1. Introduction
The poor early fossil record makes investigations of sponge evolution difficult. Ediacaran sponge-like fossils are questionable (e.g. Gehling & Rigby, Reference Gehling and Rigby1996; Brasier et al. Reference Brasier, Green and Shields1997; Li et al. Reference Li, Chen and Hua1998; Reitner & Wörheide, Reference Reitner, Wörheide, Hooper and Van Soest2002; Maloof et al. Reference Maloof, Rose, Beach, Samuelsson, Calmet, Erwin, Poirier, Yao and Simons2010; Antcliffe et al. Reference Antcliffe, Callow and Brasier2014), and the earliest appearance of sponge spicules has been dated to c. 535 Ma (Guo et al. Reference Guo, Li, Han, Zhang, Zhang, Ou, Liu, Shu, Maruyama and Komiya2008; Chang et al. Reference Chang, Feng, Clausen and Zhang2017). Problematically, however, disarticulated spicules are of little taxonomic value. To date, articulated, well-preserved sponge fossils in Burgess-Shale-type preservation and deep-water black shales have only been reported after the beginning of Cambrian Age 3 (e.g. Steiner et al. Reference Steiner, Mehl, Reitner and Erdtmann1993; Luo et al. Reference Luo, Zhao and Zeng2020). Furthermore, Luo & Reitner (Reference Luo and Reitner2019) described three-dimensionally preserved sponge skeletal frames from the basal Niutitang Formation (Stage 2) near Sancha in China that have illuminated the nature of stem-group hexactinellids.
Here, new occurrences of sponge fossils of no later than the Cambrian Age 2 are described from phosphorite deposits near the village of Fontanarejo in central Spain (Fig. 1). The Fontanarejo phosphorites belong to the Pusa Formation and were targets of mining exploration led by the Geological Survey of Spain (IGME). Perconig et al. (Reference Perconig, Vázquez Guzmán, Velando and Leyva1983, Reference Perconig, Vázquez Guzmán, Velando, Leyva, Cook and Shergold1986) provided the first summary of the formation’s stratigraphy, sedimentology and fossil content (particularly sponge spicules) in Fontanarejo. While these early studies considered the Fontanarejo phosphorites to be of Ediacaran age, Jensen et al. (Reference Jensen, Palacios and Mus2010) suggested a Lower Cambrian age (Fortunian) for the Pusa Formation. Reitner et al. (Reference Reitner, Luo and Duda2012) and Reitner & Luo (Reference Reitner and Luo2019) provided the first overview of the different sponge types contained within the Fontanarejo deposits, highlighting their stratigraphic value. The aim of this paper is to describe the different types of sponge remains found in the Fontanarejo phosphorites in greater detail and to discuss them in a taxonomic frame advocated by the authors. In addition to sponges, other fossils recovered from the phosphorites, including small shelly fossils (SSF) and microbial fossils, were studied. A further goal of the paper is to examine the microbial impact on early diagenetic apatite formation that facilitated the exceptional preservation of fossils.

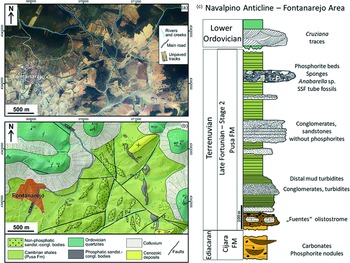
Fig. 1. (a) Latest available orthophotograph (2019) of the Fontanarejo area (obtained from the Spanish Geographical Survey, IGN) showing the current outcrop conditions, dominated by vegetation and agricultural lands. (b) Geological map of the Fontanarejo area, same extension as in (a), at the western end of the Navalpino Anticline. Map is drawn over a lidar digital elevation model (obtained from the Spanish Geographical Survey, IGN) and based on our own fieldwork and on the previous geological maps of the area. (c) Reconstructed stratigraphic section of the Fontanarejo area, based on Perconig et al. (Reference Perconig, Vázquez Guzmán, Velando, Leyva, Cook and Shergold1986), Picart Boira (Reference Picart Boira1988) and our own fieldwork. The Ediacaran Cijara Formation and the so-called ‘Fuentes’ olistostromes (base of the Pusa Formation), which roughly represent the Precambrian–Cambrian boundary, are not exposed in this area. Data used in this reconstruction are from Álvaro et al. (Reference Álvaro, Shields-Zhou, Ahlberg, Jensen and Palacios2016) and Jensen et al. (Reference Jensen, Palacios and Mus2010). This section shows the stratigraphic base, which is exposed in the Valdelacasa Anticline west of Fontanarejo.
Assuming that animal life had a long evolutionary history before the Cambrian, palaeontologists have been tracing the earliest fossil evidence of sponges for many decades. Putative Precambrian spicule-like structures have been reported from analyses of thin-sections (e.g. Brasier et al. Reference Brasier, Green and Shields1997; Li et al. Reference Li, Chen and Hua1998; Reitner & Wörheide, Reference Reitner, Wörheide, Hooper and Van Soest2002) and macroscopic Ediacaran fossils (e.g. Gehling & Rigby, Reference Gehling and Rigby1996; Serezhnikova & Ivantsov, Reference Serezhnikova and Ivantsov2007; Clites et al. Reference Clites, Droser and Gehling2012). Furthermore, enigmatic structures observed in various Neoproterozoic successions were interpreted to be poriferan (e.g. Maloof et al. Reference Maloof, Rose, Beach, Samuelsson, Calmet, Erwin, Poirier, Yao and Simons2010; Brain et al. Reference Brain, Prave, Hoffmann, Fallic, Botha, Herd, Sturrock, Young, Condon and Allison2012; Wallace et al. Reference Wallace, Hood, Woon, Hoffmann and Reed2014). These microscopic and macroscopic structures have mostly been discounted by later studies (e.g. Antcliffe et al. Reference Antcliffe, Callow and Brasier2014; Muscente et al. Reference Muscente, Hawkins and Xiao2015; Cunningham et al. Reference Cunningham, Liu, Bengtson and Donoghue2016; Luo et al. Reference Luo, Pan and Reitner2017). The most plausible alternative evidence for Precambrian sponges, i.e. molecular fossils (e.g. Love et al. Reference Love, Grosjean, Stalvies, Fike, Grotzinger, Bradley, Kelly, Bhatia, Meredith, Snape, Bowring, Condon and Summons2009; Zumberge et al. Reference Zumberge, Love, Cárdenas, Sperling, Gunasekera, Rohrssen, Grosjean, Grotzinger and Summons2018) and exceptionally preserved fossils from the Weng’an Biota (Yin et al. Reference Yin, Zhu, Davidson, Bottjer, Zhao and Tafforeau2015), is also still under debate (e.g. Antcliffe et al. Reference Antcliffe, Callow and Brasier2014; Botting & Muir, Reference Botting and Muir2018).
Several reasons may plausibly explain the poor fossil record of early sponges. For instance, the earliest sponges may have been too small or too labile to be fossilized, or they were morphologically very different from their Phanerozoic descendants.
Regardless of this question, the earliest indisputable sponge fossils are disarticulated spicules recognized from the Fortunian (538.8–529.0 Ma; Peng et al. Reference Peng, Babcock, Ahlberg, Gradstein, Ogg, Schmitz and Ogg2020). These fossil spicules include pentactins and hexactins macerated from the Protoherzina biozone of the Yangjiaping Formation of Hunan, China (Ding & Qian, Reference Ding and Qian1988), and stauractins and pentactins from the same biozone of the Soltanieh Formation in North Iran (Antcliffe et al. Reference Antcliffe, Callow and Brasier2014). The spicules from Iran occur right above the Basal Cambrian Carbon Isotope Excursion (BACE). Probably even earlier occurrences of spicules were reported from the Yanjiahe Formation of Yangtze Gorges which were correlated to around the nadir of the BACE, although the referred geochemical profiles were not directly obtained from the fossil-bearing outcrop (Chang et al. Reference Chang, Feng, Clausen and Zhang2017, Reference Chang, Zhang, Clausen, Bottjer and Feng2019).
Taxonomically more informative sponge fossils (i.e. those preserved with articulated skeletal frames) are mostly known from fossil Lagerstätten that slightly predate the Cambrian Age 3. Famous examples include black shales of the Niutitang and Hetang Formations in South China (Steiner et al. Reference Steiner, Mehl, Reitner and Erdtmann1993; Xiao et al. Reference Xiao, Hu, Yuan, Parsley and Cao2005), the Chengjiang Biota (e.g. Chen et al. Reference Chen, Hou and Lu1989, Reference Chen, Hou and Li1990; Rigby & Hou, Reference Rigby and Hou1995), the Sirius Passet Biota (Botting & Peel, Reference Botting and Peel2016) and the Burgess Shale fossil Lagerstätte (e.g. Rigby, Reference Rigby1986; Rigby & Collins, Reference Rigby and Collins2004). The recently described three-dimensionally preserved hexactinellid sponge fossils and diverse disarticulated spicules from cherty phosphorites at the basal Niutitang Formation of Hunan, China, are probably older than 521 Ma, thus providing a valuable window for studying sponge palaeobiodiversity, palaeoecology and evolution preceding the Cambrian Epoch 2 (Luo & Reitner, Reference Luo and Reitner2019).
The Fontanarejo Lagerstätte is of similar age to – or perhaps slightly older than – the Niutitang phosphorites as indicated by recent discoveries of Anabarella and an improved sedimentological correlation (Álvaro et al. Reference Álvaro, Cortijo, Jensen, Lorenzo, Palacios and Pieren2019; Álvaro & Lorenzo, Reference Álvaro and Lorenzo2021; this study). It therefore provides unique and valuable insights into early metazoan diversity, particularly allowing for the reconstruction of the major sponge branches.
Sponges are famous for their morphological plasticity and richness of homoplastic characters. The fact that even these characters were only partly preserved in fossils increases the difficulty of establishing a natural classification for distinct taxa, which is one of the major objectives of palaeontological research. Generally, the classification used here follows the traditional concept of the Systema Porifera: A Guide to the Classification of Sponges (Hooper & Van Soest, Reference Hooper and Van Soest2002). Recent phylogenomic studies have partly challenged the sponge taxonomy advocated in this book (e.g. Morrow & Cárdenas, Reference Morrow and Cárdenas2015; Erpenbeck et al. Reference Erpenbeck, Galitz, Ekins, Cook, van Soest, Hooper and Wörheide2020). Nevertheless, it is still the best reference work for palaeontologists because it is based on a balanced consideration of both morphological and molecular features. For this reason, we integrate findings from the most recent phylogenetic models (e.g. Erpenbeck & Wörheide Reference Erpenbeck and Wörheide2007; Dohrmann et al. Reference Dohrmann, Janussen, Reitner, Collins and Wörheide2008; Wörheide et al. Reference Wörheide, Dohrmann, Erpenbeck, Larroux, Maldonado, Voigt, Borchiellini, Lavrov, Becerro, Uriz, Maldonado and Xavier2012; Redmond et al. Reference Redmond, Morrow, Thacker, Diaz, Boury-Esnault, Cárdenas, Hajdu, Lôbo-Hajdu, Picton, Pomponi, Kayal and Collins2013) into the taxonomic frame of Hooper & van Soest (2002).
A different phylogenetic framework for sponges has recently been proposed by Botting & Muir (Reference Botting and Muir2018). This concept, however, is not adopted here since the underlying data require re-examination and some key phylogenetic interpretations need further discussion. For example, the authors stated that a tetraradial symmetry was ancestral to all sponges but secondarily lost. This is neither supported by phylogenetic analyses of living sponges, nor by sufficient palaeontological evidence except for a superficial similarity between the Burgess Shale fossil Takakkawia and the ctenophore Thaumactena ensis from the Chengjiang Biota. In fact, some phylogenetically distant modern sponge taxa show tetraradial symmetry, such as the hexactinellid Composocalyx (Tabachnick & Menshenina Reference Tabachnick, Menshenina, Hooper and Van Soest2002) and the carnivorous demosponges Chondrocladia lyra (Lee et al. Reference Lee, Reiswig, Austin and Lundsten2012). The latter even exhibits other symmetrical patterns within the single species, including bilateral, triradial and pentaradial forms, and, this seems to be of ecological rather than phylogenetic significance (e.g. Tabachnick, Reference Tabachnick, Reitner and Keupp1991). Indeed, these symmetrical patterns are absent in closely related lineages of the symmetric taxa (Morrow & Cárdenas, Reference Morrow and Cárdenas2015; Dohrmann et al. Reference Dohrmann, Kelley, Kelly, Pisera, Hooper and Reiswig2017). From a palaeontological point of view, there is obviously no robust evidence to support the claim that Cambrian tetraradial sponges evolved earlier than other fossil taxa. A detailed account of the controversy surrounding this new phylogenetic frame is beyond the scope of this paper and will be discussed in future publications.
2. Material and methods
Over the last two decades, we conducted several field campaigns to the phosphorite deposits in the Fontanarejo area, with the aim of mapping the outcrop and collecting samples. In the field, we collected data for preparing a new geological map of the area, using previous maps as a starting point (Perconig et al. Reference Perconig, Vázquez Guzmán, Velando and Leyva1983, Reference Perconig, Vázquez Guzmán, Velando, Leyva, Cook and Shergold1986; Monteserín López et al. Reference Monteserín López, Nozal Martín, López Díaz, Rubio Pascual and Serrano García1989; López Díaz, Reference López Díaz1995) (Fig. 1). The background geographic information used was the latest available topographic maps, and lidar digital elevation models, obtained from the Spanish Geographical Survey (IGN) in 2019. The geographic data were processed and managed using QGIS software, and the geological map was drawn using CorelDraw.
Additional photos of the outcrop geology and deposits are provided in Supplementary Figures S1 and S2 (in the Supplementary Material available online at https://doi.org/10.1017/S001675682100087X).
We collected more than 200 rock samples in the field. From these, we prepared 120 large thin-sections (10 × 5 cm, 5 × 5 cm, and 15 × 10 cm). The thin-sections are archived in the museum collection of the Faculty of Geoscience and Geography at the University of Göttingen, Germany (inventory number GZG.INV.808).
