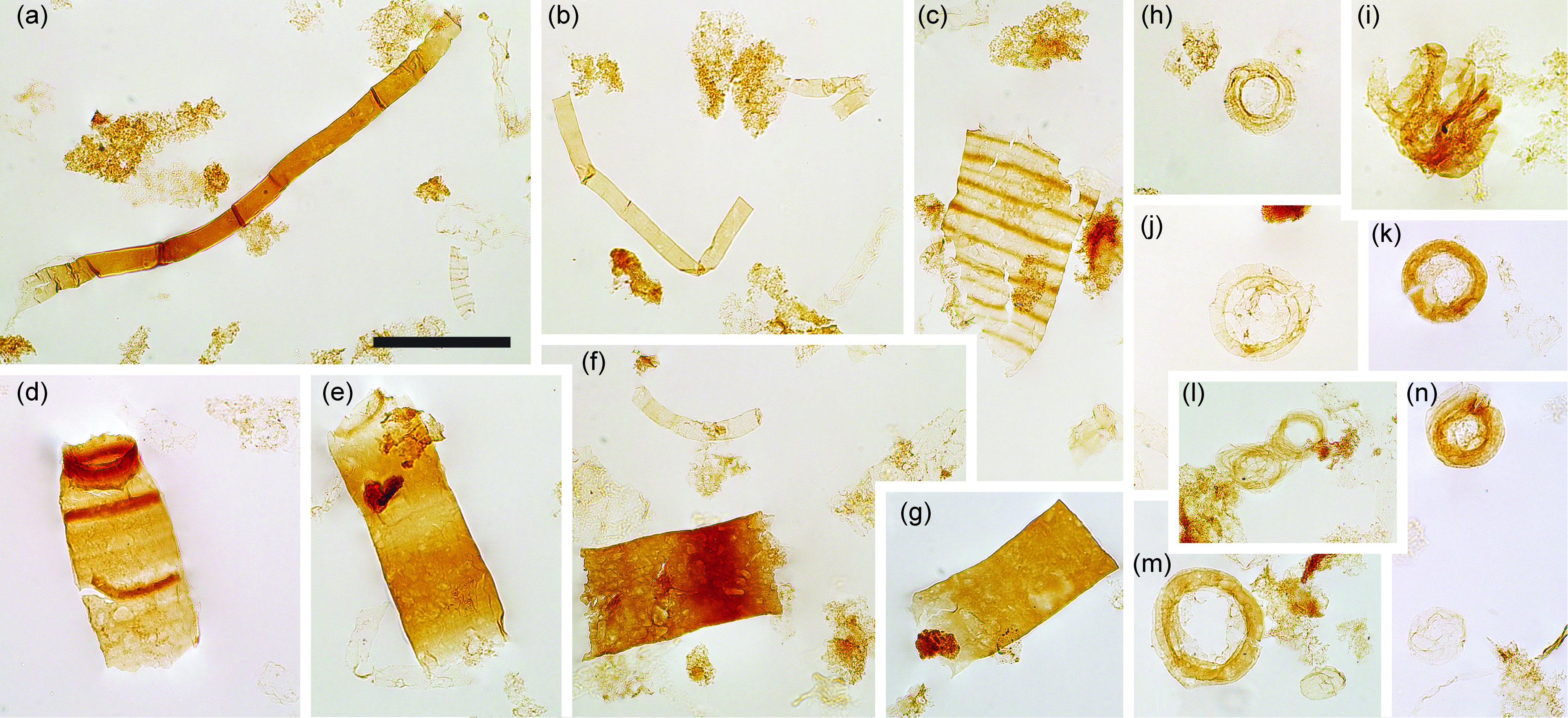
Fig. 8. Filaments and acritarchs (small). (a–b) Smooth sheaths of Siphonophycus. (c) Possible fragment of Cephalonyx-type filament showing regular transverse banding. (d–g) Typical fragments of Rugosoopsis. (h–n) Specimens of Obruchevella, comprising chains of cell rings. (i, l) Show laterally displaced rings. Scale bar represents 100 µm. (a–n) 7.53 m Saarijärvi M52/3533/84/313 core. All specimen numbers have the prefix PMU 38: (a) 165/9; (b) 165/10; (c) 165/11; (d) 165/12; (e) 165/13; (f) 165/14; (g) 165/15; (h) 165/16; (i) 165/17; (j) 165/18; (k) 165/19; (l) 165/20; (m) 165/21; (n) 165/22.
