Antibiotic resistance is a major threat to patient safety, leading to an estimated 2 million infections and 23,000 deaths per year in the United States. 1 Antibiotic stewardship programs (ASPs) coordinate interventions directed toward curbing inappropriate antibiotic use and improving overall antibiotic prescribing practices. 2 , Reference Sanchez, Fleming-Dutra, Roberts and Hicks 3 Despite advances in antibiotic stewardship programs in the acute-care setting, it is widely acknowledged that most organizations do not have formal outpatient ASPs. 2 This is true even in institutions with robust inpatient ASPs. Annually, 154 million ambulatory visits result in an antibiotic prescription, and ~30% of antibiotic use in outpatient settings is inappropriate. 4
In terms of targeted stewardship efforts in the ambulatory arena, the need for improved prescribing for acute respiratory infections (ARIs) is a fruitful starting point.Reference Fleming-Dutra, Hersh and Shapiro 5 – Reference Meeker, Linder and Fox 7 An estimated 44% of all outpatient antibiotic prescriptions are written for ARIs (eg, sinusitis, otitis media, pharyngitis, and bronchitis), 4 many of which are caused by viruses and often resolve without antibiotics. Outpatient prescribing practices vary based on geography, patient population, insurance, and provider specialty.Reference Hicks, Bartoces and Roberts 8 , Reference Roberts, Hicks and Bartoces 9 Although New York City is the most densely populated metropolitan area in the United States and one with substantial antibiotic resistance, to our knowledge, a widespread assessment of ambulatory prescribing practices there has not been published.
In 2016, United Hospital Fund, an independent nonprofit organization whose mission is to develop a more effective health care system for every New Yorker, issued a request for proposals to engage hospital-owned outpatient practices in a grant-funded initiative to better elucidate the current state of outpatient antibiotic stewardship and to describe factors influencing antibiotic prescribing practices, with a focus on adult patients with ARIs. Stage 1 of this initiative sought to assess the status of outpatient ASPs in New York City, focusing on ARI antibiotic prescribing patterns; stage 2 is using the information from stage 1 to assist and direct participating organizations implementing site-specific plans to improve outpatient prescribing practices. This report describes the findings of stage 1.
Methods
This mixed-methods study was designed to assess current practices around outpatient prescribing of antibiotics. The Biomedical Research Alliance of New York Institutional Review Board reviewed the study protocol and deemed it exempt from review. Individual health systems received local institutional review board approval if appropriate. The collaborative activities occurred from May 2016 through January 2017.
Surveys and data collection tools were developed in consultation with an advisory group including members from UHF, 10 the New York State Department of Health, the Greater New York Hospital Association, the Centers for Disease Control and Prevention,Reference Sanchez, Roberts, Albert, Johnson and Hicks 11 and inpatient and outpatient clinicians from most of the participating hospitals and health systems. The tools and a description of the tools are included as supplemental materials. These include an assessment of current outpatient stewardship practices, a chart abstraction tool, and a survey of providers.
The participating clinics piloted and tested the tools prior to data abstraction. In addition, throughout the initiative, a collaborative approach was used: subject matter advisors educated and provided guidance through a series of in-person meetings and webinars. The UHF staff provided technical assistance and individual feedback to clinics to improve consistency and accuracy of abstraction.
Data were collected at each of the participating clinics and were entered into a web-based survey tool (SurveyMonkey, San Mateo, CA). Each clinic was asked to use codes from the International Classification of Diseases, Tenth Revision to select possible charts for review. If >30 charts met these criteria, each site chose charts randomly. The method of randomization was chosen by each site’s principal investigator. One site elected to use the ordering of diagnostic testing (eg, respiratory viral panel and/or sputum culture) to select potential charts rather than primary or secondary diagnosis codes. Patient-level data from chart abstraction were deidentified prior to submission. Data from the assessment of the current practices, survey of prescribers, and chart review were aggregated across the clinics and by hospital or health system. Sites received results comparing clinic-specific data to the aggregate for all sites.