Thin-section analyses were conducted using a Zeiss SteREO Discovery.V12 stereo microscope and a Zeiss AxioImager Z1 microscope. Both microscopes were connected to a Zeiss AxioCam MRc camera. A fluorescence microscope equipped with an ebx 75 isolated power unit was mounted on a Zeiss AxioImager Z1 microscope. The instrument was fitted with a Zeiss A 10 Alexa Fluor 488 filter, with an excitation wavelength of BP 450–490 nm and an emission wavelength of BP 515–565 nm. For cathodoluminescence (CL) microscopy, a Cambridge Instrument Citl CCL 8200 Mk3A cold-cathode system (operating voltage of c.15 kV; electric current of c. 250–300 μA) linked to a Zeiss Axiolab microscope and AxioCam 703 camera was used. Field emission scanning electron microscopy (Fe-SEM) was performed with a Carl Zeiss LEO 1530 Gemini system. The instrument was coupled to an Oxford Instruments INCA X-act energy-dispersive X-ray spectrometry (EDX) detector, to additionally obtain EDX single spectra and elemental maps.
Elemental distributions in the phosphorite samples were mapped using a Bruker M4 Tornado micro X-ray fluorescence (μ-XRF) instrument equipped with a XFlash 430 Silicon Drift Detector. Analyses were carried out at a spatial resolution of 70 µm. Each pixel covers 30 ms. Measurements were performed at 50 kV and 400 µA at a chamber pressure of 20 mbar. Additionally Raman spectroscopy was used for point measurements and area mapping. Raman spectra were collected using a WITec alpha300 R fibre-coupled ultra-high throughput spectrometer. Before analysis, the system was calibrated using an integrated light source. The experimental set-up included a 405 nm laser (chosen to reduce fluorescence effects), 10 mW laser power, and 50x and 100x long working distance objectives with a numerical aperture of 0.55 and a grating of 1200 g mm–1. This set-up had a spectral resolution of 2.6 cm–1. The spectrometer was centred at 1530 cm–1, covering a spectral range from 122 cm–1 to 2759 cm–1. Each spectrum was collected by two accumulations, with an acquisition time of 5 s. Raman spectra were processed with the WITec project software. The background was subtracted using a rounded shape, and band positions were determined by fitting a Lorentz function. Both analytical facilities (µ-XRF and Raman spectroscopy) are located at the Geosciences Center of the University of Göttingen. Detailed µ-XRF and Raman maps are provided in Supplementary Figures S3–S5 (in the Supplementary Material available online at https://doi.org/10.1017/S001675682100087X).
3. Regional geology and stratigraphy
3.a. Regional geological setting
The studied deposits crop out near the village of Fontanarejo (Ciudad Real province, central Spain) (Fig. 1). Geographically, the successions are located at the southern margin of the Toledo Mountains, and geologically they are part of the southeast area of the Central Iberian Zone (CIZ) of the Iberian Variscan Massif. This area of the CIZ consists of large-scale, NW–SE-trending upright folds, topographically highlighted by the relief of the Ordovician quartzites. The folds include wide anticlines containing pre-Ordovician materials and narrower synclines containing Ordovician–Devonian deposits (Díez Balda et al. Reference Díez Balda, Vegas, Gonzáles Lodeiro, Dallmeyer and Martínez García1990; Vidal et al. Reference Vidal, Palacios, Gámez-Vintaned, Balda and Grant1994; Liñán et al. Reference Liñán, Gozalo, Palacios, Gámez Vinaned, Ugidos, Mayoral, Gibbons and Moreno2002; Valladares et al. Reference Valladares, Barba, Ugidos, Gibbons and Moreno2002; Martínez Catalán et al. Reference Martínez Catalán, Martínez Poyatos, Bea and Vera2004; Álvaro et al. Reference Álvaro, Cortijo, Jensen, Lorenzo, Palacios and Pieren2019). The Fontanarejo area represents the northeastern border of the Navalpino Anticline, which accordingly exhibits a core of folded and deformed Neoproterozoic and Cambrian rocks unconformably overlain by Ordovician quartzites (San José, Reference San José1984; López Díaz, Reference López Díaz1994, Reference López Díaz1995; Gutiérrez-Marco et al. Reference Gutiérrez-Marco, Lorenzo and Sá2017) (Fig. 1b, c; Supplementary Fig. S1, in the Supplementary Material available online at https://doi.org/10.1017/S001675682100087X). The studied phosphate-rich deposits are part of the Cambrian succession that directly underlies the Ordovician quartzites of the northeastern margin of the Navalpino Anticline (cf. Perconig et al. Reference Perconig, Vázquez Guzmán, Velando and Leyva1983, Reference Perconig, Vázquez Guzmán, Velando, Leyva, Cook and Shergold1986; Picart Boira, Reference Picart Boira1988) (Fig. 1b, c; Supplementary Fig. S1. in the Supplementary Material available online at https://doi.org/10.1017/S001675682100087X).
3.b. Stratigraphy
The stratigraphic terminology and age of the pre-Ordovician deposits of the CIZ have been controversial for several decades (see discussions in San José et al. Reference San José, Pieren, García-Hidalgo, Vilas, Herranz, Peláez, Perejón, Dallmeyer and Martínez García1990; Vidal et al. Reference Vidal, Palacios, Gámez-Vintaned, Balda and Grant1994; Rodríguez Alonso et al. Reference Rodríguez Alonso, Díez Balda, Perejón, Pieren, Liñan, López Díaz, Moreno, Gámez Vintaned, González Lodeiro, Martínez Poyatos, Vegas and Vera2004; Jensen et al. Reference Jensen, Palacios and Mus2010; Álvaro et al. Reference Álvaro, Cortijo, Jensen, Lorenzo, Palacios and Pieren2019). Typically, these deposits have been divided into three large lithostratigraphic units, with varying names and boundaries. The Fontanarejo phosphorites belong to the youngest of these groups, termed ‘Pusian’ (San José et al. Reference San José, Pieren, García-Hidalgo, Vilas, Herranz, Peláez, Perejón, Dallmeyer and Martínez García1990), ‘Valdelacasa Group’ (Monteserín López et al. Reference Monteserín López, Nozal Martín, López Díaz, Rubio Pascual and Serrano García1989) or ‘Río Huso Group’ (Vidal et al. Reference Vidal, Palacios, Gámez-Vintaned, Balda and Grant1994). Within this group, the studied deposits belong to a shale-dominated unit termed the Pusa Formation, whose composition and boundaries have also been studied previously (e.g. San José et al. Reference San José, Pieren, García-Hidalgo, Vilas, Herranz, Peláez, Perejón, Dallmeyer and Martínez García1990; Vidal et al. Reference Vidal, Palacios, Gámez-Vintaned, Balda and Grant1994; Álvaro et al. Reference Álvaro, Cortijo, Jensen, Lorenzo, Palacios and Pieren2019).
From a sedimentological point of view, the Pusa Formation in the study area has six sequences or subunits (San José, Reference San José1984; Gabaldón López et al. Reference Gabaldón López, Hernández Urroz, Lorenzo Álvarez, Picard Boira, Santamaría Casanova, Solé Pont, Notholt, Sheldon and Davidson1989), with the Fontanarejo phosphorites being part of subunit 5. San José (Reference San José1984) even defined them as a member within the unit (‘Fontanarejo Member’). From a cartographic point of view, the Pusa Formation has only two (Monteserín López et al. Reference Monteserín López, Nozal Martín, López Díaz, Rubio Pascual and Serrano García1989) or three (San José, Reference San José1984; López Díaz, Reference López Díaz1994; Vidal et al. Reference Vidal, Palacios, Gámez-Vintaned, Balda and Grant1994) mappable subunits. The latest stratigraphic review (Álvaro et al. Reference Álvaro, Cortijo, Jensen, Lorenzo, Palacios and Pieren2019) considers three mappable lithostratigraphic members, with the Fontanarejo phosphorites being part of the middle, conglomerate-rich member (Fig. 1c; Supplementary Fig. S1, in the Supplementary Material available online at https://doi.org/10.1017/S001675682100087X). According to this recent stratigraphic framework, the Pusa Formation is as thick as 3500 m. Lithologically, the formation is dominated by shales interbedded with breccia, conglomerates and sandstones, but also locally contains carbonate and phosphorite seams (Álvaro et al. Reference Álvaro, Cortijo, Jensen, Lorenzo, Palacios and Pieren2019).
The precise age of the Pusa Formation remains uncertain, but most interpretations agree that it includes deposits from the latest Neoproterozoic and earliest Cambrian (Brasier et al. Reference Brasier, Perejón and De San1979; Perconig et al. Reference Perconig, Vázquez Guzmán, Velando and Leyva1983, Reference Perconig, Vázquez Guzmán, Velando, Leyva, Cook and Shergold1986; San José, Reference San José1984; Monteserín López et al. Reference Monteserín López, Nozal Martín, López Díaz, Rubio Pascual and Serrano García1989; Vidal et al. Reference Vidal, Palacios, Gámez-Vintaned, Balda and Grant1994; Jensen et al. Reference Jensen, Palacios and Mus2010). However, a zircon age of 533 ± 17 Ma from the middle part of its lower member (Talavera et al. Reference Talavera, Montero, Martínez Poyatos and Williams2012), and the discovery of Anabarella cf. plana fossils, have led the Pusa Formation to be assigned exclusively to the Lower Cambrian, mainly to the Terreneuvian. The presence of trilobites, small shelly fossils and archaeocyaths evidences that the stratigraphic position of the upper Pusa Formation corresponds to the Cambrian Series 2 (Álvaro et al. Reference Álvaro, Cortijo, Jensen, Lorenzo, Palacios and Pieren2019).
Notably, we also found Anabarella-like fossils within the Fontanarejo phosphorite deposits. This finding aligns with occurrences of Anabarella cf. plana in stratigraphically equivalent sedimentary rocks from the Alcudia anticline close to Ciudad Real in central Spain (i.e. ‘Conglomerados de San Lorenzo’, Pusa or Fuentepizarra formations: Pieren & García-Hidalgo, Reference Pieren and García-Hidalgo1999; Vidal et al. Reference Vidal, Palacios, Moczydłowska and Gubanov1999; Pieren Pidal, Reference Pieren Pidal2000; Álvaro et al. Reference Álvaro, Cortijo, Jensen, Lorenzo, Palacios and Pieren2019; Álvaro & Lorenzo, Reference Álvaro and Lorenzo2021). Given this stratigraphic framework, the phosphorite deposits of Fontanarejo we studied are likely late Fortunian – earliest Age 2 in age. Figure 1c summarizes the main sedimentary structures and the stratigraphy of the Fontanarejo area.
4. Outcrop description and sedimentology
The landscape of the Fontanarejo area is mainly controlled by the weathering susceptibility of shales from the Pusa Formation underlying the main agriculture fields (Fig. S1, in the Supplementary Material available online at https://doi.org/10.1017/S001675682100087X). Interbedded with the shales are more weathering-resistant sandstone–conglomerate beds that form the small hills of the landscape. The phosphorites we studied crop out ∼2 km east of the village of Fontanarejo and are embedded in the youngest series of the sandstone–conglomerate beds (Perconig et al. Reference Perconig, Vázquez Guzmán, Velando and Leyva1983, Reference Perconig, Vázquez Guzmán, Velando, Leyva, Cook and Shergold1986; Picart Boira, Reference Picart Boira1988) (Fig. 1b, c; Supplementary Figs S1, S2, in the Supplementary Material available online at https://doi.org/10.1017/S001675682100087X). These phosphorite deposits were first identified in the course of extensive field campaigns conducted by IGME during the 1980s. Since then, the Fontanarejo outcrops have been significantly degraded due to agricultural activities (Supplementary Fig. S1). This makes it almost impossible to find in situ outcrops larger than a couple of square metres, and those are significantly weathered. A further problem is that different phosphorite beds cannot be directly correlated due to fault displacement.
Given the current poor outcrop conditions, it is difficult to accurately assess the geometry and macroscopic features of the sandstone–conglomerate beds in greater detail. However, they are clearly discontinuous and locally seem to pinch out laterally. Furthermore, they appear to be 100–500 m wide and 20–70 m thick. All these characteristics are consistent with the previous descriptions of the surface and subsurface. Notably, earlier studies have reported that some of the beds display erosional bases and flat to undulated tops and internally include fining-upward, cross-bedded sediments (Perconig et al. Reference Perconig, Vázquez Guzmán, Velando and Leyva1983, Reference Perconig, Vázquez Guzmán, Velando, Leyva, Cook and Shergold1986; Picart Boira, Reference Picart Boira1988; Álvaro et al. Reference Álvaro, Shields-Zhou, Ahlberg, Jensen and Palacios2016). We found these features only to be present in sandstone–conglomerate beds that do not contain a significant number of phosphorite pebbles (Supplementary Fig. S2e, in the Supplementary Material available online at https://doi.org/10.1017/S001675682100087X), while they appear to be absent in the youngest, phosphorite-rich beds in the succession (Supplementary Figs S1, S3). Regardless of these differences, however, integrated map and field evidence suggests that the phosphorite-rich and phosphorite-poor bodies are both channel deposits.
Detailed microfacies analyses of the youngest, phosphate-rich sandstone–conglomerate beds revealed that these deposits exhibit a typical rudstone fabric (Supplementary Figs S1d, S3, in the Supplementary Material available online at https://doi.org/10.1017/S001675682100087X). About 90 % of the beds consist of phosphate clasts that are ovoidal to subspherical in shape and display black, grey and brown colours. The remaining 10 % comprise reworked, sub-rounded blocks of Pusa shale, often containing angular grey mudstone intraclasts and quartzite grains with minor mica. Iron oxide crusts surround many phosphate clasts and are likely remnants of former framboidal pyrites. Two types of phosphatic sediments are present: one is more weathered and iron oxide rich, the other is fresher, with black to grey clasts with less iron oxide.
The phosphate clasts consist of apatite and display diverse morphologies. Ovoid-shaped clasts, some millimetres in size (‘oncoid-like microbialites’), are abundant and have distinct nuclei. They are often composed of sponge remains, SSF relics and mud clasts. Anatase (TiO2) is common and often associated with microbial remains, rendering an authigenic origin possible. The oncoid-like microbialites do not show any clear laminated fabric but instead display cloudy colloform, often thrombolitic structures that are enriched in organic matter. Clay minerals form the nucleic area of the oncoid-like microbialites (Supplementary Fig. S3, in the Supplementary Material available online at https://doi.org/10.1017/S001675682100087X).
In addition to the oncoid-like microbialites, mm-sized microcrystalline structureless apatite clasts are also common and often include sponge spicules. The microcrystalline apatite components usually show shrinkage patterns and are brown or dark grey in colour. The spicule-rich apatite, in contrast, has an irregular shape, is generally honey-yellow in colour and is locally enriched in organic matter. Such micro-peloidal textures typically result from the taphonomic mineralization of microbially interspersed sponge tissue (Reitner & Neuweiler, Reference Reitner and Neuweiler1995; Reitner & Schumann-Kindel, Reference Reitner and Schumann-Kindel1997). For this reason, we interpret these components as permineralized sponge tissue.
