A β-coronavirus, SARS-CoV-2, was recognized in a cluster of patients with community-acquired pneumonia in Wuhan, Hubei Province, China, in December 2019.Reference Zhu, Zhang and Wang1 With the establishment of high-speed rail within China and international travel, this novel coronavirus rapidly disseminated to all provinces of China and 25 countries in the Asia-Pacific region, North America, Europe, and South America within 1 month of its discovery.2 Similar to the other β-coronaviruses, such as severe acute respiratory syndrome–associated coronavirus (SARS-CoV) and Middle East respiratory syndrome–associated coronavirus (MERS-CoV), the SARS-CoV-2 is postulated to have originated from bats and to have been transmitted to intermediate hosts before jumping to humans, causing community and nosocomial pneumonia.Reference Cheng, Lau, Woo and Yuen3–Reference Chan, Lau, To, Cheng, Woo and Yuen5 Before February 11, 2020, the disease caused by this novel coronavirus was temporarily named the 2019 novel coronavirus (2019-nCoV) disease. On February 11, 2020, the World Health Organization renamed the disease the coronavirus disease 2019 (COVID-19), and the virus was classified as SARS-CoV-2 by the International Committee on Taxonomy of Viruses (ICTV). By February 17, 2020, a total of 71,429 people had been infected globally, including 70,635 cases (98.9%) in China. With the addition of 3 patients who died in the Philippines, Japan, and France, 1,772 deaths have been reported in China, with a crude mortality of 2.5%.6 Two healthcare workers (HCWs) succumbed as a result of nosocomial acquisition of SARS-CoV-2 in China. In Hong Kong, 8 HCWs died in the outbreak of SARS-CoV in 2003.Reference Cheng, Lau, Woo and Yuen3 Considering our experience with SARS-CoV, it is important to respond to this emerging infectious disease with proactive infection control measures to prevent importation and nosocomial transmission of SARS-CoV-2 in Hong Kong. Here, we report our infection control measures in the first 6 weeks, and the admission of 42 confirmed cases, after the official announcement of a cluster of pneumonia of unknown etiology in Wuhan, Hubei Province, by the National Health Commission of the People’s Republic of China (PRC).
Methods
Epidemiology and infection control preparedness for SARS-CoV-2 in Hong Kong
According to the infection control preparedness plan for emerging infectious disease in Hong Kong,Reference Wong, Chen and Liu7 a series of proactive infection control measures were activated by the Hospital Authority, a governing body of all 43 public hospitals responsible for 90% of inpatient service in Hong Kong, immediately after the official announcement of a cluster of pneumonia of unknown etiology in Wuhan, Hubei Province, by the National Health Commission of the PRC on December 31, 2019 (day 1). The key measures included a bundle of infection control measures for early recognition, isolation, notification, and molecular diagnostic testing for all suspected cases.Reference Cheng, Wong, To, Ho and Yuen8 Active surveillance was performed on patients presenting to the hospital according to a set of clinical and epidemiological criteria, which were adjusted during the evolution of SARS-CoV-2 in China and Hong Kong (Table 1). All suspected cases were isolated in airborne infection isolation rooms (AIIRs) for contact, droplet, and airborne precautions. The Centre for Health Protection, Department of Health, and the Hospital Authority were notified of suspected cases. Enhanced laboratory surveillance was also conducted to widen the scope of screening (Table 1). Patients received AIIR care when available; otherwise, patients received care in a ward with 1-m spacing between patients.
Table 1. Surveillance Program for Early Recognition of Patients With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Hong Kong
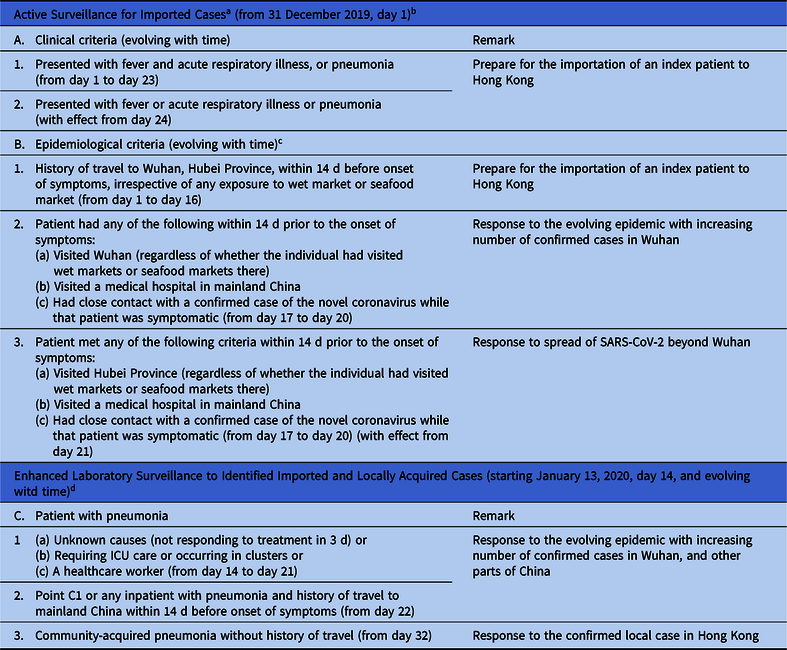
a Application for Accidental and Emergency Department (AED), outpatient clinics, and day centers to prevent the importation of a patient with SARS-CoV-2. Patients fulfilling clinical and epidemiological criteria are to be isolated in airborne infection isolation room, reported to the Centre for Health Protection, Department of Health, and tested for SARS-CoV-2 by reverse transcription polymerase chain reaction (RT-PCR).