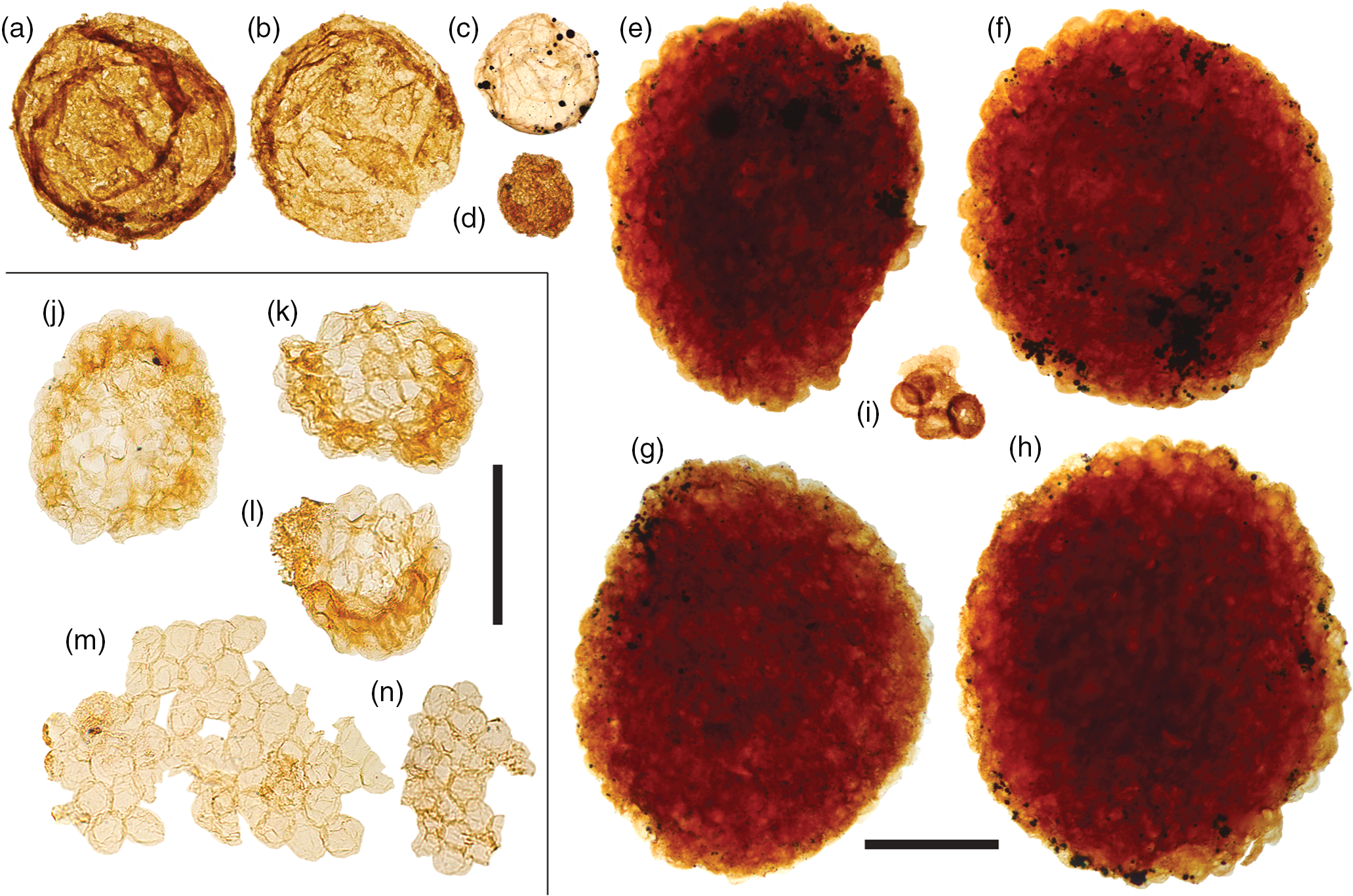
Fig. 9. Acritarchs (large). (a, b) Chuaria sp. (c) Large leiosphaerid encrusted with pyrite framboids. (d) Cell aggregate mass. (e–h) Large, densely packed cell aggregates. (i) Cluster of sphaeromorphic acritarchs. (j–l) Compact, regular spheroid clusters assigned to Symplassosphaeridium. (m, n) Irregular aggregates of loosely-bound spheroids assigned to Synsphaeridium. Scale bars represent 200 µm (a–i); 100 µm (j–n). (a, b, i) 72.20 m M52-Hail-04-004 core; (c–h) 72.70 m M52-Hail-04-004 core; (j–n) 7.53 m Saarijärvi M52/3533/84/313 core. All specimen numbers have the prefix PMU 38: (a) 158/7; (b) 157/8; (c) 159/7; (d) 161/13; (e) 156/4; (f) 156/5; (g) 160/9; (h) 160/10; (i) 162/9; (j) 165/1; (k) 165/2; (l) 165/3; (m) 165/4; (n) 165/5.
4.c. Acritarchs
The majority of samples from both Hailuoto and Saarijärvi produced irregular, aggregate clusters of spheroids (Fig. 9m, n). Comparable sheet-forming clusters of spheroids are frequently assigned to the form-taxon Ostiana (e.g. fig. 5J of Samuelsson & Butterfield, Reference Butterfield2001); however, the more irregular aggregates recovered here are more appropriately assigned to the form-taxon Synsphaeridium Eisenack Reference Eisenack1965 (Fig. 9m, n; cf. fig. 13 of Riedman & Porter, Reference Riedman and Porter2016). The individual spheroids range between 15 and 25 µm in diameter, and form clusters up to ˜350 µm in maximum dimension, consisting of up to ˜100 individual spheroids. More tightly bound and regular-shaped clusters of spheroids (e.g. Fig. 9j–l), where the vesicles are often deformed by compression, are assigned to Symplassosphaeridium Timofeev Reference Timofeev1959, Reference Timofeev;1966 (cf. fig. 18.6 of Hofmann & Jackson, Reference Hofmann and Jackson1994). Occasionally, similar forms have been ascribed to Squamosphaera colonialica Jankauskas Reference Jankauskas1979, but Squamosphaera do not possess true vesicles, displaying only hemispherical protrusions from the vesicle wall (see fig. 17 of Porter & Riedman, Reference Porter and Riedman2016).