Early diagenetic phosphatic cements are quite common. Early fibrous prismatic phosphatic cements surround spicules and other components. This early cementation also locally stabilized and preserved original spicule arrangements, facilitating the interpretation of the sponge fossils. Later diagenetic phosphatic cements exhibit larger crystals, and it seems that phosphate minerals replaced siliceous (spicules) and calcareous materials (cf. Perconig et al. Reference Perconig, Vázquez Guzmán, Velando and Leyva1983, Reference Perconig, Vázquez Guzmán, Velando, Leyva, Cook and Shergold1986; Picart Boira, Reference Picart Boira1988; Álvaro et al. Reference Álvaro, Shields-Zhou, Ahlberg, Jensen and Palacios2016).
Most studies interpret the Fontanarejo phosphorites (and equivalent deposits in adjacent areas) as allochthonous channel deposits formed in an offshore shelf/platform setting, below wave base level, probably close to the platform margin or to the beginning of the slope (Picart Boira, Reference Picart Boira1988; Gabaldón López et al. Reference Gabaldón López, Hernández Urroz, Lorenzo Álvarez, Picard Boira, Santamaría Casanova, Solé Pont, Notholt, Sheldon and Davidson1989; Pieren Pidal, Reference Pieren Pidal2000; Álvaro et al. Reference Álvaro, Shields-Zhou, Ahlberg, Jensen and Palacios2016). However, Perconig et al. (Reference Perconig, Vázquez Guzmán, Velando and Leyva1983, Reference Perconig, Vázquez Guzmán, Velando, Leyva, Cook and Shergold1986) interpreted the Fontanarejo phosphorites as intertidal and subtidal channel deposits, based on the sorting and rounding of the clasts, the biogenic features recognized in the rocks, and the local presence of mud cracks. Our findings clearly support the first, more traditional interpretation. This conclusion is based on map and field evidence as well as on microfacies characteristics (see above). For instance, we found no sedimentological evidence for subaerial exposure (e.g. mud cracks). Furthermore, the channel infill is polymictic and poorly sorted, suggesting relatively rapid sedimentation after high-energy episodes that remobilized material from shallower areas as well as material from adjacent zones. The interpretation that some of the Pusa shales are distal mud turbidites interbedded with channels and turbiditic sandstone layers (Fig. 1c; Supplementary Fig. S2a, b, d, in the Supplementary Material available online at https://doi.org/10.1017/S001675682100087X) is consistent with findings from other studies on the formation (San José, Reference San José1984; Monteserín López et al. Reference Monteserín López, Nozal Martín, López Díaz, Rubio Pascual and Serrano García1989; López Díaz, Reference López Díaz1994; Vidal et al. Reference Vidal, Palacios, Gámez-Vintaned, Balda and Grant1994; Rodríguez Alonso et al. Reference Rodríguez Alonso, Díez Balda, Perejón, Pieren, Liñan, López Díaz, Moreno, Gámez Vintaned, González Lodeiro, Martínez Poyatos, Vegas and Vera2004; Álvaro et al. Reference Álvaro, Shields-Zhou, Ahlberg, Jensen and Palacios2016, Reference Álvaro, Cortijo, Jensen, Lorenzo, Palacios and Pieren2019).
5. Results and discussion: Animal fossils in Fontanarejo phosphorites
5.a. Phylogenetic status of Hexactinellida (Schmidt, Reference Schmidt1870)
As outlined in the Introduction and discussed below, it is overwhelmingly difficult to reconstruct the phylogeny of fossil sponges. Therefore, our study is based on the careful examination and morphological classification of fossil sponge remains contained in the investigated materials.
Hexactinellids are a monophyletic group characterized by triaxon spicules. Based on distinct microscleres, two major clades are defined in this class: Hexasterophora and Amphidiscophora (Schulze, Reference Schulze1886). The ancestral hexactinellid spicule was interpreted to be regular hexactin (Reitner & Mehl, Reference Reitner and Mehl1996; Dohrman et al. Reference Dohrmann, Janussen, Reitner, Collins and Wörheide2008, Reference Dohrmann, Kelley, Kelly, Pisera, Hooper and Reiswig2017). It has long been debated how the palaeontological record can be reconciled with modern molecular phylogenetic data. The skeletal architecture of early Palaeozoic hexactinellids includes two major morphological groups, the Reticulosa Reid, Reference Reid1958 and the ‘Rossellimorpha’ (Mehl, Reference Mehl1996), both of which could be paraphyletic or polyphyletic (Mehl-Janussen, Reference Mehl-Janussen1999; Botting & Muir, Reference Botting and Muir2018).
Most of the Fontanarejo articulated hexactinellid sponges exhibit a lyssacine body plan, i.e. a primarily non-cemented spicular skeleton. These early lyssacine hexactinellids fit best with the morphological group ‘Rossellimorpha A’ defined by Mehl-Janussen (Reference Mehl-Janussen1999). Some Fontanarejo hexactinellids display a cemented skeleton resembling a type of ‘dictyonal’ body plan (description see Section 5.b), in which the spicules are cemented by controlled secondary silica cementation. This type of skeleton may have evolved independently and therefore represent a polyphyletic characteristic. Steiner et al. (Reference Steiner, Mehl, Reitner and Erdtmann1993) reported the first more typical dictyonal hexactinellid from China (Sanshadictya microreticulata Mehl & Reitner 1993), which also has an early Cambrian (Age 3) age. To date, the earliest convincing modern-type dictyonal skeletons are of middle Devonian age (Mehl, Reference Mehl1996).
5.b. Fontanarejo hexactinellid spicule record
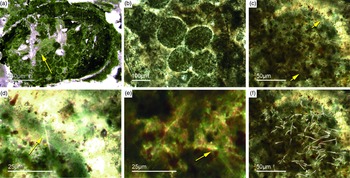
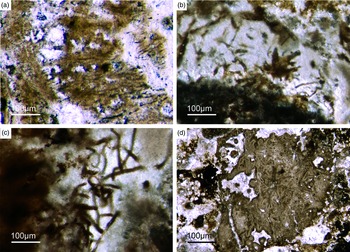
Most of the observed hexactinellid spicules are adaptions of simple hexactins (Fig. 2). The average size of the spicules is c. 500 µm. Many of these show a residual axial canal that originally contained a protein-rich organic fibre but now is often filled with iron oxide, likely resulting from the diagenetic alteration of primary framboidal pyrite. Some spicules are entirely replaced by iron oxide (Fig. 2 d, e, f, h). Silicious spicules tend to be completely recrystallized and are typically overgrown by the first generation of carbonate cement that was then diagenetically phosphatized. In addition to simple hexactins, differentiated triaxon spicules were observed, including (possibly dermal) pentactins (Figs 2d, 3d, 4d), stauractins (Figs 2f, h, 4b) and maybe diactins (Fig. 2h). Differentiation in mega- and microscleres, as seen in the ball-shaped hexactinellids of the Niutitang Formation basal phosphorites in China (Luo & Reitner, Reference Luo and Reitner2019), was not observed.
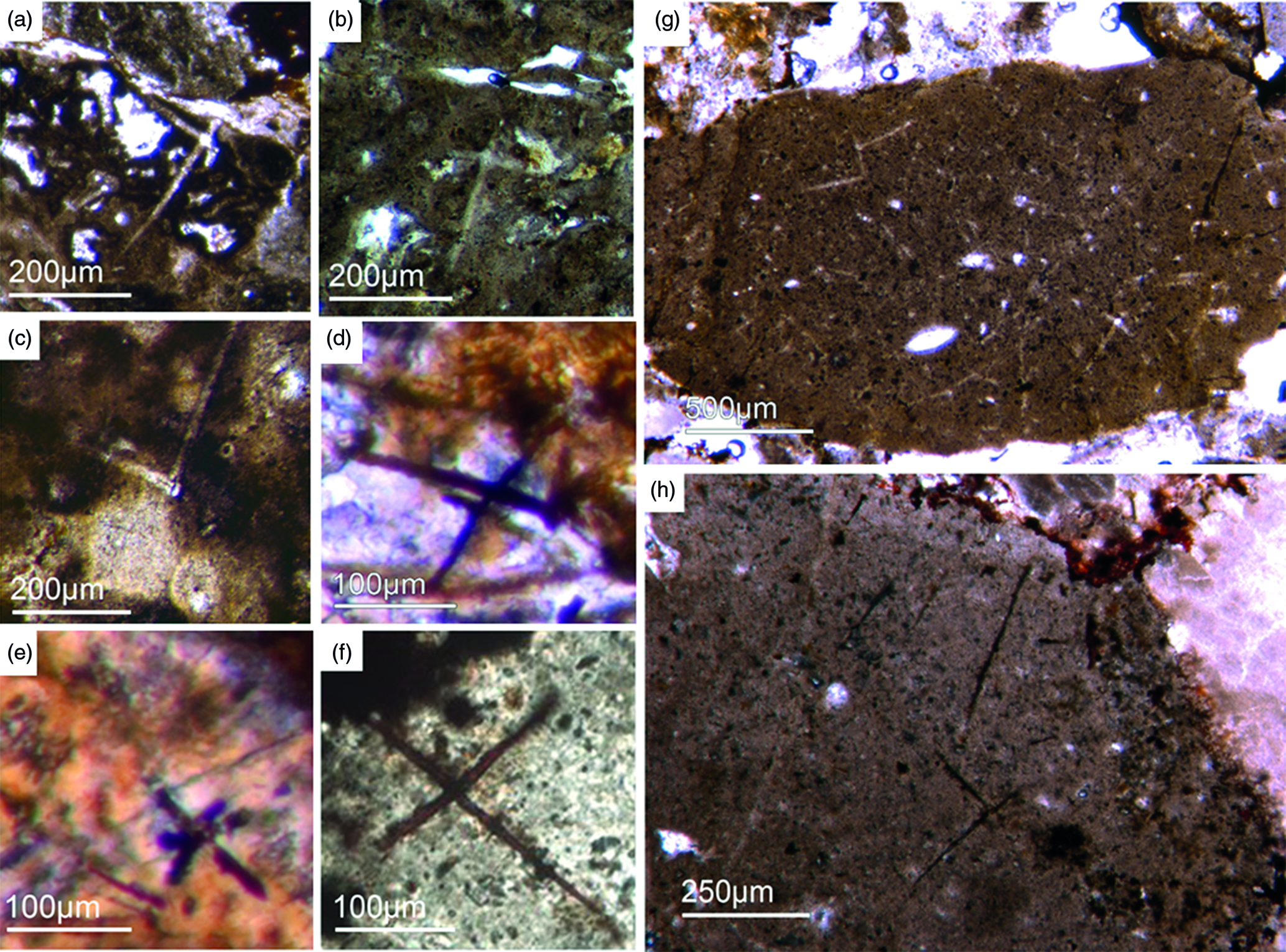
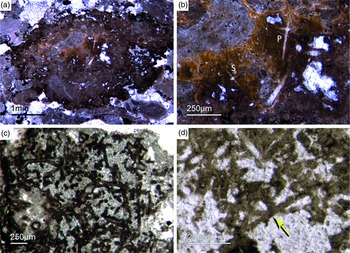
Fig. 2. Hexactinellid spicule types. (a, b) Oblique cuts of regular hexactins (Fon1, 26). (c) Hexatin with preserved axial canals (Fon36). (d) Pentactin and stauractin embedded in yellow-brownish phosphate, preserved in Fe-oxide that derived from pyrite (Fon27). (e) A regular hexactin (Fon29). (f) A stauractin preserved in Fe-oxide embedded in bright phosphatic material (Fon02). (g) Lyssacine hexactinellid skeleton with parenchymal hexactinellid spicules contained in fine-grained dark-brown phosphate (Fon27). (h) Monaxon and pentactin spicules preserved in Fe-oxide (formerly pyrite).
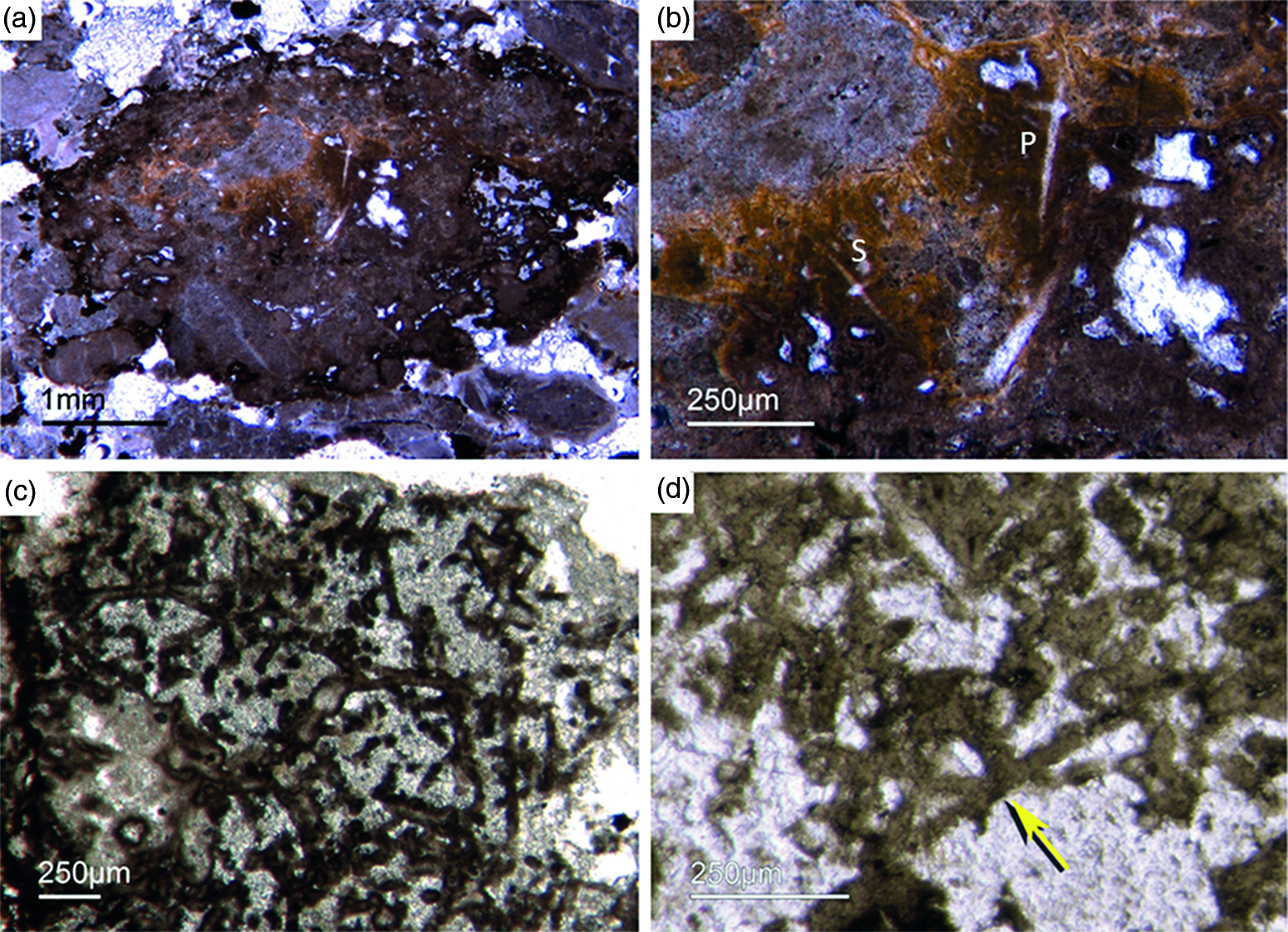
Fig. 3. (a) A lyssacine hexactinellid preserved in honey-yellow phosphate (Fon38). (b) Detail of Figure 6a exhibiting large pentactins (P) and stauractins (S). The honey-yellow phosphate shows in the upper margin a tent-like fabric, indicating that the early rapid phosphatization may have permineralized the soft tissue of the sponge. (c) Cemented parenchymal hexactinellid skeleton (Fon20). (d) Fused hexactinellid skeleton. Primarily articulated fused spicular skeleton, mainly preserved in brownish phosphate (Fon16). Space between spicules cemented by bright phosphate. Remains of dermal skeleton exhibiting fused pentactins (arrow).