b Day 1 is denoted as the day of official announcement of a cluster of pneumonia in Wuhan, Hubei Province, by the PRC National Health Commission.
c Epidemiological criteria have been updating according to the spread of SARS-CoV-2.
d Serving as safety net to detect infected patient without a clear epidemiological exposure.
Upper respiratory specimens (ie, nasopharyngeal aspirates, or flocked swabs, and throat swabs) were collected for all cases under active and enhanced laboratory surveillance, whereas lower respiratory specimens (ie, sputum, endotracheal aspirates, or bronchoalveolar lavage) were collected for rapid molecular diagnostic testing if it was available. The molecular diagnostic testing was simultaneously performed by the Public Health Laboratory Service, the Centre for Health Protection, and Queen Mary Hospital, The University of Hong Kong, during the initial phase of the preparedness measures, with a turnaround time of 4–8 hours, depending on the number of specimens per batch. With the increasing number of tests, molecular diagnostic testing has been performed by 7 microbiology laboratories in 7 regional hospitals, including Queen Mary Hospital in Hong Kong, since February 1, 2020 (day 33).
The epidemiology of confirmed cases was analyzed. Imported cases were defined as patients with history of travel to the affected areas 14 days before symptom onset. A local case was defined as a patient with no history of travel to the affected areas 14 days before onset of symptoms. Enhanced infection control measures with clear illustrations regarding the choice of personal protective equipment (PPE) were enforced (Table 2). Regular, open-staff forums were held, along with face-to-face education sessions, to provide “right-on-time” infection control updates and to address staff concerns. Practical training sessions using PPE were performed by the hospital infection control team. Hand hygiene compliance assessments were conducted regularly in our hospitals.
Table 2. Enhanced Infection Control Measures to Prevent Nosocomial Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Hong Kong

a Suspected or confirmed cases of SARS-CoV-2 receive care in airborne infection isolation rooms.
b Including triage stations of emergency rooms and outpatient clinics.
c Aerosol generating procedures included endotracheal intubation, cardiopulmonary resuscitation, bronchoscopy, and open suction of respiratory tract, sputum induction, use of nebulizer therapy, noninvasive positive pressure ventilation, and high-frequency oscillatory ventilation.
d Including outpatient clinics, radiological facilities, physiotherapy, occupation therapy, and day centers.
e Surgical mask could be used as an alternative based on risk assessment.
f AMMI, Association for the Advancement of Medical Instrumentation PB70:2003 is to define the liquid barrier performance and classification of protective apparel and drapes intended for use in healthcare facilities (https://www.fda.gov/medical-devices/personal-protective-equipment-infection-control/medical-gowns). AAMI level 1 isolation gowns are used when small amounts of fluid exposure are anticipated, and AAMI level 3 isolation gowns are used when large amounts of fluid exposure are anticipated.
Investigating possible nosocomial transmission of SARS-CoV-2
Upon laboratory confirmation of a patient with SARS-CoV-2, the infection control team immediately followed up to identify HCWs and patients with unprotected exposure. This procedure basically followed the contact-tracing protocol of avian influenza A H7N9 in Hong Kong.Reference Cheng, Tai and Lee9 Briefly, close contact refers to those with unprotected exposure, defined as HCWs who had provided care for a case patient with inappropriate PPE and patients who had stayed within the same cubicle of the index case regardless of the duration of exposure. Persons deemed to have had close contact with unprotected exposure were required to remain in quarantine for 14 days since last exposure, followed by medical surveillance for 14 days after completion of the quarantine period. During medical surveillance, these people were advised to wear surgical masks in the hospital and the community.