Larger cell-aggregates with denser walls were found in most samples, but were particularly abundant in samples from Hailuoto (Fig. 9e–h). Fragmentary forms of identical morphology to these acritarchs were described by Tynni & Donner (Reference Tynni and Donner1980) from the same sediments on Hailuoto, which they ascribed to the extant prasinophycean alga genera Cymatiosphaera as a new species, Cymatiosphaera precambrica (a holotype was not formally designated in that paper, but was rectified in Tynni & Donner, Reference Tynni and Donner1982). Tynni & Donner (Reference Tynni and Donner1980) extrapolated the size of the fragmentary remains to be derived from a spherical body c. 250 µm in diameter when complete; this falls towards the lower end of the size range of forms recovered in this study (˜300–700 µm), perhaps reflecting the differing processing techniques. Assignment of fossil material to the extant genus Cymatiosphaera is not unknown (e.g. Cymatiosphaera are reported from the Early Devonian Rhynie Chert; Dotzler et al. Reference Dotzler, Taylor and Krings2007). However, the surface sculpture of specimens recovered by Tynni & Donner (Reference Tynni and Donner1980) was originally interpreted as reticulate, as in extant Cymatiosphaera. Our recovery of intact specimens here nevertheless demonstrates that the apparently polygonal surface texture actually results from the intersection of compacted adjacent spheroids, which clearly protrude at the flattened cluster margins (Fig. 9e–h). In light of this, these densely clustered cell aggregates are instead more appropriately compared to cell colonies found within organic cysts such as those reported in some Chuaria (see fig. 3 of Tang et al. Reference Tang, Pang, Yuan and Xiao2017).