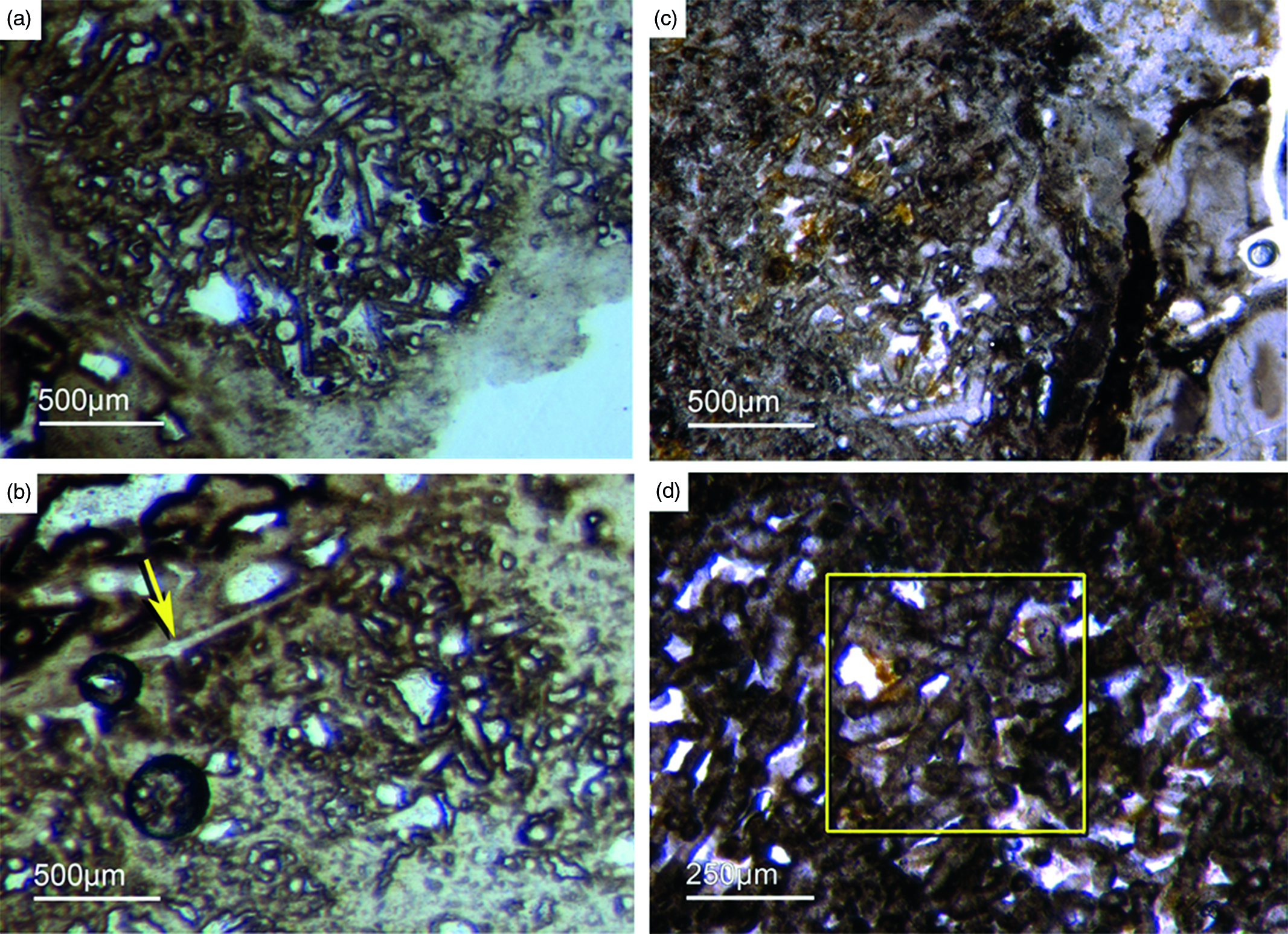
Fig. 4. Fused hexactinellid skeletons. (a, b) Nearly entirely preserved hexactinellid sponge with a cemented parenchymal and dermal spicular skeleton (Fon29). The marginal portions completely impregnated by honey-yellow phosphate and show ghost-remains of spicules and permineralized tissue. Preserved are large dermal spicules like stauractins (arrow in Fig. 7b). (c, d) Best preserved skeleton with large and small parchenchymal hexactin spicules and pentactin dermal spicules (rectangle) (Fig. 7d) (Fon26).
The lyssacine morphotype of nearly articulated hexactinellids displays only weakly developed regularities in spicule arrangement. In some cases, only pentactins and stauractins are located at the sponges’ marginal areas (‘Dermalia’ sensu Schulze, Reference Schulze1887). The basic hexactins (‘Principalia’ sensu Schulze, Reference Schulze1887) are randomly orientated and not attached to each other within the lyssacine sponge tissue (Fig. 3a, b).
This is in contrast to almost entirely preserved hexactinellids that have fused spicule arrangements (Figs 3c, d, 4). The choanosmal spicules in the latter seem to be fused as in cemented skeletons. However, they do not exhibit a rectangular architecture known from typical Dictyospongidae. It is possible to differentiate a few large (0.5 mm) fused hexactins from the abundant smaller ones (250 µm), with the latter being much more abundant (Fig.4a, b). The dermal area exhibits large pentactins (Fig. 4d) and possible tangentially arranged stauractins (Fig. 4b). The sponges are strongly impregnated with light brownish phosphate that likely replaced the primary sponge tissue. The spicules are often surrounded by dark, dense prismatic phosphate, and the inner parts are filled with a bright phosphatic cement (Fig. 4a, b). A second type of preservation displays a brownish phosphatic spicule replacement, though a few have bright phosphatic cements. We observed fused spicule arrangement in a few choanosomal spicules. It is not clear whether this type of preservation is related to rapid phosphatization or if the cementation was mediated by the sponge. If the latter is the case, then this would be the oldest appearance of an advanced rigid hexactinellid skeleton.
5.c. Phylogenetic status of Demospongiae (Sollas, Reference Sollas1885)
Demosponges and hexactinellids are phylogenetically closely related and form a monophylum (e.g. Wörheide et al. Reference Wörheide, Dohrmann, Erpenbeck, Larroux, Maldonado, Voigt, Borchiellini, Lavrov, Becerro, Uriz, Maldonado and Xavier2012). The elementary demosponge spicule types are monaxons and a four-rayed spicule called ‘caltrops’. The caltrop is an advanced characteristic demosponge spicule (van Kempen, Reference van Kempen and Rützler1990; Reitner & Mehl, Reference Reitner and Mehl1995, Reference Reitner and Mehl1996; Reitner & Wörheide, Reference Reitner, Wörheide, Hooper and Van Soest2002) (Fig. 5a, b, d). Hexactinellids also harbour monaxon spicules. In living sponges, it is possible to distinguish between hexactinellid and demosponge monaxons by the cross-section shape of the axial filament: it is more or less quadratic in hexactinellids, while it is triagular or hexagonal in demosponges (e.g. Hartman, Reference Hartman, Hartman, Wendt and Wiedenmayer1980; Reitner & Mehl, Reference Reitner and Mehl1996; Leys et al. Reference Leys, Mackie and Reiswig2007). In the fossil record, however, this structure is rarely preserved pristinely.
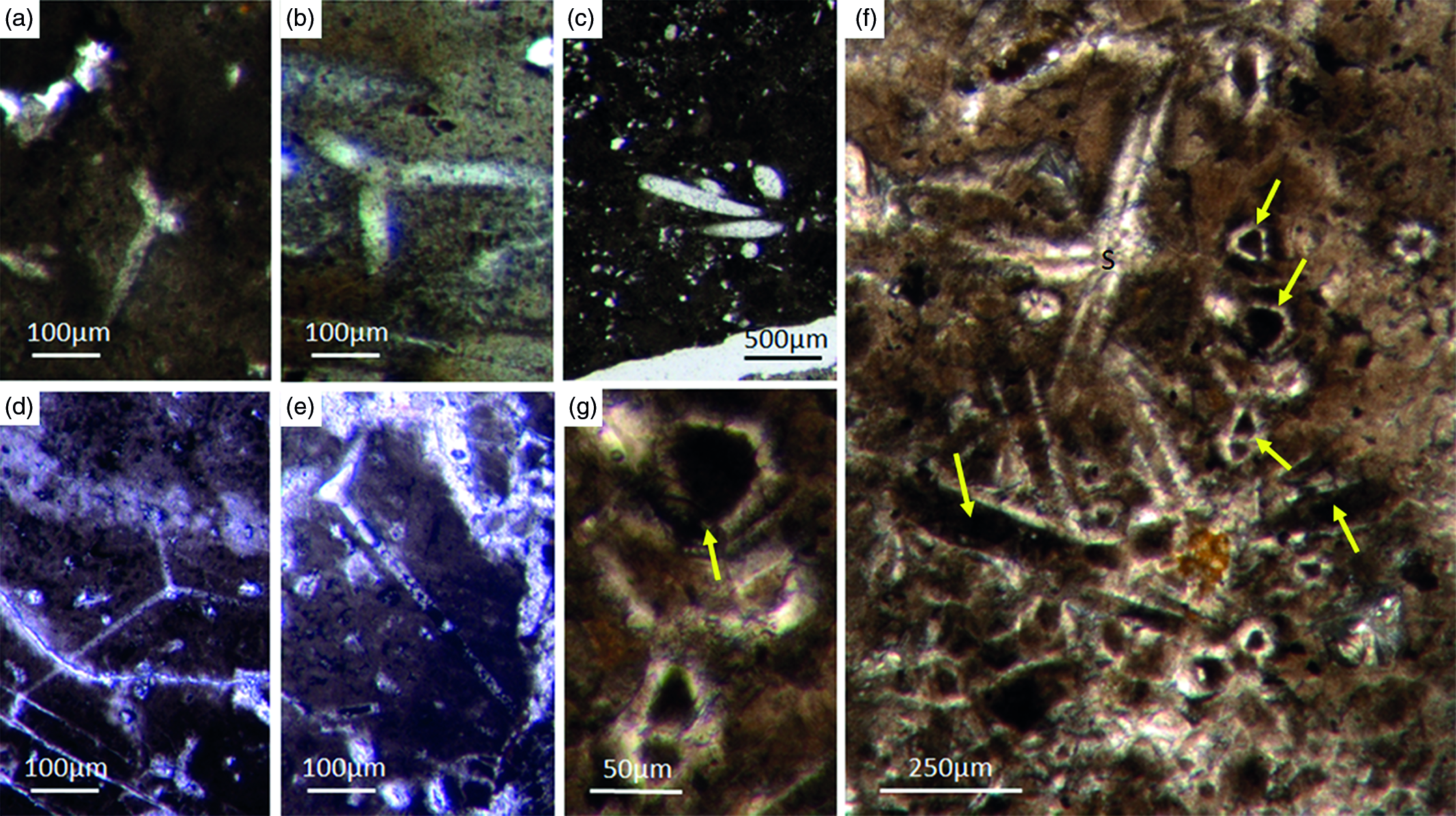
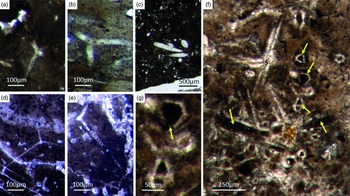
Fig. 5. Basic demosponge spicules. (a, b, d) Various types of four-rayed spicules with an angle of about 120° between the rays (Fon31, 59, 11). (c) Group of thick monaxon-style spicules (Fon11). (e) Typical triaene (orthotriaene) spicule (Fon11). (f, g) Aggregates of spicules (S) with possible remains of enlarged axial filament structure (arrows). Axial canal filled with organic matter (kerogen) and exhibiting hexagonal cross-sections as known from demosponges (Fon10).
Botting & Muir (Reference Botting and Muir2013) illustrated the axial canals of a reticulosan which, in their opinion show hexagonal cross-section. There are several problems with this interpretation. First, the depicted axial canals do not appear to be hexagonal but rather round in shape. Furthermore, the presented data are insufficient to support the conclusion. More specifically, the feature would need to be found in many more fossil sponge taxa to establish a phylogenetic significance. This is an essential prerequisite, since the claimed presence of hexagonal cross-sections in a single taxon could likewise be interpreted as an evolutionary experiment of the specific group. It is thus neither necessarily representative nor a plesiomorphic strait. For these reasons, we do not accept the premature claim that some reticulosans represent stem group demosponges (Botting & Muir Reference Botting and Muir2013, Reference Botting and Muir2018) and refrain from considering it in our discussion. Notably, demosponges are characterized by a great diversity of monaxonic spicule types that are distinctly different to hexactinellid monaxons.