Chart abstraction data were analyzed in aggregate and are shown in Table 1. Descriptive statistical analysis was performed using SAS version 9.4 software (SAS Institute, Cary, NC). Univariate associations were compared using the χ2 or the Mantel-Haenszel χ2 when applicable. Those factors achieving a P value of <.10 were included in a stepwise multivariable logistic regression model to identify potential independent predictors of antibiotic prescribing.
Table 1 Demographic and Clinical Characteristics for Antibiotic Prescribing for Acute Respiratory Infections in All Participating Outpatient PracticesFootnote a
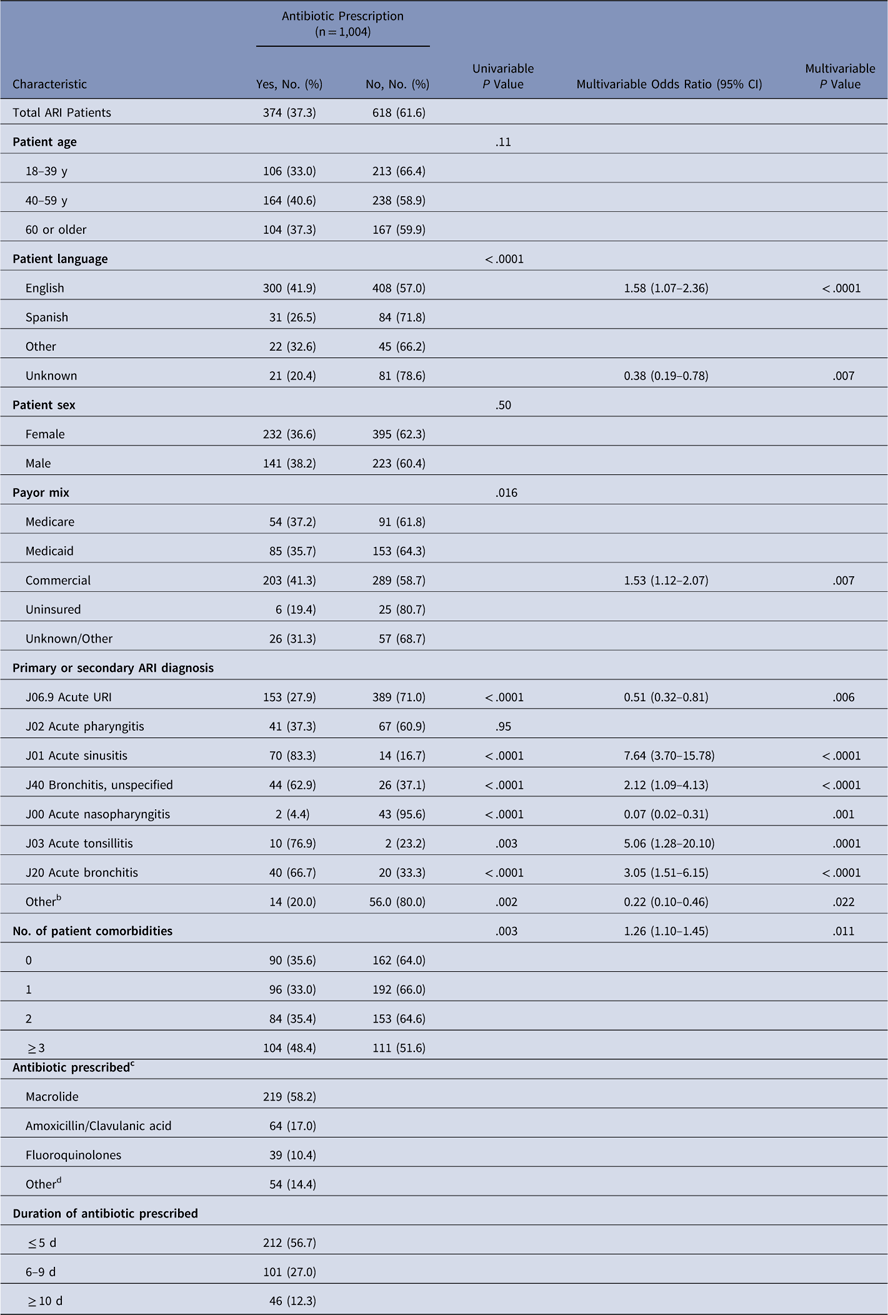
Note. ARI, acute respiratory infection; URI, upper respiratory infection.
a n=1,004 patient encounters from 31 clinics.
b Case selection was based on ordering of diagnostic testing (eg, respiratory viral panel and/or sputum culture) and not by a primary diagnosis code.
c In several cases, an individual patient was prescribed >1 type of antibiotic. The types of antibiotic prescribed should not be considered mutually exclusive categories.
d Penicillin, cephalosporins, clindamycin, and other.
