Introduction
Pharmacoequity is defined as the goal of ensuring that all individuals, regardless of race, ethnicity, and socioeconomic status, have access to the highest-quality medications required to manage their health needs. Pharmacoequity is increasingly recognized as a priority to reduce health disparities. Reference Essien, Dusetzina and Gellad14 Antibiotics are the most commonly prescribed class of medications to children Reference Hales, Kit, Gu and Ogden15 and are crucial in the management of infections. Racial and ethnic variations in antibiotic prescribing to children are well-reported in outpatient, emergency department, and urgent care settings. Reference Gerber, Prasad and Localio1–Reference Nedved, Lee and Wirtz6 In studies evaluating ambulatory prescribing data, White children consistently receive more antibiotics than children of other races or ethnicities, both at a population level Reference Fleming-Dutra, Shapiro, Hicks, Gerber and Race2,Reference Wattles, Vidwan, Feygin, Jawad, Creel and Smith7,Reference Kilgore, Lanata and Willis8 and within healthcare systems. Reference Gerber, Prasad and Localio1,Reference Wattles, Feygin and Jawad5,Reference Nedved, Lee and Wirtz6
Because race is a social construct with no biological meaning, Reference Yudell, Roberts, DeSalle and Tishkoff9 these differences likely reflect non-clinical factors and potential inequities in over or under utilization. Although demographic and between-hospital variations in antibiotic utilization are reported in pediatric outpatient settings, Reference Gerber, Newland and Coffin10,Reference Griffith, Dantuluri and Thurm11 little is known about racial and ethnic patterns of antibiotic utilization in inpatient settings. In a recent scoping review examining race and antibiotic prescribing, only one of 61 eligible studies was conducted in an acute care setting. Reference Kim, Kabbani and Dube12 The study, an evaluation of adults with skin and soft tissue infections, found that race was associated with differential management. Reference Wurcel, Essien and Ortiz13 The authors of the review provide a framework of factors contributing to health inequities in antibiotic prescribing in the United States (US), including national, community, healthcare, and individual factors. Reference Kim, Kabbani and Dube12
The frequency of antibiotic prescribing in hospitalized children, and the gap in literature on racial and ethnic differences in inpatient antibiotic prescribing, makes this an important area to study inequities. Identifying variations and inequities in treatment is an important step toward understanding downstream racial and ethnic disparities in health outcomes during and after hospitalization. Our objective was to provide a broad overview of national inpatient antibiotic utilization among children by race and ethnicity. We hypothesized that there would be racial and ethnic variations in antibiotic prescribing to children in hospital settings. The aforementioned framework Reference Kim, Kabbani and Dube12 guided variable selection for our study.
Methods
Data source
This study was a retrospective, observational, cross-sectional study of the Pediatric Health Information System (PHIS), an administrative database managed by Children’s Hospital Association (Lenexa, KS) that contains data from 46 tertiary referral children’s hospitals in the US. The database captures demographics, diagnoses and procedures (using the International Classification of Diseases version 10), and daily billing data for all inpatient, observation, emergency room, and ambulatory surgery centers at participating hospitals. The study was reviewed by the University of Louisville Institutional Review Board and classified as Non-Human Subjects Research.
Study population
We collected hospitalization (inpatient and observation) data for all patients <20 years old who were admitted to a participating PHIS hospital between January 1, 2022 and December 31, 2022. All patients with an inpatient hospitalization encounter within these age limits and timeframes were included. Data were analyzed at the individual-visit level, meaning that patients could have more than one included visit within the study period.
Exposure
The exposure of interest was patient race and ethnicity. Race and ethnicity in PHIS are submitted by hospitals based on local hospital collection practices (e.g., parent/guardian self-report, assignment at registration) and categorized separately as race (White, Black, Asian, Native Hawaiian or Other Pacific Islander, American Indian or Alaska Native, other) and ethnicity (Hispanic or Latino, not Hispanic or Latino (NH)). We combined race and ethnicity variables to create the following mutually exclusive categories: Hispanic (including any race), NH American Indian, NH Asian, NH Black, NH Native Hawaiian and Other Pacific Islander, NH White, and NH Other (including a non-listed race, more than one race, or missing race and ethnicity). Visits with an unassigned ethnicity were recategorized as NH and then classified by race. The majority group (NH White) was used as the reference group in comparison analyses, though we note that race is a social construct and does not imply that the majority group represents the preferred standard of care. In certain stratified analyses, NH American Indian, NH Asian, NH Native Hawaiian and Other Pacific Islander and NH Other were combined as “NH Other” due to low sample sizes, though our intention is not to imply that these groups experience the same potential social and structural drivers of differential antibiotic utilization.