Despite these morphological differences, both sponge clades form spicules by a fairly similar process. More precisely, amorphous hydrated opaline silica is precipitated on an organic matrix that forms a proteinaceous fibre (axial filament). However, the fibre’s proteinaceous composition in demosponges is formed of silicatein α, a cathepsin L-like lysosomal proteolytic enzyme (Shimizu et al. Reference Shimizu, Cha, Stucky and Morse1998). In hexactinellids, in contrast, the fibre usually consists of cathepsin instead of silicatein; in only one case a modified silicatein (AuSil-Hex protein in Aulosaccus schulzei) was detected (Veremeichik et al. Reference Veremeichik, Shkryl, Bulgakov, Shedko, Kozhemyako, Kovalchuk, Krasokhin, Zhuravlev and Kulchin2011). In hexactinellids, collagen is also an important spicule secondary protein (Uriz et al. Reference Uriz, Turon, Becerro and Agell2003; Müller et al. Reference Müller, Li, Schröder, Qiao and Wang2007; Ehrlich, Reference Ehrlich2010). This is in contrast to the sponge clade Calcarea which secrete calcitic spicules extracellularly and not around an organic fibre (Jones, Reference Jones and Fry1970). The capability to form spicules developed multiple times and in phylogenetically independent groups of sponges (Wörheide et al. Reference Wörheide, Dohrmann, Erpenbeck, Larroux, Maldonado, Voigt, Borchiellini, Lavrov, Becerro, Uriz, Maldonado and Xavier2012; Voigt et al. Reference Voigt, Adamska, Adamski, Kittelmann, Wencker and Wörheide2017).
Notably, various demosponges are non-spicular, and such forms were formerly described as ‘Keratosa,’ or ‘horny sponges’ (Minchin, Reference Minchin and Lankester1900; Lévi, Reference Lévi1957). Recent phylogenomic studies have separated the non-spicular taxa into the subclass ‘Keratosa’ (orders Dendroceratida and Dictyocertida) and the subclass Verongimorpha–Myxospongia (orders Chondrosiida and Verongiida) (Borchiellini et al. Reference Borchiellini, Chombard, Manuel, Alivon, Vacelet and Boury-Esnault2004; Erpenbeck et al. Reference Erpenbeck, Sutcliffe, Cook, Dietzel, Maldonado, van Soest, Hooper and Wörheide2012; Wörheide et al. Reference Wörheide, Dohrmann, Erpenbeck, Larroux, Maldonado, Voigt, Borchiellini, Lavrov, Becerro, Uriz, Maldonado and Xavier2012; Redmond et al. Reference Redmond, Morrow, Thacker, Diaz, Boury-Esnault, Cárdenas, Hajdu, Lôbo-Hajdu, Picton, Pomponi, Kayal and Collins2013; Morrow & Cárdenas, Reference Morrow and Cárdenas2015). The organic skeletons of these sponges are mainly composed of spongin, a composite of protein and/or chitin (Ehrlich et al. Reference Ehrlich, Bazhenov, Debitus, de Voogd, Galli, Tsurkan, Wysokowski, Meissner, Bulut, Kaya and Jesionowski2017). Spongin is still an enigmatic proteinaceous material that contains halogenated residues and until recently had not been sequenced (Ehrlich, Reference Ehrlich and Ehrlich2019). This composite material is extremly resistant, as demonstrated by findings of chitin in middle Cambrian vauxiid keratose sponges (Ehrlich et al. Reference Ehrlich, Rigby, Botting, Tsurkan, Werner, Schwille, Petrášek, Pisera, Simon, Sivkov, Vyalikh, Molodtsov, Kurek, Kammer, Hunoldt, Born, Stawski, Steinhof, Bazhenov and Geisler2013). Luo & Reitner (Reference Luo and Reitner2014) demonstrated, for the first time, that ‘keratose’ non-spicular demosponge fossils are abundantly preserved in Phanerozoic carbonates. They showed that the rapid micritization of the soft tissue and relatively slower decay of the spongin–chitin skeleton resulted in spar-filled moulds of the skeleton scaffolds.
5.d. Fontanarejo spicule record of basic Demospongiae
5.d.1. Non-articulated demosponge spicules
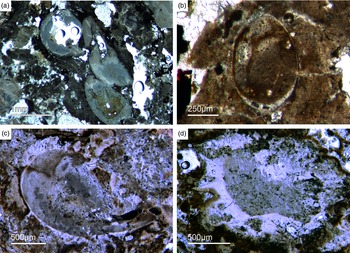
Different types of simple isolated four-rayed spicules were found within the Fontanarejo phosphorites (Fig. 5a, b, d). In a few cases, triaene dermal spicules (e.g. orthotrianes), which are likely modified caltrops (Fig. 5e), were recorded. Large, thick spicule styles (c. 0.5–1 mm long, 50–100 µm thick) are abundant and often arranged in bundles (Figs 5c, 6a–d). Most of the spicules are embedded in yellow-brownish phosphorite, and often held together by a dark rim of phosphatic cement (Fig. 6a, c, d). Some of the spicules exhibit a clear hexa- to triangular central part formed by organic matter. This part could be the remains of a spicule’s central fibre; however, the preservation is unusual (Fig. 5g, f) and not yet understood.
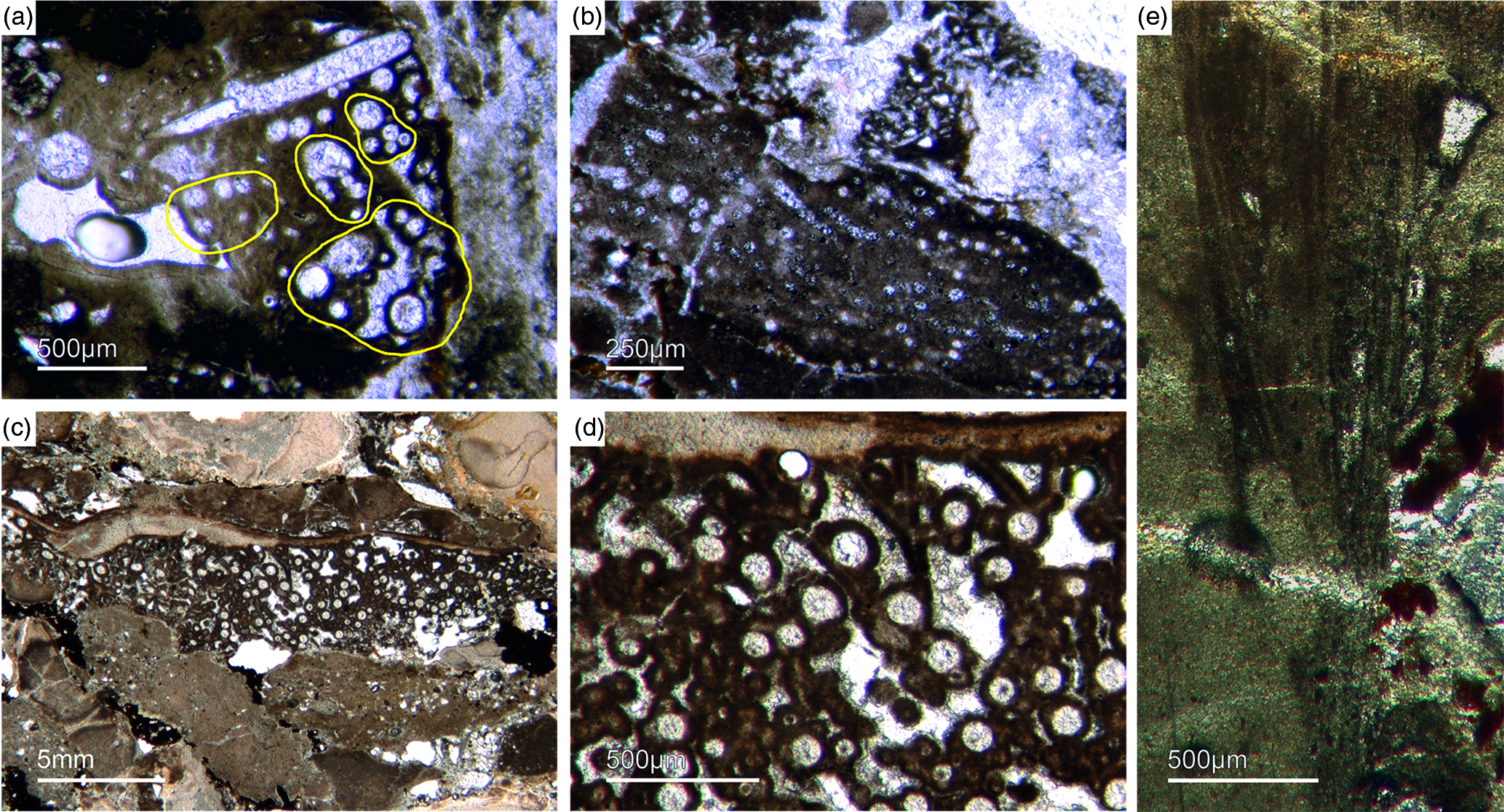
Fig. 6. Articulated demosponge spicular skeletons. (a) Plumose bundle of large styles (Fon1). This arrangement of spicules is known from the ‘axinellid–halichondrid’ group. (b) Cross-sections of styles that are mostly arranged in rows and probably part of the dermal skeleton (Fon1). In (a, b) the sponges are preserved in honey-yellow to brownish apatite, representing permineralized soft tissue. (c, d) Cross-sections of monaxon spicules grouped in bundles (Fon4). The individual spicules are glued by dark phosphatic cements. The arrangement of upright spicule bundles is known from early Cambrian sponges like Halichondrites and Hamptonia. (e) A typical hamptonid spicule bundle is formed by very long thin styles (Fon20, 19, 0007).
5.d.2. ‘Axinellid–halichondrid ’ spicule architectures
Some of the observed sponge specimens exhibit well-preserved spicule architecture (Figs 6a, b, c, d, 7c, d). For instance Figure 6a shows an association of thick and small styles with spicule bundles. The plumose arrangement of some of the spicule bundles resembles an ancient ‘axinellid’ bauplan (e.g. Reitner, Reference Reitner1992; Reitner & Wörheide, Reference Reitner, Wörheide, Hooper and Van Soest2002). Figure 6b, c and d show cross-cuts through a demosponge dermal layer comprising vertically orientated monaxons. The monaxons are cemented by fibrous brown apatite (Fig.6c, d). In some of the spicules the axial canals are still visible as small dark spots (Fig. 6b, d). Figure 7c and d show a demosponge with plumose bundles of long and short styles in the outer dermal parts and tangentially orientated monaxons in the inner dermal parts (arrow in Fig. 7d). Figures 6e and 7c show further examples of plumose spicule arrangements. The long, thin styles are nearly radially arranged, a structure known from lower Cambrian taxa such as Choia, Choiaella, Hamptoniella, Hamptonia, Halichondrites and Pirania (Reitner & Wörheide, Reference Reitner, Wörheide, Hooper and Van Soest2002; Finks & Rigby, Reference Finks, Rigby, Finks, Reid and Rigby2004; Rigby & Collins, Reference Rigby and Collins2004; Botting et al. Reference Botting, Muir and Lin2013). In some cases, bouquet-like arrangements of long styles (some millimetres) are observed close to a plumose arrangement of spicules (Fig. 7d). These bouquet types are present in ball-shaped modern taxa of Tethyidae and closely related to Hemiastrellidae (Hooper, Reference Hooper, Hooper and Van Soest2002; Sará, Reference Sará, Hooper and Van Soest2002; Sorokin et al. Reference Sorokin, Ekins, Yang and Cárdenas2019). A particularly remarkable demosponge specimen exhibits a well-developed, very dense etcosomal spicule architecture composed of short strongyles (c. 50 µm) (Fig. 8). The strongyles are interwoven and display a preferred tangential orientation (Fig. 8b, e). The endosomal spicules are large, thick styles (c. 200–300 µm long, 50 µm diameter) surrounded by long (200–400 µm), thinner styles (Fig. 8a, c, d). Some accessory curved monaxons are also present. This morphological character is similar to an ‘axinellid–halichondrid’ sponge type, but the ectosomal dermal layer is unusual.

Fig. 7. Articulated demosponge spicular skeletons. (a, b) Representing a ‘hadromerid’ skeleton. The sponge shows a dense crust of putative asterose dermal microsclers (adm) and randomly orientated choanosomal, probably monaxon spicules (cms) (Fon 38). (c) Radially orientated long styles typical for various early Cambrian sponges like Choia, Choiaella, Hamptoniella (Fon20). (d) Plumose arrangement of short dermal styles (ds) and tangentially arranged monaxons (arrow) on the outer part of the skeleton (Fon20). (e, f) The fossil represents asterose microsclers characteristic for chondrillid hadromerids ((f) enlarged area of (e)) (Fon17).

Fig. 8. Almost entirely preserved sponge exhibiting a dense dermal layer (ECT – ectosomal spicules). (a) Plumose arranged thick large styles (yellow lines) within the choanosomal part of the sponge (CHO) and (c, d) smaller styles. (b, e) The dense dermal layer exhibits a network of interwoven short strongyles (ECT) (Fon41).
5.d.3. ‘Hadromerid’ spicule arrangements
In addition to these spicule arrangements, we observed demosponges with a thick dermal layer of small ball-shaped microscleres (c. 10–15 µm), probably former asterose spicule types (Fig. 7a, b). The choanosomal part of these sponges exhibits randomly orientated, poorly preserved monaxone spicules, probably styles (Fig. 7a). This bauplan is known from ‘tetractinellid’ demosponges. A ‘hadromerid’ affinity is most probable. In one case, only a dense dermal crust of putative asterose microscleres is observed (Fig. 7e, f). This sponge resembles the modern hadromerid Chondrillidae and the Cretaceous fossil taxon Calcichondrilla crustans Reitner Reference Reitner, Reitner and Keupp1991, which show similar dermal asterose microsclers (e.g. Reitner, Reference Reitner, Reitner and Keupp1991; Boury-Esnault, Reference Boury-Esnault, Hooper and Van Soest2002; Fromont et al. Reference Fromont, Usher, Sutton, Toze and Kuo2008).