Results
Participant demographics and current state of outpatient stewardship
In total, 31 clinics representing 9 hospitals or health systems participated. They represented diverse patient and provider populations and were located throughout the New York City region: Manhattan (n=7), Queens (n=9), Bronx (n=4), Brooklyn (n=8), Long Island (n=2), and Westchester (n=1) (Tables 1 and 2). The payer mix varied by site; the median percentage covered by Medicare was 22%, by Medicaid 29%, by commercial payers 24%, and by other/unknown 0.5%; 5% were uninsured.
Table 2 Provider Characteristics and Antibiotic and Prescribing Knowledge

Note. EHR, electronic health record; CDC, Centers for Disease Control and Prevention.
a The vignette in the survey of antibiotic prescribers described a healthy, 36-year-old patient with fever and nasal discharge for 5 days with a temperature <37.7°C (<100°F), erythematous and enlarged nasal turbinates, cloudy discharge on the right, and tenderness over the right maxillary sinus.
b Results from the “often impacts decision to prescribe” category was included in the aggregate response for all outpatient practices. Providers were allowed to select >1 response.
Overall, 68% of practices responded that there were ASPs in their health system. Although 25% of practices reported having institutional guidelines for antibiotic use and selection for ARIs as part of their program, only 11% had any ambulatory-specific guidance. A high proportion of providers, close to 40%, stated that there was an identified leader for outpatient ASP. Although all the practices stated they had an electronic health record system, only 7% reported embedded computer decision support for antibiotic use in that system.
Antibiotic prescribing practices
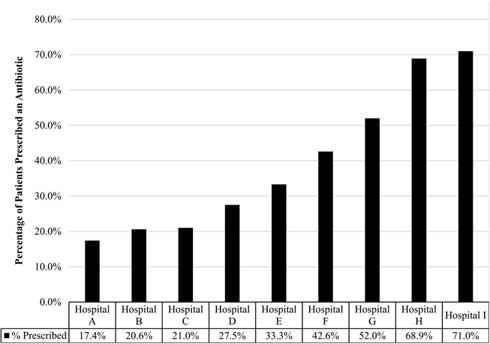
Across the clinics, 1,004 charts were reviewed; all clinics provided chart reviews. Moreover, 37% of patients diagnosed with an ARI received a prescription for antibiotics. Statistically significant variation was observed in the rate of prescribing based on the hospital or health system in which the patient sought treatment, with prescribing rates ranging from 17.4% to 71.0% (P<.001) (Fig. 1).
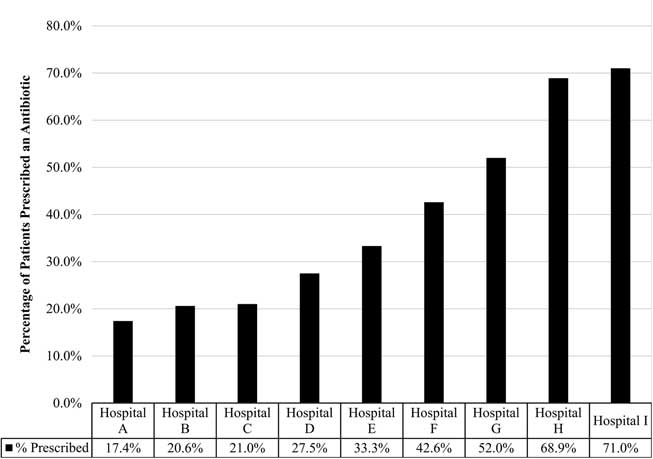
Fig. 1 Percent of patient visits prescribed an antibiotic for an acute respiratory infection. Reflects the percentage of patients with adult acute respiratory infection that received an antibiotic from participating hospital or health system, which varied significantly (17·4%–71·0%; P<.001).