Covariates
Other covariates included age at admission, sex, rural-urban continuum area based on each patient’s residential zip code (metropolitan, micropolitan, or rural), 16 diagnosis antibiotic frequency, hospital region (Midwest, Northeast, South, West), Child Opportunity Index (COI version 2.0) (classified as very low, low, moderate, high or very high opportunity areas), Reference Acevedo-Garcia, Noelke and McArdle17 payor (government, private, other), Pediatric Medical Complexity Algorithm (PMCA) (no chronic condition, chronic condition, complex chronic condition), Reference Simon, Cawthon and Stanford18 inpatient unit (NICU or PICU vs. non-ICU), and Hospitalization Resource Intensity Scores for Kids (H-RISK). To represent the likelihood that a patient’s diagnosis was associated with antibiotic administration, we determined the proportion of inpatient encounters associated with each All Patients Refined Diagnosis Related Group (APR-DRG) (3M Health, version 38) for which an antibiotic was prescribed. APR-DRGs for which >75% of inpatient encounters were prescribed antibiotics were labeled as “very high frequency”, >50%–75% as “high frequency”, >25%–50% as “intermediate frequency”, and 0%–25% as “low frequency” (eTable 1). The COI is an index of neighborhood resources and conditions that help children develop in a healthy way Reference Acevedo-Garcia, Noelke and McArdle17 and is classified based on residential zip code in PHIS. The COI combines the 3 subdomains of education, health-environment, and social-economic indices. H-RISK is a surrogate for severity of illness and is calculated by assigning cost-based relative weights to each APR-DRG and severity of illness level, enabling comparison of severity across APR-DRGs. Reference Richardson, Rodean, Harris, Berry, Gay and Hall19
We also chose five diagnostic groups—categorized by infection-related APR-DRG categories—to stratify for a secondary analysis: infections of the upper respiratory tract; bronchiolitis and respiratory syncytial virus (RSV) pneumonia; other pneumonia; cellulitis and other skin infections; and septicemia and disseminated infections.
Outcomes
The primary outcome was antibiotic use, which was measured (1) as the percentage of all hospitalizations that received an antibiotic, and (2) as days of therapy (DOT) per 1000 patient days. Antibiotic prescriptions were identified using billing data. Secondary outcomes included length of stay (LOS) in days, days of broad-spectrum antibiotic therapy per 1000 patient days (“Broad DOT”), and intravenous (IV) DOT per 1000 patient days (“IV DOT”). Broad-spectrum antibiotics were classified using previously published studies Reference Gerber, Newland and Coffin10,Reference Griffith, Dantuluri and Thurm11,Reference Gerber, Hersh, Kronman, Newland, Ross and Metjian21 and author consensus (eTable 2). DOT was calculated such that multiple antibiotics on the same calendar day each counted individually as a “day of therapy”; thus, DOT could exceed LOS for a given encounter.
Statistical analysis
We summarized visit characteristics, stratified by child race and ethnicity. Additional descriptive analyses were performed for percent of antibiotic visits by hospital, DOT outcomes, and outcomes by APR-DRGs of interest, all stratified by race and ethnicity. Outcomes by hospital and APR-DRGs were risk-adjusted using mean H-RISK scores for each racial-ethnic group within the individual hospital or diagnosis group. Pairwise chi-square tests were used to compare outcomes stratified by APR-DRGs of interest.
Mixed-effect logistic regression models were used to assess the likelihood of receiving an antibiotic, overall and stratified by race-ethnicity, after adjusting for covariates and accounting for clustering by hospital using random intercepts. A quasi-Poisson mixed-effect regression with a log-link was used to model antibiotic DOT, using LOS as the offset, and clustering by hospital using random intercepts. All regression models controlled for all covariates. Collinearity was assessed between all variables. To address the impact of variations in NICU or newborn nursery visits within our sample, we performed a sensitivity analysis excluding children less than 2 months of age. Analyses were conducted using R version 4.2.3 (2023–03–15 ucrt).
Results
There were 846,530 pediatric hospitalizations included in the analysis. Demographic characteristics and percentage of antibiotic visits for the overall study sample and stratified by race and ethnicity are presented in Table 1. Most hospitalizations were for NH White (45.2%) children, followed by Hispanic (27.1%), NH Black (19.2%), NH Other (4.5%), and NH Asian (3.5%) children. Children identified as NH NHPI and NH American Indian represented 2,665 (0.3%) and 2,054 (0.2%) visits, respectively. There were 15,672 visits (1.9%) with missing race and ethnicity variables, who were included in the NH Other category. The median length of stay was 2 days (IQR 1–4) for the study sample and across all racial-ethnic groups.
Table 1. Unadjusted visit demographics and antibiotic visits by race and ethnicity

NH, Non-Hispanic; Child Opportunity Index (a neighborhood measure of resources and conditions for healthy development) was assigned using patient zip code; CC, chronic condition.