Laboratory diagnosis of SARS-CoV-2
Clinical specimens including nasopharyngeal aspirates, nasopharyngeal swabs, throat swabs, saliva, sputum, endotracheal aspirates, or bronchoalveolar lavage were first mixed into 2 mL viral transport medium (VTM), and 250-μL samples were subjected to nucleic acid extraction by the eMAG extraction system (bioMérieux, Marcy-l’Étoile France), with an elution volume of 55 μL.
Before the identification of SARS-CoV-2, a pan-coronavirus polymerase chain reaction (PCR) assay with modification to detect 23 coronaviruses known to be present in human, animals, and bats was used.Reference Cheng, Wong, To, Ho and Yuen8,Reference Yip, Lam and Luk10 Subsequently, real-time PCR targeting the E gene of the SARS-CoV-2/SARS-like coronavirus was performed using the LightMix Modular SARS and Wuhan CoV E-gene mix (TIB Molbiol, Berlin, Germany) and the LightCycler Multiplex RNA Virus Master Kit (Roche Diagnostics, Mannheim, Germany). Briefly, a 20-μL reaction contained 10 μL RNA templates, 4 μL 5 × RT-qPCR reaction buffer, 0.5 μL LightMix reagent mix, 0.1 μL 200 × RT enzyme, and 5.4 μL nuclease-free H2O. Thermal cycling was performed at 55°C for 5 minutes for reverse transcription, followed by 95°C for 5 minutes, then 45 cycles of 95°C for 5 seconds, 60°C for 15 seconds, and 72°C for 15 seconds on the LightCycler 480 II system (Roche Diagnostics, Mannheim, Germany). The SARS-CoV-2 RNA loads in patient and environmental samples were determined using a real-time RT-PCR assay developed in house to target the SARS-CoV-2 RdRp geneReference Chan, Yip and To11.
Environmental surveillance for SARS-CoV-2
Air samples for SARS-CoV-2 RNA were collected for the first confirmed case in Hong Kong by an air sampler, SAS Super ISO 180 model 86834 (VWR International PBI Srl, Milan, Italy) with modification as previously described.Reference Cheng, Wong, Chiu, Yip, Wong and Yuen12,Reference Cheng, Wong and Wong13 Briefly, the air sampler was perpendicularly positioned at a distance of 10 cm at the level of patient’s chin, and 1,000 L air at a rate of 180 L per minute was collected for each culture plate containing 3 mL of VTM. The patient was instructed to perform 4 different maneuvers (ie, normal breathing, deep breathing, speaking “1, 2, 3” continuously, and coughing continuously) while putting on and putting off the surgical mask, which complied with the ASTM F2100 level 1 standard. The VTM was transferred to the laboratory within 2 hours and was subjected to RT-PCR for the detection of SARS-CoV-2.
Swab samples (Oxoid Transport Swabs, Copan Italia, Italy) from the patient’s environment including bench, bedside rail, locker, bed table, alcohol dispenser, and window bench, before and after collection of air samples, were collected and tested for SARS-CoV-2 using RT-PCR. Briefly, swab samples covering a mean surface area of 9 cm2 (3 cm × 3 cm) were submerged in 2 mL VTM. The VTM was further centrifuged at 13,000 ×g for 1 minute, and 1 mL of the supernatant was used for nucleic acid extraction. A nasopharyngeal flocked swab, throat swab, and saliva of this patient were collected on the day of environmental surveillance and were subjected to viral load assay.
This study was approved by the Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong West Hospital Cluster.
Statistical analysis
We used the Fisher exact test to compare independent categorical variables between groups. All reported P values were 2-sided. P < .05 was considered statistically significant. Computation was performed using the SPSS version 15.0 software for Windows (IBM, Armonk, NY).
Results
Epidemiology and infection control preparedness for SARS-CoV-2 in Hong Kong
Up to February 10, 2020 (day 42 after official announcement of a cluster of pneumonia of unknown etiology in Wuhan, Hubei Province), 1,275 patients fulfilled the clinical and epidemiological criteria for active and enhanced surveillance upon presentation to our public hospitals, of whom 42 of these 1,275 patients (3.3%) were confirmed to be a cases of COVID-19 in Hong Kong (Fig. 1). Among these patients, 20 were male and 22 were female, with a median age of 59 years (range, 22–91 years); 9 of these patients were residents of mainland China (7 from Wuhan, 1 from Shenzhen, and 1 from Zhuhai), who had arrived by high-speed train (n = 6), by flight (n = 2), and by bus (n = 1). The remaining 33 patients were Hong Kong residents, 5 of whom had a history of travel to mainland China in the 14 days before the onset of symptoms. Exposure to a wet or seafood market was reported by 2 patients. The first patient was confirmed on January 21, 2020 (day 22). From day 22 to day 32, only 1 of 13 confirmed cases (7.7%) was locally acquired. The number of locally acquired cases significantly increased to 27 of 29 confirmed cases (93.1%) from day 33 to day 42 (P < .001, Fisher exact test). These cases occurred in 8 family cluster involving 28 patients. One patient (2.4%) died and 4 patients (9.5%) remained in critical condition requiring mechanical ventilation as at day 42.