Among patients with a diagnosis of ARI, the diagnoses associated with the highest antibiotic prescribing rates were sinusitis (83.3%) and bronchitis (62.9% bronchitis-unspecified, 66.7% acute bronchitis). The rate of antibiotic prescribing varied based on patient-level characteristics including primary spoken language, insurance type, and number of comorbid conditions. Patients who reported their preferred language as English were more likely to be prescribed an antibiotic than non-English speaking patients (P<.001). In addition, patients with commercial insurance were more likely to receive a prescription than patients with Medicare, Medicaid, or no insurance (P=.016). The presence of 3 or more comorbidities also increased the likelihood that a patient would be prescribed an antibiotic (P=.003). We detected no difference in prescribing rates based on patient age or sex. In multivariable analysis, having commercial insurance and speaking English were both independent predictors of receiving a prescription.
In this sample, attending physicians prescribed antibiotics more often than other prescribing providers. While attending physicians comprised 35.6% of providers across all sites, the charts reviewed in the sample indicated that 74.1% of the antibiotics were prescribed by attending physicians when they were not overseeing a resident. The remaining prescriptions were written by nurse practitioners, physician assistants, and resident physicians in training.
Furthermore, 58% of patients receiving an antibiotic prescription were prescribed a macrolide, and 17% were prescribed amoxicillin or clavulanic acid. Fewer patients were prescribed fluoroquinolones (10.4%) or other antibiotics. Of those patients receiving an antibiotic prescription, 56.7% were prescribed the antibiotic for <5 days, 27.0% were prescribed the antibiotic for 6–9 days, and 12.3% were prescribed the antibiotic for ≥10 days.
In addition, 45% of patients received education on their diagnosis and/or treatment, and follow-up was recommended in nearly 62% of cases. However, fewer than 44% of patient records included documentation of any follow-up.
Survey of provider knowledge, attitude, and perceptions
The findings from the provider survey (Table 2) are based on 302 surveys received, representing all 31 clinics. The total number of providers (eg, attending physicians, residents, nurse practitioners, and physician assistants) at all sites was 1,029; the response rate was 29.3%. Providers responding to the survey consisted largely of attending physicians (42.1%) and residents (50.7%).
In terms of provider knowledge, most respondents acknowledged the difference between broad- and narrow-spectrum antibiotics, and most considered spectrum of activity in prescribing. In response to a clinical vignette in which current guidelines would not support antibiotic prescribing, 24% of respondents indicated they would prescribe an antibiotic.
When asked to select the top 3 factors in the decision to prescribe antibiotics, severity of illness, clinical practice guidelines, and patient comorbidities were the most frequently selected. Moreover, 35% percent of providers identified “concern for antibiotic resistance” as one of the top 3 considerations, and 7% of providers cited patient request or satisfaction as a concern.
When asked to choose methods or tools that would likely improve decision making and antibiotic prescribing for ARIs in their practice, providers were most interested in reference guides and clinical guidelines, educational materials for patients and families, and decision support tools.
Discussion
We sought to better define the current outpatient antibiotic prescribing landscape in the greater NYC area by assessing provider perceptions and prescribing patterns for adult ARIs among diverse clinics associated with 9 hospitals and healthcare systems. We found very little activity directed specifically toward improving outpatient antibiotic use, and none of the clinics had outpatient-specific strategies in place to improve antibiotic prescribing.
Antibiotic prescribing for the treatment of bronchitis and sinusitis was high, with nearly 67% of patients with acute bronchitis and >80% of patients with acute sinusitis prescribed an antibiotic, despite guidelines and endorsed metrics against routine use for these indications.Reference Roberts, Hicks and Bartoces 9 , Reference Harris, Hicks and Qaseem 12 , Reference Rosenfeld, Piccirillo and Chandrasekhar 13
Interestingly, as in previous studies,Reference Jones, Sauer and Jones 14 provider knowledge or experience did not appear to influence prescribing; most antibiotic prescriptions were authored by attending physicians. One motivation for antibiotic prescribing may be these clinicians’ long-standing relationships with patients and perception of patient pressure or satisfaction.Reference Sanchez, Roberts, Albert, Johnson and Hicks 11 , Reference McKay, Mah, Law, McGrail and Patrick 15 However, the provider survey did not identify this as a major contributor to antibiotic prescribing.