1 Broad spectrum visit percentages are the proportion of antibiotic visits that included at least one order for a broad-spectrum antibiotic.
2 Antibiotic frequency for diagnosis is the proportion of encounters with a given APR-DRG code received inpatient antibiotics. “Very high” refers to APR-DRG codes for which >75% of encounters were associated with an antibiotic. “High” refers to APR-DRG codes for which >50%–75% of encounters were associated with an antibiotic. “Intermediate” refers to APR-DRG codes for which >25%–50% of encounters were associated with an antibiotic. “Low” refers to APR-DRG codes for which 0%–25% of encounters were associated with an antibiotic.
Overall, 41.6% of hospitalizations included an antibiotic. The percentage of antibiotic visits ranged from 38.4% in NH Black children to 47.5% in NH NHPI children. Compared to NH White children, who had an antibiotic visit rate of 42.4%, Hispanic children had a higher antibiotic visit rate (42.9%, P <.001) and NH Black children had a lower rate (38.4%, P <.001). Among children prescribed an antibiotic, NH Black children had a higher rate of broad-spectrum antibiotic visits (72.9%) compared to NH White children (71.2%, P <.001). Among NH White children, antibiotic visits decreased with increasing COI, but a reverse relationship was observed for NH Black children. H-RISK-adjusted variations in antibiotic visits by race and ethnicity across and within individual hospitals are shown in Figure 1.

Figure 1. Hospitalization Resource Intensity Scores for Kids (H-RISK)-adjusted racial and ethnic variations by individual hospital. Adjusted by case mix index (using mean H-RISK for each racial-ethnic group within each hospital). Hospitals are ordered by percent of antibiotic visits for NH Black children (lowest to highest). The outlier hospital with <10% of visits including an antibiotic had >50% of visits in children ages <2 months.
The H-RISK-adjusted distribution of DOT per 1000 patient days, IV DOT, and broad-spectrum DOT stratified by racial-ethnic groups are shown in Figure 2. In general, compared to patients who were NH White, patients who were NH Black, NH Other, and NH Asian received fewer DOT, IV DOT, and broad-spectrum DOT, and patients who were NH NHPI received more DOT, IV DOT, and broad-spectrum DOT.

Figure 2. Days of therapy by race-ethnicity. NH NHPI = Non-Hispanic Native Hawaiian/other Pacific Islander; NH AI = Non-Hispanic American Indian. Error bars represent standard error of the mean.
Adjusted outcomes
Adjusted odds of receiving an antibiotic and incident rate ratios of antibiotic DOT are shown in Figure 3, and presented in eTable 3. Adjusting for all covariates, NH Black children had lower odds of an antibiotic visit compared to NH White children (aOR 0.96 [95% CI 0.94–0.97]), while NH NHPI had higher odds of an antibiotic visit (aOR 1.16 [95%CI 1.05–1.29]). Compared to NH White children, NH NHPI children received more antibiotic DOT (adjusted incident rate ratio [aIRR] 1.06 [95%CI 1.04–1.08]), while patients who were Hispanic (aIRR 0.99 [95%CI 0.99–0.99]), NH Other (aIRR 0.97 [95%CI 0.96–0.98]), NH Asian (aIRR 0.94 [95%CI 0.93–0.94]), and NH American Indian (aIRR 0.99 [95%CI 0.98–0.99]) received fewer antibiotic DOT (Figure 3, eTable 4). A sensitivity analysis excluding infants <2 months old revealed similar findings (eTable 5).
