To the Editor—Central venous catheter (CVC)–related bloodstream infections (CRBSIs) are potentially preventable complications associated with high morbidity, especially in patients with hematological malignancies.Reference Zakhour, Chaftari and Raad 1 Neutropenia is one of the most important risk factors for CRBSIReference Zakhour, Chaftari and Raad 1 , Reference Hentrich, Schalk and Schmidt-Hieber 2 because of insufficient immune control of the patient’s microbial flora or pathogens when absolute neutrophil counts (ANC) are <500/µL.Reference Bodey, Buckley, Sathe and Freireich 3 , Reference Holland, Gallin and Kasper 4 Neutropenia as a risk factor for CRBSI is only well defined for the time of CRBSI onset.Reference Howell, Walters, Donowitz and Farr 5 , Reference Wisplinghoff, Seifert, Wenzel and Edmond 6 So far, no reliable data are available that address the impact of neutropenia on CRBSI at the time of CVC insertion. This impact is of special interest for hematology patients because CVCs are often inserted during neutropenia either due to the underlying malignancy, like acute myeloid leukemia (AML), or after application of chemotherapy. In addition, CVC reinsertions after CVC removal due to CRBSI are also common, especially during long-lasting neutropenia, for example, after remission-inducing chemotherapy in AML patients or after conditioning therapies before hematopoietic stem-cell transplantation (HSCT). Therefore, we aimed to investigate the impact of neutropenia on subsequent CRBSI at the time of insertion of short-term, nontunneled CVCs in adult patients with hematological malignancies.
We analyzed data from the prospective multicenter SECRECY study (German Clinical Trial Register, no. DRKS00006551), a CRBSI registry conducted in 6 German hematology and oncology centers, including the aforementioned group of high-risk patients receiving AML induction or HSCT. Inclusion criteria encompassed short-term, nontunneled jugular and subclavian vein CVCs with ≥1 day in situ, and CRBSI was classified according to the 2012 Infectious Diseases Working Party (AGIHO) of the German Society for Hematology and Medical Oncology (DGHO) CRBSI definition.Reference Hentrich, Schalk and Schmidt-Hieber 2 Only CRBSIs classified as definite or probable were considered. Hence, we identified 2,368 patients with a total follow-up of 37,932 CVC days. In 2,158 cases (91.1%), the underlying diseases were hematological malignancies. Among them, we identified 406 cases (17.1%) with neutropenia defined as ANC <500/µL or white blood cell counts <1,000/µL at the time of CVC insertion. Of 406 cases, 400 could be matched for age, sex, underlying disease, site of CVC insertion, use of chlorhexidine-coated CVC dressings, use of chlorhexidine-, antimicrobial- or silver sulfadiazine-coated CVCs, and complications during CVC insertion.
The median age of the patients was 59 years, and 60.3% were male (Table 1). Approximately 75% of CVCs were inserted in patients suffering from acute leukemia, and almost all CVCs were placed in the jugular vein (388 of 400, 97.0%). In a minority of patients, coated CVCs were inserted (23 of 400, 5.8%), whereas in half of the cases chlorhexidine-coated CVC dressings were used (196 of 400, 49.0%). Known high-risk CVC features (ie, male sex, complicated CVC insertion, diagnosis of AML, multiple myeloma, or non-Hodgkin lymphomaReference Schalk, Toelle and Schulz 7 ) were present in half of the patients (203 of 400, 50.8%). The median CVC time in situ was nonsignificantly shorter in CVCs inserted during neutropenia compared to CVCs in nonneutropenic patients (14 vs 18 days; P = .39). We found no differences in CRBSI rates comparing neutropenic to nonneutropenic patients (45 of 400 [11.3%] vs 50 of 400 [12.5%]; P = .66). However, median time to CRBSI diagnosis was shorter in patients who received the CVC in neutropenia compared to non-neutropenic controls (10 vs 15 days; P = .002). Generally, definitive CRBSIs were more often diagnosed than probable CRBSIs without differences between the neutropenic and the nonneutropenic patient groups. Furthermore, there were no differences in the CRBSI incidence (7.1 vs 7.1 per 1,000 CVC days; P = .96) or in the CRBSI probability over the time on day 14 (hazard ratio [HR], 1.64; 95% confidence interval [CI], 0.96–2.49; P = .07) or on day 21 (HR, 1.09; 95% CI, 0.70–1.70; P = .71). Predominantly, coagulase-negative staphylococci (∼80%) were documented as the causative pathogens for CRBSI without differences in both groups. Approximately half of the CVCs inserted during neutropenia were removed during neutropenia (190 of 400, 47.5%), while this was only the case in one-third of the CVCs inserted in nonneutropenic patients (134 of 400, 33.5%; P < .001). At the time of CRBSI diagnosis, we found no significant impact of neutropenia during CVC insertion (38 of 45 [84.4%] vs 38 of 50 [76.0%]; P = .44). We found no positive impact for chlorhexidine-coated CVC dressings compared to standard dressings (HR, 0.92; 95% CI, 0.48–1.75; P = .79) regarding CRBSI probability on day 21 for patients with neutropenia at the time of CVC insertion. The same could be shown for patients without neutropenia at the time of CVC insertion (HR, 0.75; 95% CI, 0.41–1.39; P = .36).
