Appropriate use of antibiotics through antibiotic stewardship is essential to curb the increase in antimicrobial resistance.Reference Holmes, Moore and Sundsfjord1,Reference Dyar, Huttner, Schouten and Pulcini2 Clinicians often face uncertainty whether a bacterial infection is present or not in a given patient. Biomarkers such as procalcitonin (PCT), by serving as decisional support, may potentially help avoid unnecessary antimicrobial prescribing.Reference Christ-Crain and Müller3,Reference Neeser, Branche, Mueller and Schuetz4
Evidence from meta-analyses of randomized clinical trials (RCTs) suggests that PCT-guided therapy reduces antibiotic use without harm to patients and may even have potential benefits.Reference Schuetz, Wirz and Sager5 The vast majority of these studies were, however, undertaken in patients with lower respiratory tract infections (LRTIs) in the emergency department or sepsis/septic shock in the intensive care unit (ICU).Reference de Jong, van Oers and Beishuizen6,Reference van der Does, Rood, Haagsma, Patka, van Gorp and Limper7
Diagnostic stewardship, that is, the responsible use of diagnostic tests to avoid unnecessary costs and negative consequences associated with false-positive findings, is also a key priority for healthcare systems in an era in which “less is more” and where “smarter medicine” campaigns advocate for rational use of resources.Reference Lehmann, Berner and Bogner8 Overuse and misuse of procalcitonin is frequent in routine practice,Reference Garin, Poffet, Harbarth and Perrier9 as is the case with many other diagnostic tests.Reference Huang, Yealy and Filbin10,Reference Bremmer, Moffa and Ma11 Furthermore, its added value over C-reactive protein remains controversial.Reference Afshari and Harbarth12,Reference Albrich and Harbarth13
The primary objective of this study was to analyze the effect of restricting PCT measurements on antibiotic use. Secondary objectives were to assess the impact of the restriction on clinical outcomes and costs.
Methods
This quasi-experimental study was conducted in Geneva University Hospitals (HUG), a tertiary care center in Switzerland. HUG had an antibiotic stewardship program over the entire study period with regularly updated guidelines, review of all positive blood cultures, and dedicated stewardship rounds in certain high-risk wards (eg, ICU). Although the infectious diseases (ID) service issued guidance on the use of PCT (Appendix online), PCT results were not reviewed by the ABS program. The estimated PCT reagent (technical laboratory supplies) cost in our hospital is ~400’000 CHF (US$408,998) per year, which represents 2.4% of the global annual reagent budget. In contrast, total antibiotic expenditure is ~1.5 million CHF (US$1.53 million) per year. Because of this substantial burden, PCT measurements were restricted as of February 3, 2016, except for the pediatric emergency department, the adult and pediatric ICUs and transplant units. PCT measurements could still be prescribed in other wards with prior approval by an ID consultant and the head of the laboratory division, at their discretion.
We obtained numbers of PCT measurements from the laboratory database as well as patient-level (admission/discharge dates, ward location, in-hospital mortality) and systemic (oral and parenteral) antibiotic administration (ATC class J01 and oral metronidazole) data from the electronic health record system.
We included patients in all inpatient wards (and the emergency department) in HUG, except those with preintervention low PCT test usage, defined as <1 PCT measurement per week, on average, over a 12-month period.
The primary outcome was the change in antibiotic use in defined daily doses (DDD) per 1,000 patient days (PD) per month. The secondary outcomes included days of therapy (DOT) per 1,000 PD per month, in-hospital mortality, length of stay (LOS), and cost savings.
Statistical analysis
We conducted an interrupted time-series analysis of monthly rates of antibiotic use in DDD per 1,000 PD before and after the start of the intervention using a Prais-Winsten regression, which is based on generalized least-squares method accounting for serial autocorrelation. We used the Durbin-Watson d statistic to evaluate how well the model took into account first-order correlation. We hypothesized the absence of an impact on antibiotic use and overall in-hospital mortality in wards where PCT was discontinued.
We calculated monthly LOS as a ratio of total patient days and number of admissions. In-hospital mortality was calculated as a monthly proportion of deaths by admission. We estimated the cost savings as a percentage reductions of PCT-reagent–related costs and in percentage reductions of the department’s global reagent costs. Costs are provided in Swiss Francs (CHF) and US dollars (US$) with an exchange rate of 1CHF = US$0.978 (ie, the average exchange rate for 2018, OECD).