5.d.4. Putative ‘keratose’ demosponges
Non-spicular demosponges are a sister group of spicular demosponges (e.g. Maldonado, Reference Maldonado2009; Erpenbeck et al. Reference Erpenbeck, Sutcliffe, Cook, Dietzel, Maldonado, van Soest, Hooper and Wörheide2012; Morrow & Cárdenas, Reference Morrow and Cárdenas2015). Recent investigations demonstrated a widespread fossil record of this group, which is due to the decay-resistant skeletal spongin and chitinous fibres (e.g. Luo & Reitner, Reference Luo and Reitner2014, Reference Luo and Reitner2016; Friesenbichler et al. Reference Friesenbichler, Richoz, Baud, Krystyn, Sahakyan, Vardanyan, Peckmann, Reitner and Heindel2018). The best-known fossil taxon is the middle Cambrian Vauxia from the Burgess Shale, which has recently also been discovered in the lower Cambrian Chengjiang Biota (Series 2, Stage 3) (Luo et al. Reference Luo, Zhao and Zeng2020). The presence of chitin in this genus is in good accordance with the skeletal composition of the Verongiida (subclass Verongimorpha) (Ehrlich et al. Reference Ehrlich, Rigby, Botting, Tsurkan, Werner, Schwille, Petrášek, Pisera, Simon, Sivkov, Vyalikh, Molodtsov, Kurek, Kammer, Hunoldt, Born, Stawski, Steinhof, Bazhenov and Geisler2013).
Some components in the Fontanarejo phosphorites may be fossils of keratose sponges – (Fig. 9). This is because they exhibit an irregular network of fibres that is remarkably similar to those known from younger Phanerozoic keratose fibre networks (Fig. 9a, b, c). Most of the fibre networks are embedded in dark-brown phosphate matrices. They often form connecting points, and in some cases the fibres are curved. The fibres are preserved as a bright phosphatic cement. Micro-peloids, a characteristic taphonomic end product of sponge tissue alteration (Reitner et al. Reference Reitner, Neuweiler, Gautret, Reitner and Neuweiler1995; Reitner & Neuweiler, Reference Reitner and Neuweiler1995, Reitner & Schumann-Kindel, Reference Reitner and Schumann-Kindel1997), fill the spaces between the fibres (Fig. 9d). All these features are typical characteristics of keratose sponges and are interpreted here accordingly. An alternative interpretation of these structures as boring traces is not favoured here but might be tested in future studies.

Fig. 9. (a) A dark-brownish phosphorite component with a clear fibre network (Fon19). (b) Network of thin filamentous fabrics within a dark-brownish phosphorite, remarkably similar to typical ‘keratose’ organic fibre skeletons (Fon77). (c) Within a brownish phosphatic clast thin filamentous fabrics are seen, interpreted as remains of spongin fibres – alternatively, boring traces may also be possible (Fon56). (d) Dark micropeloids enriched in phosphate and organic matter, likely the taphonomic end-product of microbial sponge tissue degradation (Fon16).
5.d.5. Putative sponge larvae or resting bodies
Bundles of long styles with egg-shaped stuctures are observed within a few sponge ‘balls’ (Fig. 10). These egg-shaped structures have a diameter of c. 100–200 µm and resemble viviparous sponge larvae or gemmules. They have a distinct c. 10 µm thick bright rim that consists of radially orientated phosphatic crystals, a dark inner granular matrix, and potentially small monaxon spicule remains. The dark inner granular matrix consists of abundant iron oxides (probably remains of pyrite), and dark-grey, irregular phosphatic organic matter. The observed potential spicules are c. 20–50 µm long.
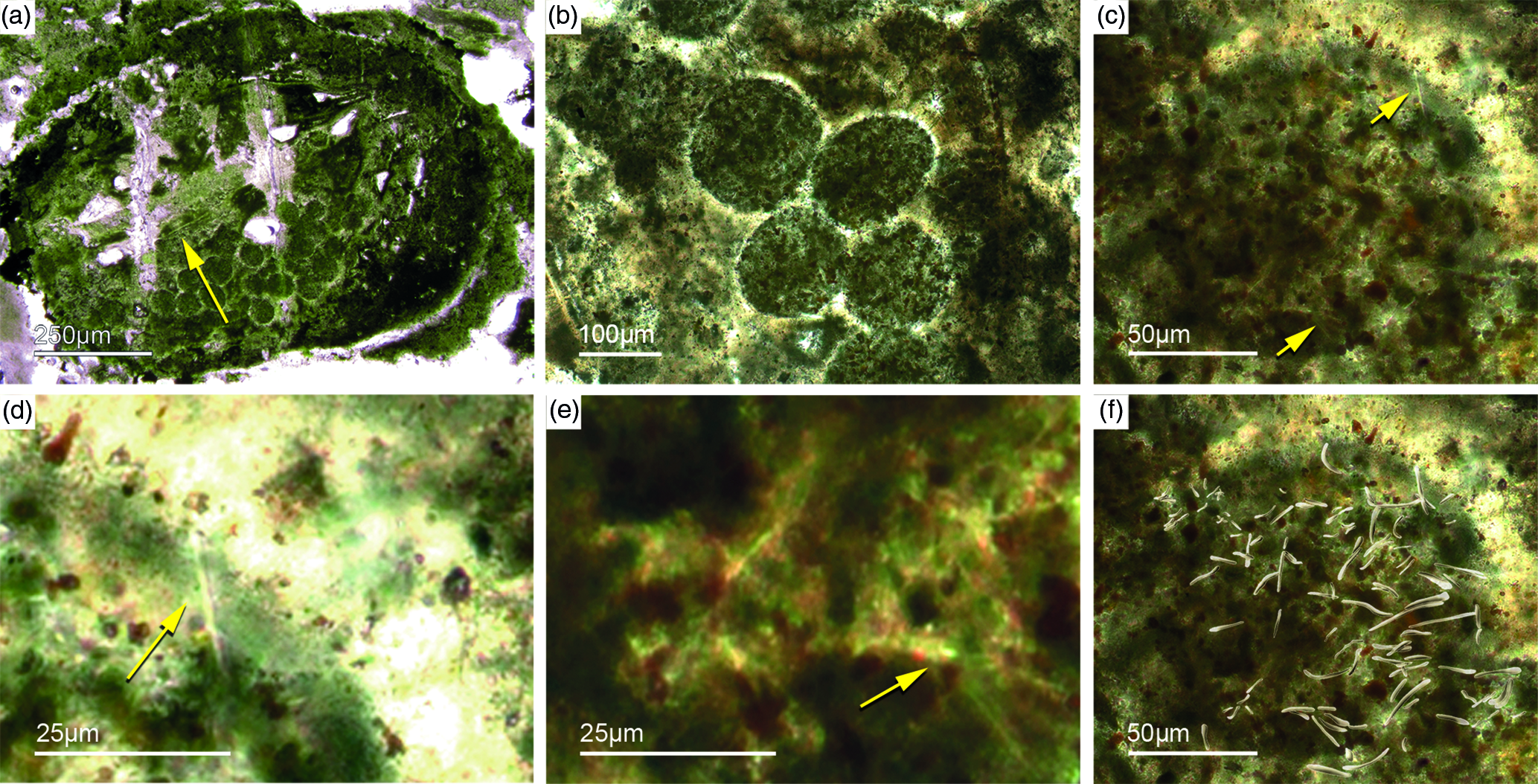
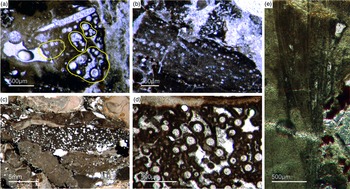
Fig. 10. (a, b) Demosponge with a central bundle of styles associated with abundant egg-shaped structures (Fon 27). (c, e) These egg-shaped bodies show a distinct margin (c) and a central area with abundant small Fe-rich small grains (e). A further important characteristic is abundant small spicules (styles, oxea) (yellow arrows in (c)). (f) Sketch of recognized spicules. The egg-shaped bodies are interpreted as larvae or resting cysts. These bodies show best analogy with demosponge parenchymella larvae.
A possible (and in our view most suitable) analogue is the parenchymella larva of viviparous demosponges (e.g. known from haplosclerids, poecilosclerids and ‘Keratosa’), the only larva type with simple monaxonic spicules (Leys, Reference Leys2003; Maldonado, Reference Maldonado2006; Ereskovsky, Reference Ereskovsky2010). To the best of our knowledge, fossil vivaparous sponge larvae (e.g. parenchymella-type) have not been observed until this study.
Alternatively, the observed structures could be resting bodies like those of gemmulae. Gemmulae structures are very rarerly preserved in the fossil record (Volkmer-Ribeiro & Reitner, Reference Volkmer-Ribeiro, Reitner, Reitner and Keupp1991; Pronzato et al. Reference Pronzato, Pisera and Manconi2017). Today, marine sponges with gemmulae are also rare and restricted to a few taxa of the Haplosclerida (Acervochalina loosanoffi, family Chalinidae) and Hadromerida (Protosuberites sp.) (Connes et al. Reference Connes, Carriére and Paris1978; Arp et al. Reference Arp, Reimer and Reitner2004).
5.e. Putative small shelly fauna
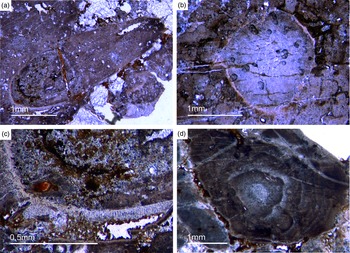
As already discussed, determining the age of the Fontanarejo phosphorites is a central problem. No stratigraphically important fossils have been previously found within the phosphorite-containing layers, and so the existing age was determined based on sedimentological correlation with nearby geological structures (Pieren Pidal, Reference Pieren Pidal2000; Jensen et al. Reference Jensen, Palacios and Mus2010; Álvaro et al. Reference Álvaro, Cortijo, Jensen, Lorenzo, Palacios and Pieren2019) and one zircon age published by Talavera et al. (Reference Talavera, Montero, Martínez Poyatos and Williams2012). Sponge spicules in the phosporites indicate a Cambrian age, but unfortunately do not allow for a more precise biostratigraphic interpretation. From the Alcudia anticline, the early mollusc Anabarella was described, providing an important stratigraphic age (Pieren & García-Hidalgo, Reference Pieren and García-Hidalgo1999; Vidal et al. Reference Vidal, Palacios, Moczydłowska and Gubanov1999; Pieren Pidal, Reference Pieren Pidal2000). For the first time, we also found SSF remains in the Fontanarejo phosphorites. Unfortunately, these microfossils could not be isolated due to the high amounts of siliceous cement, and therefore have only been examined in thin-sections. However, many specimens observed in the thin-sections show strong similarities to Anabarella (Figs 11, 13a), while others classify as tube fossils (Fig. 12a, b). These new micropalaeontological findings match with previous findings from the Alcudia anticline near Ciudad Real (e.g. Pieren Pidal, Reference Pieren Pidal2000), and thus provide compelling evidence for a similar stratigraphic age. Furthermore, they are also in agreement with findings of SSFs (including Anabarella) that have recently been reported from the Ibor and Alcudia anticlines of the CIZ (Álvaro & Lorenzo, Reference Álvaro and Lorenzo2021).
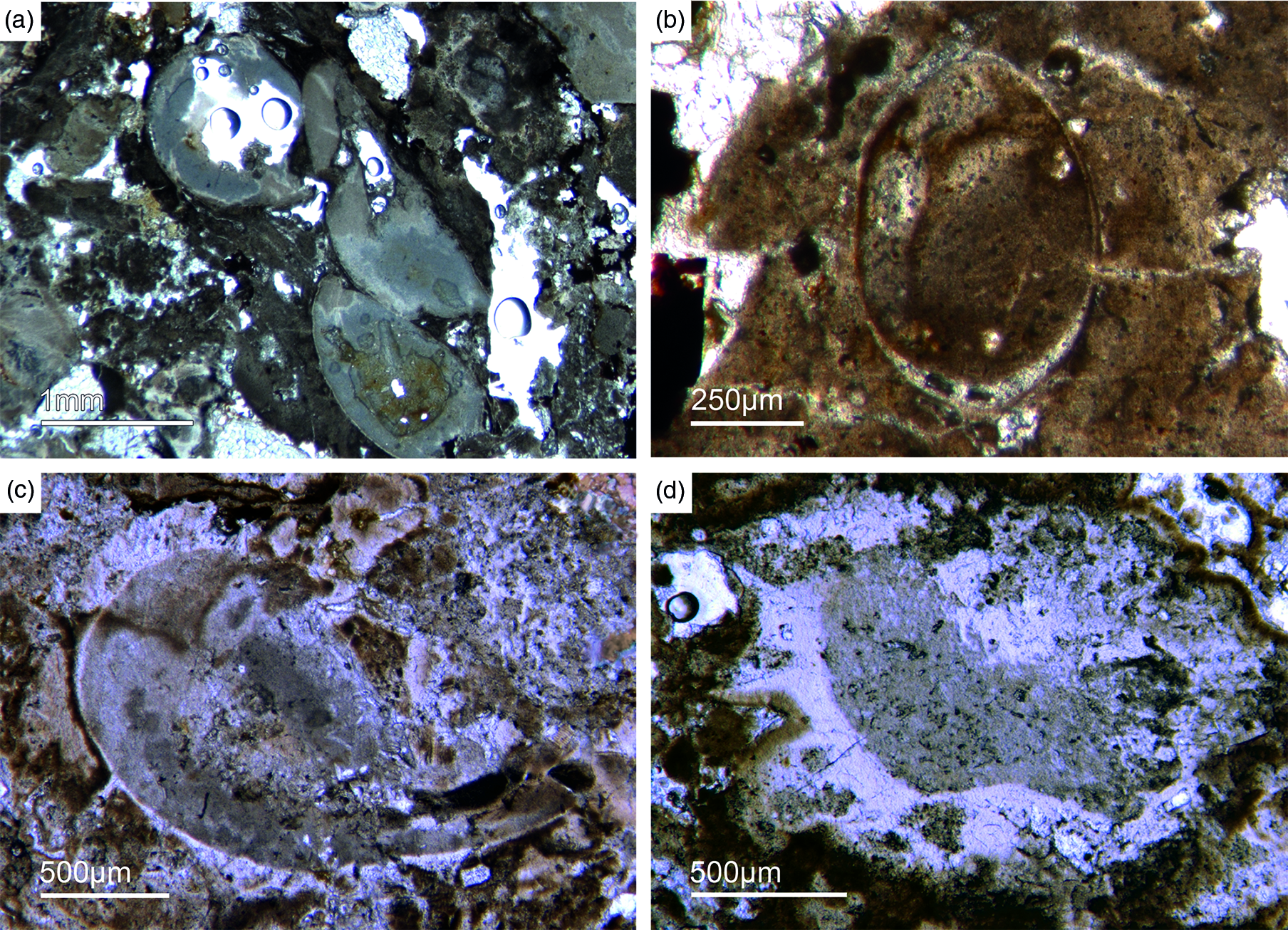
Fig. 11. Mollusc remains. (a, c) Axial and oblique cuts of Anabarella sp., a putative monoplacophoran mollusc (Fon61, 35). (b) Vertical cut of Anabarella sp. (Fon4), (d) Oblique cut of an ornamented Anabarella type (Fon35).