Table 1. Demographics and Characteristics of CVCs and CRBSI Patients
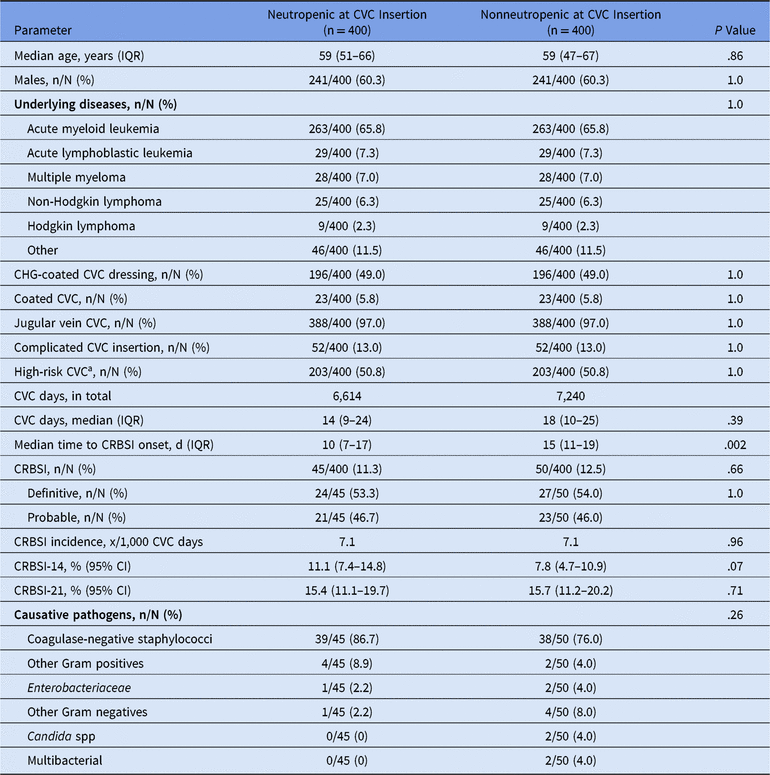
Note. CVC, central venous catheter; CRBSI, central venous catheter-related bloodstream infection; IQR, interquartile range; CHG, chlorhexidine gluconate; CRBSI-14, cumulative CRBSI probability at day 14; CRBSI-21, cumulative CRBSI probability at day 21; 95% CI, 95% confidence interval.
a 1 point for male or complicated CVC insertion; 2 points for diagnosis of acute myeloid leukemia, multiple myeloma or non-Hodgkin lymphoma; high risk, 3–4 points.Reference Schalk, Toelle and Schulz 7
In multivariate Cox regression analysis, persisting neutropenia from CVC insertion until CRBSI diagnosis (HR, 1.73; 95% CI, 1.09–2.73; P = .019) and neutropenia at the time of CRBSI diagnosis (HR, 2.57; 95% CI, 1.33–4.95; P = .005) were independent risk factors for CRBSI, but neutropenia at the time of CVC insertion (P = .89) or resolution of neutropenia from CVC insertion until CRBSI diagnosis (P = .93) had no impact. Furthermore, use of chlorhexidine-coated CVC dressings (P = .19) or coated CVCs (P = .91) did not influence CRBSI probability in our multivariate analysis.
Here, we provide data on the potential impact of neutropenia at the time of insertion of short-term, nontunneled CVCs in a large cohort of patients at high risk for CRBSI. According to our registry data, CVC insertion during neutropenia is safe and feasible and not associated with an increased CRBSI risk but with an earlier CRBSI onset. Notably, use of chlorhexidine-coated CVC dressings or coated CVCs does not significantly prevent CRBSI in patients with hematological malignancies at high risk for CRBSI. However, presence of neutropenia at the time of CRBSI diagnosis is still associated with higher morbidity, which highlights the importance of careful CVC handling and management in this vulnerable patient cohort.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.