Fig. 1. Active and enhanced laboratory surveillance for diagnosis of SARS-CoV-2 in Hong Kong. Both calendar date and day after official announcement of a cluster of pneumonia in Wuhan, Hubei Province, by the PRC National Health Commission on December 31, 2019, are shown. From day 1 to day 20, pan-coronavirus PCR with modification to detect 23 coronaviruses known to be present in human, animals, and bats was used. From day 21 onward, real-time PCR targeting the E gene of the SARS-CoV-2/SARS-like coronavirus was performed using the LightMix Modular SARS and Wuhan CoV E-gene mix (TIB Molbiol, Berlin, Germany) and the LightCycler Multiplex RNA Virus Master Kit (Roche Diagnostics, Mannheim, Germany).
Investigating possible nosocomial transmission of SARS-CoV-2
Upon epidemiological investigation of 42 confirmed cases, 36 patients were directly admitted to AIIR, and 6 patients initially received care in non-AIIR facilities. Of the 413 HCWs caring for these patients before confirmation of SARS-CoV-2, 11 HCWs (2.7%) had been in close contact with unprotected exposure and required quarantine for 14 days. None of them was infected with SARS-CoV-2 by the end of the quarantine. Nosocomial transmission was not observed in these hospitalized patients.
Environmental surveillance for SARS-CoV-2
The viral loads of the first confirmed case were 3.3 × 106 copies per mL in the pooled nasopharyngeal and throat swabs and 5.9 × 106 copies per mL in saliva on the day of air sampling. The air samples collected while the patient performed 4 different maneuvers (ie, normal breathing, deep breathing, speaking “1, 2, 3” continuously, and coughing continuously) while putting on and putting off the surgical mask were all undetectable for SARS-CoV-2 RNA. The viral load of the window bench was 6.5 × 102 copies per mL of VTM before the collection of air samples, but the other environmental samples collected before and after the air sampling had no detectable SARS-CoV-2 RNA. The environmental and air samples were collected by an experienced infection control nurse wearing full PPE including N95 respirator, face shield, cap, gloves, and gown. This nurse was in close contact with the confirmed case for a total of 63 minutes. She completed 14 days of medical surveillance without developing fever or respiratory symptoms.
Discussion
The emergence of COVID-19, the SARS-CoV-2–associated pneumonia, poses a global threat and challenges to communities as well as healthcare systems. In response to this unprecedented outbreak, which has already produced a higher number of infected cases and mortality within the first 6 weeks of its declaration than the entire outbreak of SARS-CoV in 2003,2,Reference Cheng, Lau, Woo and Yuen3 a rapid infection control response is essential to contain and mitigate the risk of nosocomial transmission and outbreak. In the SARS-CoV outbreak, almost 60% of nosocomial acquisition of SARS-CoV occurred among HCWsReference Cheng, Chan, To and Yuen4; therefore, it is critically important to implement proactive infection control measures among HCWs, and these measures must be planned in advance. In Hong Kong, a cosmopolitan city of 1,104 km2 with a population of 7.45 million in southern China, we are at a high risk of importation of infected cases from mainland China. Therefore, we progressively stepped up our infection control measures by widening the clinical and epidemiological criteria of surveillance for early recognition and isolation of index cases according to the evolution of the epidemic. In particular, having visited a hospital in mainland China was introduced as an epidemiological criterion for surveillance on day 17 of our infection control preparedness measures, even though COVID-19 was confined to Wuhan, Hubei Province, until day 20.2 The criteria of previous hospital visit was included because it had previously been determined to be a risk factor for SARS acquisition in China.Reference Wu, Xu and Zhou14 Under the surveillance program, of 42 cases of COVID-19 were identified in Hong Kong, 36 were immediately isolated in AIIR upon admission. During the SARS outbreak, the median time between index patient admission and patient isolation was 4.5 days (1–13 days), according to a review of literature.Reference Cheng, Chan, To and Yuen4