Acritarchs assigned to Chuaria were found to co-occur with these large cell-aggregate forms (Fig. 9a–b). Feasibly, the large cell aggregates represent the vegetative stage of co-occurring empty Chuaria cysts. Tang et al. (Reference Tang, Pang, Yuan and Xiao2017) suggest that although these cell aggregates could be described under a distinct form-taxonomic name, they likely represent different life-cycle stages of the same biological species, and therefore it may be suitable to expand the form taxonomy of Chuaria to encompass these aggregate forms (see also Chuaria as a subcomponent of a macroalgae, fig. 16 of Kumar, Reference Kumar2001; fig. 7 of Wang et al. Reference Wang, Wang and Du2017). Less optically dense forms could potentially fall under the definition of Leiosphaeridia jacutica (see Javaux & Knoll, Reference Javaux and Knoll2017). Other hollow cyst-like acritarchs recovered include relatively large smooth-walled Leiosphaeridia (Fig. 9c) with prominent compaction folds encrusted in pyrite (compare with fig. 1 of Slater & Budd, Reference Slater and Budd2019).
5. Discussion
5.a. Palaeoenvironment of Hailuoto/Saarijärvi OWM assemblage
As with most assemblages of acid-extracted organic-walled fossils, the Hailuoto and Saarijärvi material likely represents a combination of both benthic and planktonic organisms. Smaller vesicular acritarchs may be sourced from the water column, whereas the majority of the filamentous taxa appear to be benthic, based on their mat-forming interwoven habit. Indeed, OWM assemblages from mid- to late-Proterozoic siliciclastic sediments record a prevalence of mat-forming filaments in shallow-water assemblages, probably reflecting photic-zone colonization by cyanobacterial mats (Butterfield & Rainbird, Reference Butterfield and Rainbird1998; figs 6, 7 of Butterfield & Chandler, Reference Butterfield and Chandler1992; Butterfield, Reference Butterfield2015 b). Filamentous bacterial mats can nevertheless form at a wide range of depths on modern seafloors; whilst shallow-water mats tend to be principally composed of cyanobacteria, deeper water mats are frequently dominated by filamentous sulphur-oxidizing bacteria (Williams & Reimers, Reference Williams and Reimers1983; Jannasch et al. Reference Jannasch, Nelson and Wirsen1989; Bernard & Fenchel, Reference Bernard and Fenchel1995). Despite the inherent difficulties of determining the phylogenetic affinities of simple fossil filaments, there is a case for viewing the filamentous mats at Hailuoto and Saarijärvi as largely cyanobacterial; matted sheaths of Palaeolyngbya and Rugosoopsis share a number of features with the extant cyanobacteria Oscillatoria, Calothrix and Lyngbya; for example, lengths of Rugosoopsis are divided into a trichome and tend to break around shrivelled portions which potentially represent necridia – features associated with oscillatoriacean cyanobacteria (Lamont, Reference Lamont1969; Speziale & Dyck, Reference Speziale and Dyck1992; Butterfield et al. Reference Butterfield, Knoll and Swett1994; Samuelsson & Butterfield, Reference Butterfield2001). The cells of the trichome in Palaeolyngbya also occur as a series of stacked discs, and exhibit features resembling hormocyte cells (compare Fig. 5ac with fig. 2D of Curren & Leong, Reference Curren and Leong2018) as in extant Oscillatoria and Lyngbya (Shukovsky & Halfen, Reference Shukovsky and Halfen1976; Horodyski, Reference Horodyski1977; Nagarkar, Reference Nagarkar2002; Rani et al. Reference Rani, Perumal and Palanivel2016). Cyanobacterial mats would support a shallow-water depositional environment for the sequences at Hailuoto/Saarijärvi (Tynni & Donner, Reference Tynni and Donner1980; Kohonen & Rämö, Reference Kohonen, Rämö, Lehtinen, Nurmi and Rämö2005), although even within the photic zone such mats are likely to include a variety of other microbes (Grazhdankin & Gerdes, Reference Grazhdankin and Gerdes2007; Davies et al. Reference Davies, Liu, Gibling and Miller2016). Other probable benthic elements among the Hailuoto/Saarijärvi assemblages include a subset of the larger vesicular acritarchs (sensu Butterfield Reference Butterfield2005, Reference Butterfield2007; Knoll et al. Reference Knoll, Javaux, Hewitt and Cohen2006), and donut-shaped rings of Siphonophycus that probably grew as spherical nostocalean-like cyanobacterial colonies on late Ediacaran seafloors (see extant Nostoc; Mollenhauer et al. Reference Mollenhauer, Bengtsson and Lindstrøm1999). Larger versions of these colonies may be responsible for Ediacaran macrofossils such as Beltanelliformis (Steiner & Reitner, Reference Steiner and Reitner2001; Bobrovskiy et al. Reference Bobrovskiy, Hope, Krasnova, Ivantsov and Brocks2018 b), or even certain Aspidella and torus-shaped structures on the surface of Ediacaran microbial mats (e.g. Dzaugis et al. Reference Dzaugis, Evans, Droser, Gehling and Hughes2018).
5.b. Ediacaran microbial mats and bilaterians
Trace fossil assemblages in terminal Ediacaran (c. 555–541 Ma) sediments (globally) contain burrows that appear to have been produced by animals with a coelom/hydrostatic internal cavity and an anterior concentration of sensory systems (Budd & Jensen, Reference Budd and Jensen2000), meaning that at least stem-grade bilaterians were present in benthic communities by this time (e.g. Jensen et al. Reference Jensen, Saylor, Gehling and Germs2000, Reference Jensen, Droser and Gehling2006; Jensen, Reference Jensen2003; Narbonne, Reference Narbonne2005; Chen et al. Reference Chen, Zhou, Meyer, Xiang, Schiffbauer, Yuan and Xiao2013; Schiffbauer et al. Reference Schiffbauer, Huntley, O’Neil, Darroch, Laflamme and Cai2016; Herringshaw et al. Reference Herringshaw, Callow and McIlroy2017; Laing et al. Reference Laing, Mángano, Buatois, Narbonne and Gougeon2019; Davies et al. Reference Davies, Shillito, Slater, Liu and McMahon2020). The cuticular fragments recovered at Hailuoto (Fig. 3) demonstrate that body-fossil remains of such bilaterian-grade animals are preserved not only in the Phanerozoic but also in the Ediacaran SCF record. This raises the significance of these otherwise predominantly prokaryotic microbial mat-type fossil assemblages; given the central importance of bilaterians in shaping the nature of the Phanerozoic biosphere, the environment(s) and ecological backdrop of early bilaterian evolution are of intense palaeobiological interest (Budd & Jensen, Reference Budd and Jensen2017).