The study period was from January 2014 to May 2018, with >24 data points on either side of the intervention, which provided sufficient statistical power to analyze the primary outcome.Reference Bernal, Cummins and Gasparrini14 Data on in-hospital mortality were only available from January 2015 onward. All statistical analyses were performed using Stata version 14 software (StataCorp, College Station, TX) and, in particular, the “itsa” command.
Ethics
The study was approved by the Ethics Committee of Canton of Geneva (no. 2017-02274), which granted a waiver of informed consent.
Results
After the intervention, we observed an immediate statistically significant decrease in the level of PCT measurements per month (−637.4; 95% CI, 539.7–735.0) without a statistically significant change in slope (−0.05; 95% CI, −8.2 to 8.1) (Fig. 1A; Supplementary Tables 1 and 2 online).
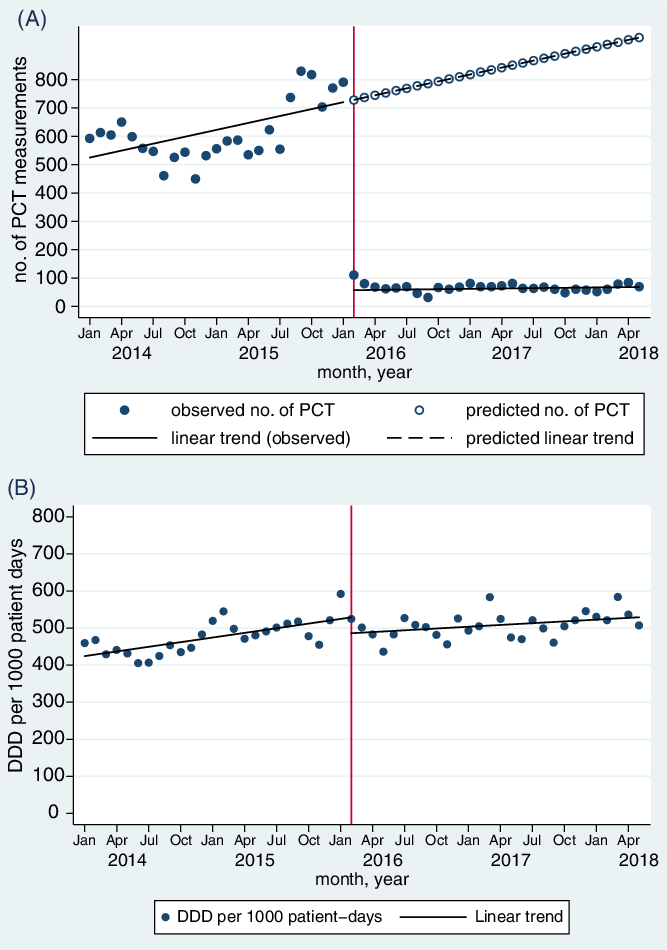
Fig. 1. Changes in (A) monthly number of procalcitonin (PCT) measurements; (B) monthly rate of antibiotic consumption, measured in defined daily doses (DDD) per 1,000 patient days, before and after restriction of PCT measurements.
Before the intervention, we observed an increasing trend of monthly antibiotic use of 4.3 DDD per 1,000 PD (95% CI, 1.4–7.1). After the intervention, there was a nonsignificant decrease in level of antibiotic use by 47 DDD per 1,000 PD (95% CI, −1.0 to 95.6), followed by an upward change in slope of 1.6 DDD per 1,000 PD (95% CI, −0.2 to 3.5) which was not statistically significant (Fig. 1B; Supplementary Table 2 online). The Durbin-Watson d statistic changed from 1.25 to 1.67, indicating that the model performed well despite residual positive autocorrelation. When analyzed by DOT, there was significant decrease in level of 36.6 DOT per 1,000 PD (95% CI, 2.8–70.3) and a significant decrease in slope of 3.6 DOT per 1,000 PD (95% CI, 1.0–6.1) after the intervention (Supplementary Table 2 and Supplementary Fig. 1 online).