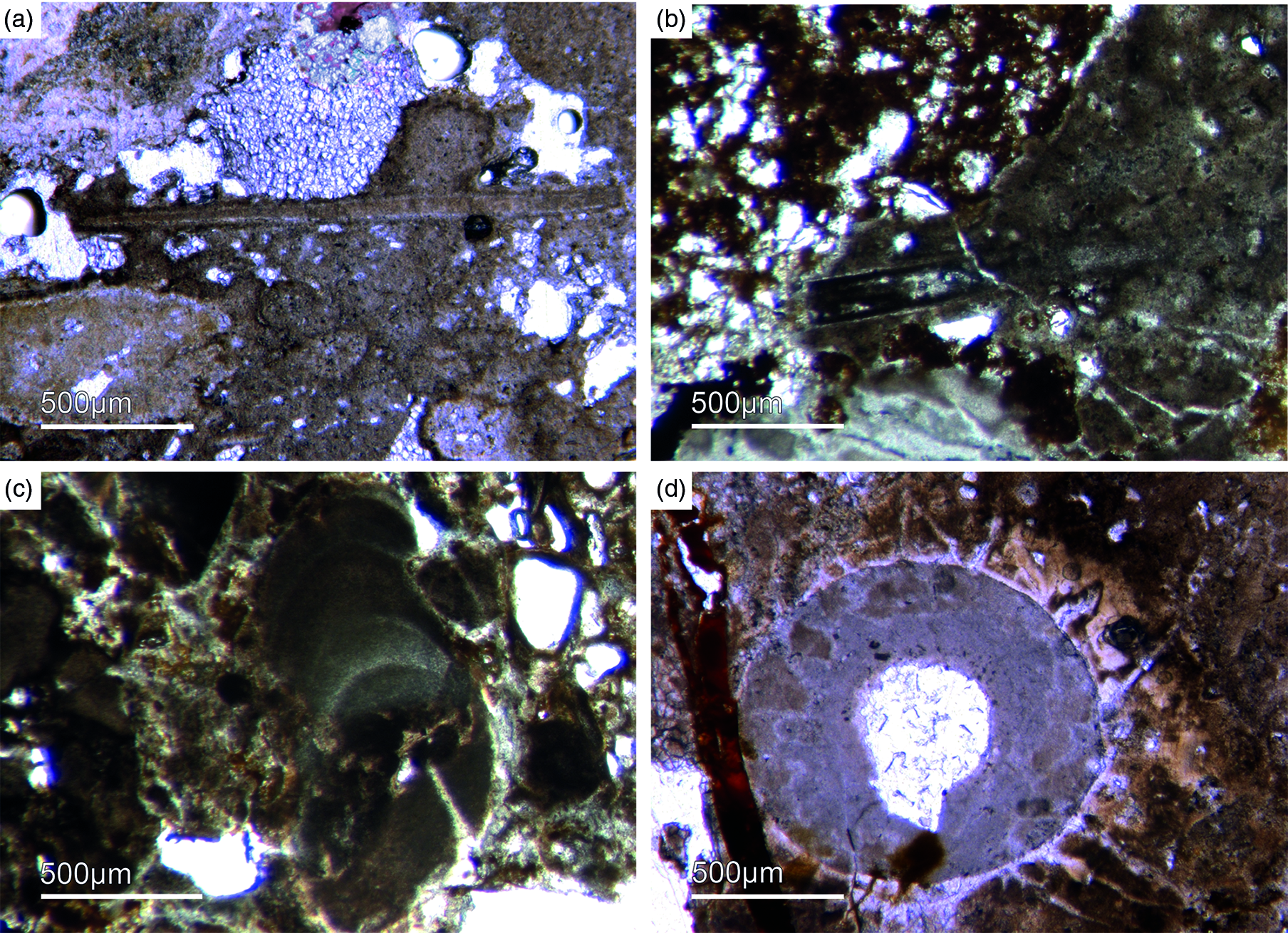
Fig. 12. Multiple types of skeletal fossils. (a, b) Long thin tube shell (Fon99, Fon44, respectively). (c) A new form of a chambered SSF (Fon31). (d) A round cross-section of a tube fossil (Fon78).

Fig. 13. (a) A median-oblique cut of Anabarella sp. (b) A vertical cut through an ornamented SSF. (c) Detail of the prismatic phosphatic shell structure of Figure 16b (Fon4). (d) Median-oblique cut of a cloudy-colloform microbialite (Fon92).
One SSF exhibits a chambered structure comparable to phragmocones of cephalopods (Fig. 12c). Up to now the only known SSF with a phragmocone-like structure is the upper Cambrian monoplacophoran Knightoconus from Antarctica (Yochelson et al. Reference Yochelson, Flower and Webers1973). However, there is a new finding of a cephalopod-type SSF from the early Cambrian (upper Terreneuvian) of Newfoundland (Hildenbrand et al. Reference Hildenbrand, Austermann, Fuchs, Bengston and Stinnesbeck2021). Together, these fossils indicate an early Cambrian origin of chambered cephalopods. On the other hand, the SFF somewhat resembles the chambered tube of ‘Hyolithes kingi’ from the Lower Cambrian of Jordan by Bandel (Reference Bandel1986). Another important group of SSFs in the studied materials are tubular remains of mollusc-type organisms. In thin-section we observe simple narrow shafts some millimetres long and 50–100 µm in diameter (Fig. 12a, b). Commonly, we observe cross-sections of putative tubular fossils with a maximum diameter of a few millimetres, often exhibiting a weak outer ornamentation. The shell structure is often prismatic (Fig. 13 b, c).
5.f. Implications of the Fontanarejo record on early metazoan evolution
Molecular clock analyses suggest that sponges evolved in the Neoproterozoic. However, no convincing body fossils or spicules have been reported from that era. For this reason, molecular clocks are usually calibrated with sedimentary hydrocarbons in Cryogenian and Ediacaran rocks and oils that are widely considered as being indicative for sponges (i.e. 24-isopropylcholestanes, 24-icp) (Sperling et al. Reference Sperling, Robinson, Pisani and Peterson2010; Erwin et al. Reference Erwin, Laflamme, Tweedt, Sperling, Pisani and Peterson2011; Cunningham et al. Reference Cunningham, Liu, Bengtson and Donoghue2016; Gold et al. Reference Gold, Grabenstatter, Mendoza, Riesgo, Ruiz-Trillo and Summons2016; Dohrmann & Wörheide, Reference Dohrmann and Wörheide2017). A recently reported steroid biomarker (26-methylstigmastane, 26-mes) might provide additional evidence for early sponges (Zumberge et al. Reference Zumberge, Love, Cárdenas, Sperling, Gunasekera, Rohrssen, Grosjean, Grotzinger and Summons2018). This is because only certain groups of extant demosponges are known to biosynthesize plausible precursor sterols for both of these distinct compounds as major lipids (e.g. Hofheinz & Oesterhelt, Reference Hofheinz and Oesterhelt1979; Djerassi & Silva, Reference Djerassi and Silva1991; Giner, Reference Giner1993; McCaffrey et al. Reference McCaffrey, Moldowan, Lipton, Summons, Peters, Jeganathan and Watt1994; Love et al. Reference Love, Grosjean, Stalvies, Fike, Grotzinger, Bradley, Kelly, Bhatia, Meredith, Snape, Bowring, Condon and Summons2009; Zumberge et al. Reference Zumberge, Love, Cárdenas, Sperling, Gunasekera, Rohrssen, Grosjean, Grotzinger and Summons2018). However, 24-icp precursors are also known as insignificant (that is, two orders of magnitude less abundant) by-products in the biosynthesis of 24-n-propylcholestane lipids by extant pelagophyte algae (Giner et al. Reference Giner, Zhao, Boyer, Satchwell and Andersen2009; Love & Summons, Reference Love and Summons2015).
Traces of putative 24-ipc and 26-mes precursor in protists (Nettersheim et al. Reference Nettersheim, Brocks, Schwelm, Hope, Not, Lomas, Schmidt, Schiebel, Nowack, De Deckker, Pawlowski, Bowser, Bobrovskiy, Zonneveld, Kucera, Stuhr and Hallmann2019) have shortly afterwards been demonstrated to be artefacts formed during closed-system pyrolysis under catalytic conditions (i.e. >300 °C: e.g. Bobrovskiy et al. Reference Bobrovskiy, Hope, Nettersheim, Volkman, Hallmann and Brocks2021; van Maldegem et al. Reference van Maldegem, Nettersheim, Leider, Brocks, Adam, Schaeffer and Hallmann2021). These high-temperature experiments do not simulate diagenesis accurately and produced traces of 24-ipc and 26-mes along with a complex array of other organic compounds, which is distinctly different to the very selective abundance pattern of C30 steranes in the Neoproterozoic–Cambrian rock record. Nevertheless, it might be possible, that low quantities of 24-ipc and 26-mes form diagenetically through secondary alkylation of C29 sterols in sedimentary environments (Duda et al. Reference Duda, Love, Rogov, Melnik, Blumenberg and Grazhdankin2020). What is important to realize, however, is that all these studies do not provide evidence that modern demosponges are incapable of synthesizing suitable C30 steroid precursors. Consequently, they cannot invalidate demosponges as a potential source of C30 steranes in Precambrian rocks; at best they might lower the level of diagnosticity of 24-ipc and 26-mes by establishing additional sources. In summary, the selective abundance pattern of C30 steranes is an established characteristic of the late Neoproterozoic–Cambrian rock record, but the interpretation of this feature is controversial and requires further investigation.
Regardless of this debate, our finding of taxonomically diverse demosponge and hexactinellid communities in the late Fortunian Fontanarejo phosphorite further corroborates a Precambrian origin of the phylum Porifera. Notably, the organismic processes involved in biomineralization are enormously complex. Furthermore, there are various extant groups of sponges that seem to belong to the non-spicular lineages and obviously lack a distinct body plan. For all these reasons, it appears likely that the most ancestral representant of the sponge phylum (or ‘Urschwamm’) solely consisted of soft tissue, perhaps somewhat resembling a microbial mat (Luo & Reitner, Reference Luo and Reitner2016). This would be in good agreement with the idea that microbial mats may have transitionally evolved into sponges, as hypothesized earlier (Reitner, Reference Reitner1998). And such a scenario would explain the seeming mismatch between palaeontological findings, the sedimentary hydrocarbon record and molecular clock data.
6. Results and discussion: Sedimentary evidence for microbial-mediated phosphate formation
The abundant cloudy textures observed in the Fontanarejo phosphorite strongly suggest a microbial origin of the deposit, as proposed earlier by Álvaro et al. (Reference Álvaro, Shields-Zhou, Ahlberg, Jensen and Palacios2016). The microbial impact on sediment formation is further evidenced by a variety of components such as mm-sized aggregates of phosphatic material that exhibit irregular to cauliflower-like shapes and often contain small allochthonous grains as for instance siliciclastic components and spicules. Notably, these components represent up to 20 % of the entire phosphatic sediment. A further example is abundant oncoid-like components with roughly laminated colloform and thrombolitic structures and a distinct nucleus (Figs 13d, 14). Raman spectroscopy revealed the presence of pure and organic-rich apatite, organic matter, as well as of accessory minerals such as anatase (TiO2), and quartz in these components (Figs S4, S5). The laminated to thrombolitic structures and the close spatial association of organic–inorganic phases indicate that apatite formation was probably mediated via microbial exopolymeric substances (EPS) and/or fuelled by phosphorus deriving from bacterial cell degradation, perhaps in biofilms (see below).
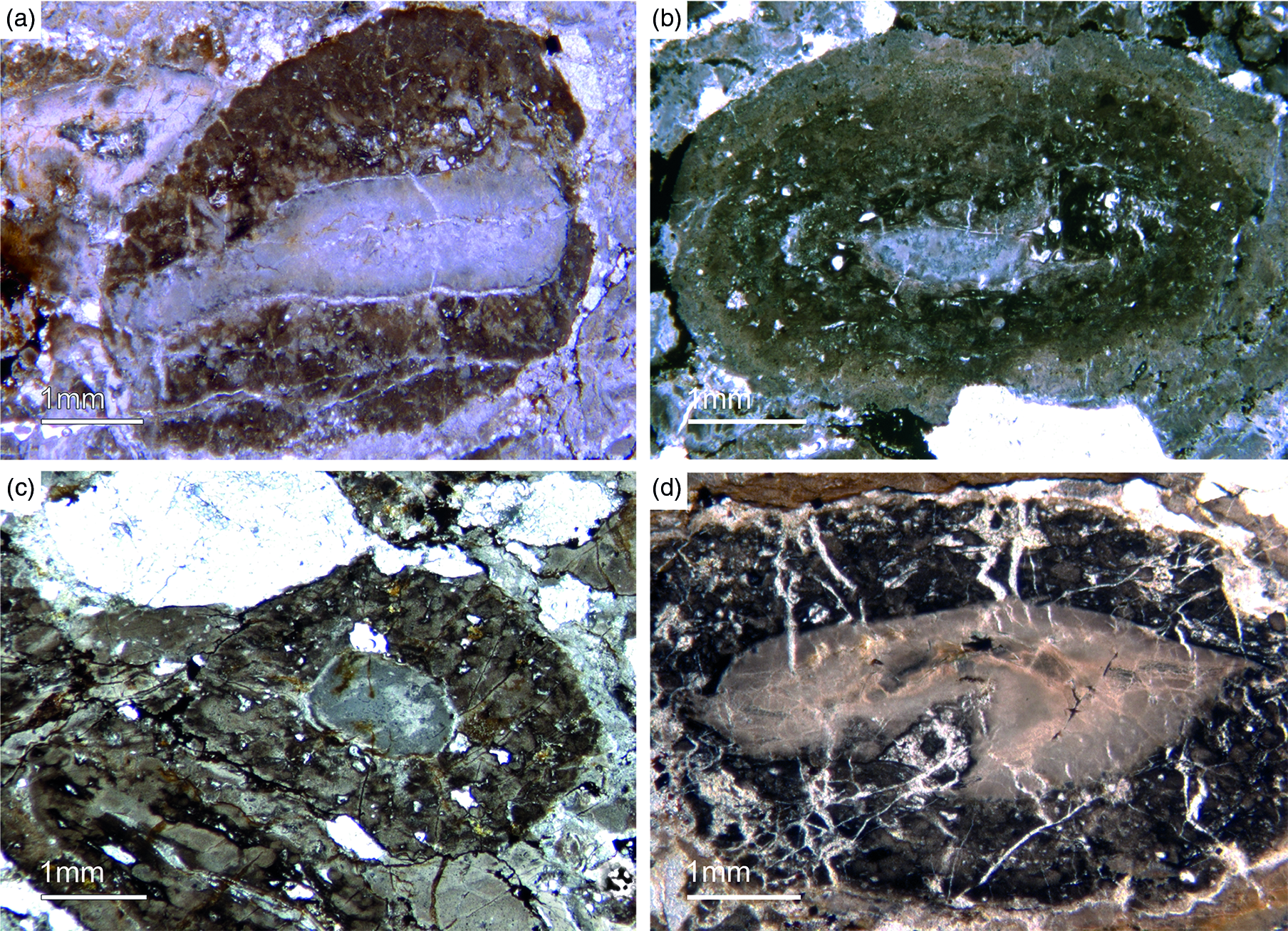
Fig. 14. Oncoid-type microbialites. (a) Encrusting phosphatic microbialite showing an irregular cauliflower growth mode (Fon18). (b) Thrombolitic growth structure on top of phosphatic component (Fon35). (c) Thrombolitic oncoid-type microbialite (Fon44). (d) Dark organic-rich thrombolitic oncoid-type microbialite (Fon21).