Previous studies have suggested that patients of certain ethnicities are more likely to desire antibiotics for ARIs.Reference Francois Watkins, Sanchez, Albert, Roberts and Hicks 16 , Reference Mangione-Smith, Elliott, Stivers, McDonald, Heritage and McGlynn 17 In our sample, patients who were English-speaking or commercially insured were more likely to be prescribed an antibiotic; further investigation into potential confounding by clinic or provider is needed.
Even more disconcerting was the use of broad-spectrum agents to treat ARIs and the variability in duration of antibiotic prescribed. Macrolides are not the first-line agent for any of the diagnoses included in our sample, but they were the most commonly prescribed antibiotic. Despite public health concerns, provider concern for antibiotic resistance was not reported as a major factor impacting prescribing. It remains unclear whether the pervasive reliance on macrolides results from gaps in knowledge of antibiotic spectrum and the common causes of ARIs or the convenience of macrolides.
The New York State Department of Health and the Centers for Disease Control and Prevention have identified a need for targeted interventions that can improve public knowledge of appropriate antibiotic use.Reference O’Sullivan, Harvey, Glasziou and McCullough 18 , Reference Fleming-Dutra, Mangione-Smith and Hicks 19 Our review showed that only 45.3% of patients diagnosed with an ARI received directed patient education. Of the 61.8% where follow-up was recommended, only 43.5% received follow-up. While our assessment did not address the reasons for this lack of education and follow-up, the discrepancy raises concerns about patient education and continuity of care that may warrant further attention.
Despite a low response rate, results from the provider survey do provide some direction for participating institutions to consider next steps. In general, providers recognized the need to improve antibiotic prescribing practices and were open to utilizing tools that will improve their practice. They expressed interest in a quick reference guide for each major diagnosis including antibiotic indication; improved use of established clinical practice guidelines for selection, dose, and duration; and clinical decision support.
Limitations of the study
Despite the overall sample size, the individual clinics reviewed a median of 30 charts (range, 28–124). There were site-specific variations in methodologies employed to randomly select charts for abstraction, which may have led to sampling bias. In addition, we hypothesize that coding bias may also have affected the findings; it is possible that physicians prescribing antibiotics may have utilized diagnosis codes for which antibiotic prescribing is not clearly contraindicated (eg, sinusitis). In addition, there were some limitations in identifying specific ARI codes where the ARI may not have been recorded as the primary diagnosis. The overall provider survey response rate was low. The discrepancy between the findings of the sampled charts and the provider survey may reflect social biases (ie, the perceived desirability of certain answers). The provider surveys may have been skewed by particular health systems with higher response rates, which may limit generalizability to the broader population. Our study was observational and largely descriptive. Although we have described the variations in prescribing practices observed in our data, we were not able to fully explore many of the underlying factors that may contribute to these differences.
In conclusion, despite evidence-based recommendations, outpatient antibiotic prescribing for ARIs continues to be high, even in hospitals and health systems with established inpatient ASPs. Using local data on antibiotic prescribing, we have been able to increase provider and leadership awareness of the importance of outpatient antibiotic stewardship for improving local ambulatory prescribing practices We remain far from resolving the problem of inappropriate antibiotic use for ARIs; however, this initiative has provided (1) a useful assessment of current outpatient antibiotic prescribing in NYC outpatient clinics, (2) a framework for site-specific and responsive actions, and (3) tools to assess the impact of improvement efforts after implementation of those actions.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2018.227
Acknowledgments
United Hospital Fund would like to thank all the organizations that participated in the Outpatient Antibiotic Stewardship Initiative for their commitment to improving stewardship in their organizations. We would also like to acknowledge the work of Deborah Halper MS, MPH, and Anne-Marie Audet MD, MSc, SM, at UHF for their guidance and input throughout the project, as well as Hillary Jalon MS, and Marit Boiler MPH, formerly at UHF, who previously led this initiative.
Financial support
This project was supported by a grant from the United Hospital Fund, which provided seed funding to each practice site.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.