During the study period, we observed a pre-existing decreasing trend of in-hospital mortality from 7.40 deaths per 100 admissions to 5.02 deaths per 100 admissions (P < .001), with no significant change associated with the intervention (Supplementary Table 2 and Supplementary Fig. 2 online). There was also a pre-existing decreasing trend in LOS from 5.12 to 3.77 days (P < .001) over the study period, with a significant decrease in level after the intervention of 0.4 days (95% CI, 0.2–0.6) (Supplementary Table 2 and Supplementary Fig. 2 online).
The annual PCT-reagent costs decreased from ~400,000 CHF (~US$409,000) to 100,000 CHF (~US$102,000), corresponding to a 75% decrease. The proportion of PCT reagent costs in the global department reagent budget decreased from 2.4% to 0.8%.
Discussion
In this study, the restriction of PCT measurements in a large tertiary-care center in Switzerland did not influence overall antibiotic use and was not associated with increases in LOS or mortality. Meanwhile, the intervention led to significant cost savings.
Although PCT has been repeatedly suggested as means to reduce antibiotic exposure and potentially mortality, mainly in LRTIs, in meta-analyses of RCTsReference Schuetz, Wirz and Sager5,Reference Meier, Branche and Neeser15 the value of PCT in real-world clinical settings has been questioned. A study including >20,000 septic ICU patients suggested that PCT use was associated with increased antibiotic consumption and poorer clinical outcomes,Reference Chu, Mehta and Walkey16 highlighting either the importance of improving implementation of adequate PCT-based strategies prior to widespread adoption or as “worst-case scenario,” the limited clinical value of PCT use.
During the study period, we observed pre-existing decreasing trends in mortality and LOS, with no effect of the intervention on in-hospital mortality but a decrease in level of LOS after the intervention. The latter effect may be an artefact; it is unclear how PCT prescription restriction would be associated with decreased LOS. Nevertheless, it is reassuring that we did not observe increases in in-hospital mortality and LOS associated with the intervention.
The main strength of our study was that the “top-down” decision to discontinue PCT measurements offered a natural quasi-experimental study design; this allowed the use of interrupted time-series, which is a robust methodology.Reference de Kraker, Abbas, Huttner and Harbarth17 One of the limitations of this study is that it evaluated hospital-level changes in antibiotic prescribing practices and therefore was prone to ecologic bias. Our negative finding does not exclude the possibility that PCT may have an impact on the management of individual patients. Also, we did not evaluate protocol adherence (ie, whether PCT levels influenced prescribing behavior), even though we suspect it to be low, as in other real-world studies.Reference Garin, Poffet, Harbarth and Perrier9,Reference Farooq and Colón-Franco18 However, since one aim of antimicrobial stewardship is to reduce overall antibiotic use, we feel that the ecologic perspective is still justified. Finally, our single-center study was conducted in a setting where antibiotic use is relatively low; thus, the generalizability of our results may be limited.
Our results do not suggest that PCT is useless; other evidence suggests that when used in specific indications, it may be an effective tool to reduce antibiotic exposure, and thus may be cost-effective.Reference Westwood, Ramaekers and Whiting19 Appropriate, evidence-based, indications of PCT include (1) initiation of antibiotic therapy in acute LRTI, including community-acquired pneumonia and COPD exacerbation and (2) reducing duration of antibiotic therapy in ICU patients with ventilator-associated pneumonia or sepsis. Like all diagnostic tests, PCT needs to be used intelligently and in conjunction with other information to have a real impact on patient outcomes. To prevent overuse and misuse of PCT, institutions may need “diagnostic stewardship” teams to enforce restrictions on use or provide real-time review of cases. Further real-life diagnostic-stewardship studies on how to implement PCT-guided therapy are needed to further inform best practices.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2020.1314
Acknowledgments
We would like to thank Dr Angela Huttner (Division of Infectious Diseases, University of Geneva Hospitals, Geneva, Switzerland) for providing input regarding the elaboration of the protocol.
Financial support
This work was partly supported by the Research Fund of the Department of Internal Medicine of the University Hospital and the Faculty of Medicine of Geneva; this Fund received an unrestricted grant from AstraZeneca Switzerland.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.