A second type of microbialite is mm-sized micro-domal stromatolites. Generally, the structure of these stromatolites is similar to the colloform structure of the phosphatic oncoid-like components described above. However, the stromatolites exhibit a distinct lamination of alternating, c. 50 µm thin dark-brownish, brownish and white layers (Supplementary Fig. S5, in the Supplementary Material available online at https://doi.org/10.1017/S001675682100087X). The dark-brown and brown layers consist of organic-rich and organic-poor apatite, respectively. The white layers, in contrast, are composed of siliceous apatite or pure silica. As in the case of the oncoid-like components (see above), the organic-rich apatite in the stromatolites is likely a product of EPS-controlled mineral precipitation or taphonomic biofilm degradation. Anatase (TiO2) is enriched in some layers and may have formed authigenically, perhaps even through microbially mediated precipitation; however, unfortunately these processes are still too poorly understood to draw robust conclusions (e.g. Taran et al. Reference Taran, Rad and Alavi2018; Izawa et al. Reference Izawa, Banerjee, Shervais, Flemming, Hetherington, Muehlenbachs, Schultz, Das and Hanan2019).
The presence of microbes in the Fontanarejo phosphorite environment is further evidenced by large filamentous fossils encapsulated in some of the observed microbialites (Fig. 15a–c). These fossils strongly resemble sheets of extant sulphide-oxidizing bacteria (SOX-B) (Peckmann et al. Reference Peckmann, Thiel, Reitner, Taviani, Aharon and Michaelis2004; Bailey et al. Reference Bailey, Corsetti, Greene, Crosby, Liu and Orphan2013; Birgel et al. Reference Birgel, Guido, Liu, Hinrichs, Gier and Peckmann2014; Andreetto et al. Reference Andreetto, Dela Pierre, Gibert, Natalicchio and Ferrando2019). This interpretation has important implications for the formation of the Fontanarejo phosphorite deposit. Today, SOX-B are particularly widespread in sediments of coastal upwelling regimes off the west coast of Africa and South America (Arning et al. Reference Arning, Birgel, Brunner and Peckmann2009 a, b). Notably, vacuolated SOX-B like the giant bacterium Thiomargarita (Schulz & Schulz, Reference Schulz and Schulz2005) as well as filamentous SOX-B like Beggiatoa and Thioploca all harbour intracellular polyphosphate inclusions (e.g. Jørgensen & Gallardo, Reference Jørgensen and Gallardo1999; Brock & Schulz-Vogt, Reference Brock and Schulz-Vogt2011; Salman et al. Reference Salman, Amann, Girnth, Polerecky, Bailey, Høgslund, Jessen, Pantoja and Schulz-Vogt2011; Crosby & Bailey, Reference Crosby and Bailey2012). It has been shown that hydrolysis of polyphosphates in Thiomargarita-rich sediments releases sufficient phosphor into the pore water to precipitate apatite (Schulz & Schulz, Reference Schulz and Schulz2005). Moreover, it has been demonstrated by 33P-labelling experiments that phosphate taken up by SOX-B is rapidly transformed from intracellular polyphosphate to apatite (Goldhammer et al. Reference Goldhammer, Brüchert, Ferdelman and Zabel2010). All these studies support the hypothesis that microbial metabolism can foster apatite formation, and we consider this as a plausible explanation for the formation of the Fontanarejo phosphorite.
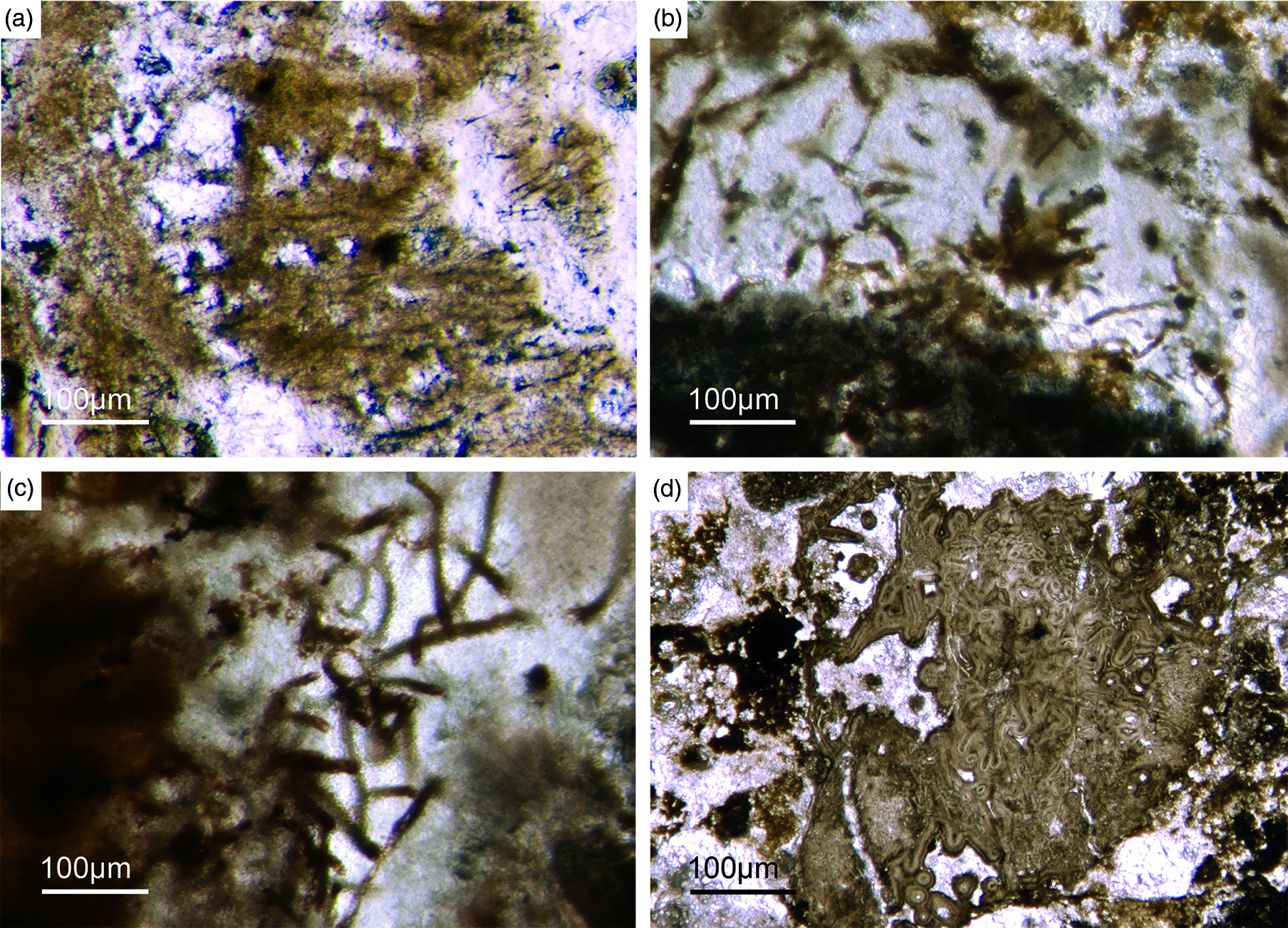
Fig. 15. Filamentous microbial remains. (a) Long filamentous structures, probably remains of sulphide-oxidizing bacteria (SOX-B) (Fon35). (b) Filamentous remains within bright phosphorite (Fon36). (c) Network of large filaments interpreted as remains of SOX-B (Fon36). (d) Sponge skeleton that is strongly cemented by multilayered phosphate, probably resulting from intense flow of P-rich fluids.
In non-upwelling systems, apatite formation appears to be linked to a non-microbial process termed ‘iron-redox pump’ (e.g. Einsele, Reference Einsele1936; Mortimer, Reference Mortimer1941; Nürnberg & Peters, Reference Nürnberg and Peters1984; Ruttenberg & Berner, Reference Ruttenberg and Berner1993). In anoxic pore-water environments, iron(III) will readily be reduced to iron(II). As a consequence, iron(III)-bearing mineral phases will dissolve, thereby releasing adsorbed phosphor into the pore water, which then further reacts to authigenic apatite. Intriguingly, fresh material from the Pusa shales is locally black and enriched in pyrite, probably indicating low-oxygen conditions (see also Vidal et al. Reference Vidal, Palacios, Gámez-Vintaned, Balda and Grant1994). These conditions would have been favourable for an ‘iron-redox pump’, which thus might account for some of the observed phosphatic cements (Fig. 15d). However, the abundance of evidence for microbial processes still strongly suggests a microbial origin for most of the observed apatite in the Fontanarejo deposit. In combination, both pathways plausibly explain the rapid precipitation of apatite (Cook & Shergold, Reference Cook and Shergold1984; Föllmi, Reference Föllmi1996) which accounts for the exceptional preservation of microbial features and lyssacine and articulated sponge fossils.
7. Conclusions
-
1. The Fontanarejo phosphorites were described decades ago, but have not been studied in detail since then. A new detailed geological map is presented in this study, updating and improving previous mappings. The stratigraphic and sedimentological aspects of these deposits have been controversial and are here reviewed and discussed, supporting the interpretation that they are late Fortunian in age and formed as channels of a turbidite system located at the platform–slope transition. Furthermore, we present the first microfacies description of these deposits, and the first comprehensive characterization of their palaeontological content, highlighting the relevance of the contained sponge fossils.
-
2. The phosphorites of Fontanarejo harbour abundant sponge remains. Hexactinellid remains are most abundant, including lyssacine and possible cemented spicular skeletons. In addition to basic hexactins, diactins, stauractins and pentactins are common. The spicules have simple morphologies, and no microsclers were observed. The lyssacine sponges are often preserved in honey-yellow phosphatic material that likely represents permineralized soft tissue. They exhibit only a few parenchymal spicules and, in rare cases, stauractin and pentactin dermal spicules. This type of hexactinellid is related to the ‘Rossellimorpha A’ sensu Mehl-Janussen (Reference Mehl-Janussen1999). In contrast, the hexactinellids with cemented skeletons show bundles of fused hexactins in the parenchymal areas, and also exhibit with hypodermal pentactins.
-
3. Demosponges are less common but show a higher morphological diversity than hexactinellids. Isolated four-rayed spicules of early demosponges, triaene spicules and diverse monaxonic spicules are common. Articulated plumose and bouquet-type spicule arrangements are related to the ‘axinellid–halichondrid’ group. An unusual type of an ‘axinellid–halichondrid’ demosponge was observed that exhibits a dense dermal layer of short strongyles. Two hadromerid-type spicule arrangements were found. One exhibits similarities to chondrillid sponges that possess only large likely dermal asterose-type microsclers. The other displays a well-developed dermal layer of putative asterose microsclers and a choanosomal skeleton of randomly oriented monaxone spicules. Some specimens of putative ‘keratose’ demosponges were also discovered.
-
4. The analysed samples contain the SFF Anabarella sp. which supports the stratigraphic assignment of the phosphates to the lower Fortunian – Stage 2. In combination, the palaeontological evidence (points 2 and 3 above) and biostratigraphic interpretation of the phosphorites strongly corroborate a Precambrian origin of the phylum Porifera.
-
5. The phosphatic sediment displays many different fabrics and other structures that are typical characteristics of microbialites. Oncoid-like microbialites are abundant; small domal stromatolites are less common. Both components contain significant amounts of organic matter. Long filamentous structures in some of the samples resemble SOX-B. These features strongly suggest that most of the Fontanarejo apatite was formed via microbial mediation. The inferred rapid precipitation of phosphatic cements was additionally driven by an intense flow of phosphor-rich fluids during early diagenesis. All these processes contributed to the exceptional preservation of fragile sponge skeletons and microbialites.
Acknowledgements
The authors gratefully acknowledge Prof. Dr Sören Jensen, Dr Iván Cortijo (Villuercas-Ibores-Jara UNESCO Global Geopark), Prof. Dr Agustín Pieren, Prof. Dr Marta Rodríguez-Martínez (Univ. Complutense Madrid) and Dr Javier Gonzáles Sanz (IGME, Madrid) for sharing their knowledge with us, and for their continuing support and valuable advice. For technical support we thank Dorothea Hause-Reitner, Axel Hackmann and Yu Pei (University of Göttingen). Ellen Kappel (NY) is thanked for checking the English version of the manuscript. PSG acknowledges the support of a Postdoctoral Fellowship from the Alexander von Humboldt Foundation to stay in Göttingen. JR and J-PD acknowledge the Göttingen Academy of Sciences and Humanities and the University of Göttingen (DFG-Courant Research Center Geobiology) for logistic and financial support. CL thanks the National Natural Science Foundation of China (Grant No. 41972016) for financial support. This is publication no. 11 of the Early Life Research Group (Department of Geobiology, Georg-August-Universität Göttingen; Göttingen Academy of Sciences and Humanities). We thank two reviewers for valuable comments that helped to improve the manuscript.
Declaration of Interest
None.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S001675682100087X