At the same time, we enhanced the infection control measures by implementing standard, contact, droplet, and airborne precautions for suspected or confirmed cases. We stepped up the use of PPE among HCWs performing aerosol-generating procedures (AGPs), even when caring for patients without clinical features and epidemiological exposure risk in the general wards. Performance of AGPs such as endotracheal intubation, open suctioning, and use of high-flow oxygen was a risk factor for nosocomial transmission of SARS-CoV among HCWs.Reference Loeb, McGeer and Henry15 In addition, provision of surgical masks to all HCWs, patients, and visitors in clinical areas was implemented on day 5. Although wearing a surgical mask alone was not clearly associated with protection from acquisition of SARS-CoV, wearing a surgical mask by either HCWs or patients reduces the risk of nosocomial transmission of pandemic influenza.Reference Cheng, Tai and Wong16,Reference Cheng, To, Tse, Hung and Yuen17 The combination of hand hygiene with face masks shows statistically significant efficacy against laboratory-confirmed influenza in the community, as illustrated in a systematic review and meta-analysis.Reference Wong, Cowling and Aiello18 Hand hygiene among HCWs and patients was promoted and enforced during the COVID-19 epidemic.Reference Cheng, Tai and Li19,Reference Cheng, Wong, Wong and Yuen20 With this bundle of infection prevention measures, we were able to maintain zero nosocomial transmission of SARS-CoV-2 after the importation of first confirmed case on day 22 in Hong Kong.
The mode of transmission of SARS-CoV-2 will undoubtedly be investigated further. Opportunistic airborne transmission was implicated in SARS-CoV.Reference Roy and Milton21 The World Health Organization recommends the use of airborne precautions whenever applicable in addition to standard, contact, and droplet precautions.22 To investigate this connection, we conducted a pilot experiment to examine the exhaled air of a confirmed patient with a moderate level of viral load in respiratory specimens, with or without wearing a surgical mask in the AIIR. Notably, the RNA of SARS-CoV-2 was undetectable in the air samples but was present in an environmental sample. We cannot make a definite conclusion based on the analysis of a single patient; however, our finding may help to reassure our staff that exhaled air may be rapidly diluted inside an AIIR with 12 air exchanges per hour, or that the SARS-CoV-2 may not be predominantly transmitted by airborne route. The presence of environmental contamination by SARS-CoV-2 highlights the importance of transmission via direct or indirect contact. SARS-CoV retained its viability on a smooth surface for >5 days at temperatures of 22–25°C and relative humidity of 40%–50%.Reference Chan, Peiris, Lam, Poon, Yuen and Seto23
Transmission within families remained a concern because 66% of confirmed cases diagnosed in Hong Kong were spread among family members. One family cluster comprised 11 cases, most probably caused by viral transmission during their gathering for hot pot, in which the use of utensils and chopsticks contaminated by saliva may occur. Saliva was shown to be positive for SARS-CoV-2 in 11 of 12 patients at a median of 3.3 × 106 copies per mL at the time of presentation.Reference To, Tsang and Chan24 In this family cluster, an asymptomatic patient was retrospectively diagnosed, a 91-year-old lady. Along with our recent report of an asymptomatic case in a pediatric patient,Reference Chan, Yuan and Kok25 we observe that asymptomatic infection can occur over a wide age range. The transmissibility of infection among asymptomatic patients deserves further investigation.
With the implementation of active and enhanced surveillance with progressively wider screening criteria during the evolution of this epidemic, we have recognized most of the confirmed cases upon hospitalization, and we have achieved zero nosocomial transmission in HCWs and patients within the first 6 weeks. However, our surveillance program may be challenged by patients with mild symptoms. In early publications, fever and cough were reported in 87% and 80% of patients, respectively, at the time of presentation.Reference Zhu, Zhang and Wang1,Reference Chan, Yuan and Kok25–Reference Chen, Zhou and Dong30 With the presence of a locally acquired case, epidemiological criteria may no longer be useful for admission screening. Vigilance in hand hygiene practice, wearing of surgical masks in the hospital, and appropriate use of PPE in patient care, especially performing AGPs, are the key infection control measures to prevent nosocomial transmission of SARS-CoV-2, even before the availability of effective antiviral agents and vaccine.
Acknowledgments
The authors thank Hospital Authority, and Centre for Health Protection Department of Health to coordinate and support the outbreak control and investigation.
Financial support
This work was supported in part by the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Diseases of the Department of Health, Hong Kong Special Administrative Region; and the Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, the Ministry of Education of China.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.