A variety of macroscopic surface textures on Ediacaran bedding planes have been attributed to microbial mats, often termed microbially induced sedimentary structures or ‘MISS’ (see a review of such structures in Davies et al. Reference Davies, Liu, Gibling and Miller2016). Microbial mats have also been widely invoked in the preservation of Ediacaran mouldic macrofossils (e.g. Gehling, Reference Gehling1999; Gehling & Droser, Reference Gehling and Droser2009; but see Bobrovskiy et al. Reference Bobrovskiy, Krasnova, Ivantsov, Luzhnaya and Brocks2019 for an alternative view). In the latter part of the Ediacaran, bedding planes exhibiting MISS are frequently associated with simple horizontal burrows (e.g. Chen et al. Reference Chen, Zhou, Meyer, Xiang, Schiffbauer, Yuan and Xiao2013). This association has led to the hypothesis that Ediacaran bilaterians exploited such matgrounds as sources of nutrient concentration (Stanley, Reference Stanley1973; Seilacher Reference Seilacher1999; Jensen et al. Reference Jensen, Droser and Gehling2005; Seilacher et al. Reference Seilacher, Buatois and Mángano2005; Buatois et al. Reference Buatois, Mángano, Noffke and Chafetz2011; Gingras et al. Reference Gingras, Hagadorn, Seilacher, Lalonde, Pecoits, Petrash and Konhauser2011; Meyer et al. Reference Meyer, Xiao, Gill, Schiffbauer, Chen, Zhou and Yuan2014; Evans et al. Reference Evans, Gehling and Droser2019; Ivantsov et al. Reference Ivantsov, Nagovitsyn and Zakrevskaya2019 b), or as oxygen-rich microenvironments if photosynthetic (Canfield & Des Marais, Reference Canfield and Des Marais1993; McIlroy & Logan Reference McIlroy and Logan1999; Gingras et al. Reference Gingras, Hagadorn, Seilacher, Lalonde, Pecoits, Petrash and Konhauser2011; Ding et al. Reference Ding, Dong, Sun, Ma, Xu, Yang, Peng, Zhou and Shen2019). Current accounts of Ediacaran matground habitats are almost entirely based on records from MISS, cast-and-mould fossils and trace fossils. Steiner & Reitner (Reference Steiner and Reitner2001) reported carbonaceous compressions of macroscopic Ediacaran taxa from the White Sea region; associated with these fossils were bedding-plane visible ‘elephant skin’ and wrinkle structures attributed to microbial mat imparted textures. Acid treatment was shown to produce Siphonophycus and other filamentous sheaths comparable to those recovered from Hailuoto and Saarijärvi (compare Figs 6–7 with fig. 6 of Steiner & Reitner, Reference Steiner and Reitner2001), including pyritized sheaths similar to those reported here (see fig. 7 of Steiner & Reitner, Reference Steiner and Reitner2001). Our data from the late Ediacaran of Finland demonstrate that such preservation is widespread, even in the absence of carbonaceous macrofossil preservation. In this light, SCF-style processing and investigation of late Ediacaran sediments can be viewed as a largely untapped taphonomic window, offering new insights into the critical change from matground to mixground seafloor environments as the Proterozoic gave way to the Phanerozoic.
6. Conclusions
Late Ediacaran sedimentary rocks from subsurface deposits in central Finland contain well-preserved carbonaceous microfossils, including an abundance of filamentous prokaryotes (probable cyanobacteria), a variety of acritarchs, and significantly, fragments of metazoan cuticle derived from bilaterians. Based on the composition of the recovered fossil assemblage, we revise previous interpretations of an early- to mid-Ediacaran age to a late Ediacaran age for the upper part of the Hailuoto Formation.
Acknowledgements
SW and BJS acknowledge joint first authorship of this paper. We thank Marko Puonti for help in collection of samples and logistical work at the Finnish national drillcore archive in Loppi, and Jussi Pokki (Geological Survey of Finland) for helpful advice. Insightful comments by Heda Agić (University of California, Santa Barbara) and an anonymous reviewer improved the manuscript. BJS acknowledges the support of Swedish Research Council (VR) grant 2020-03314.