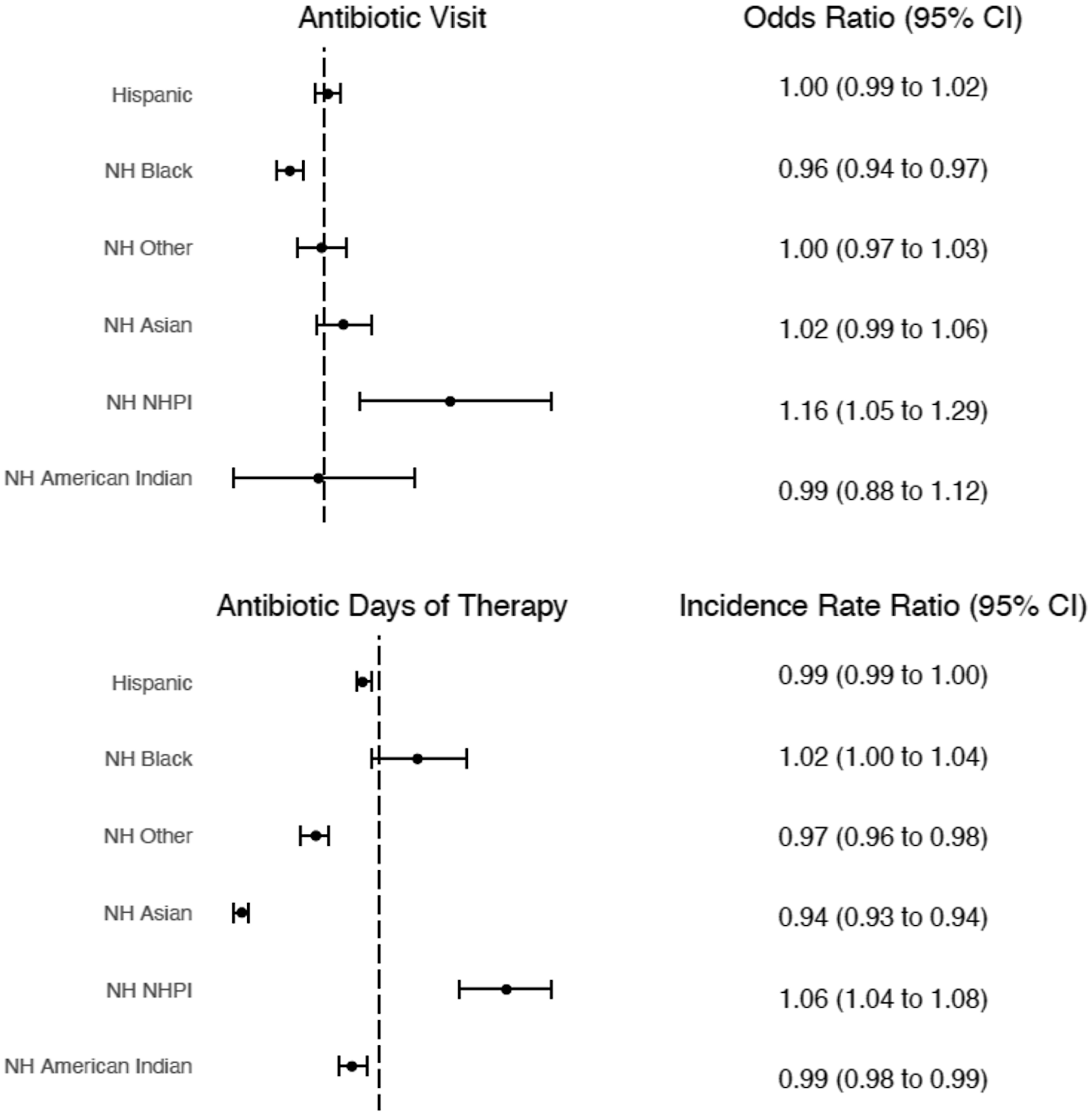
Figure 3. Mixed-effects multivariable regression models. Reference group = NH White. Models control for age, sex, RUCA, COI, antibiotic frequency, region, payor, PMCA, location, and H-RISK, and account for clustering by hospital. No collinearity was found among covariates.
Diagnoses of interest
The most common infectious diagnoses identified by APR-DRGs were bronchiolitis and RSV pneumonia (n = 44,930), infections of the upper respiratory tract (n = 18,823), other pneumonia (n = 17,220), cellulitis and skin infections (n = 8,221), and septicemia and disseminated infections (n = 8,067). Collectively, these APR-DRGs account for 11.5% of all hospitalizations. H-RISK-adjusted variations in antibiotic use by race-ethnicity and diagnoses are shown in Figure 4, using a composite “NH Other” category due to limited representation of several racial and ethnic groups in encounters with these APR-DRG codes. Trends across racial and ethnic groups within diagnoses were similar in a risk-adjusted analysis; however, trends in antibiotic variations were not consistent across diagnoses. Additional unadjusted outcomes by diagnosis group, including DOT and H-RISK, are provided in eTable 6.

Figure 4. Hospitalization Resource Intensity Scores for Kids-adjusted racial-ethnic stratification of antibiotic visits by diagnosis. The following categories were combined as “NH Other” due to small sample sizes: NH Other, NH Asian, NH Native Hawaiian/Pacific Islander, NH American Indian.
Discussion
Our analysis of more than 800,000 hospitalizations in tertiary children’s hospitals across the US in 2022 found that antibiotic visits were lower in NH Black children compared to NH White children and that antibiotic DOT varied by race and ethnicity. These differences persisted after controlling for covariates, including socioeconomic status, illness severity, and the presence of chronic conditions. These findings are important for hospital and antimicrobial stewardship program leadership seeking to improve pharmacoequity for hospitalized children.
Our study was not designed to determine whether racial variations in antibiotic utilization represent health disparities (preventable differences in the burden of disease or opportunities to achieve optimal health), or health inequities (particular types of health disparities that stem from unfair and unjust systems, policies, and practices and limit access to the opportunities and resources needed to live the healthiest life possible). Reference Kim, Kabbani and Dube12 Health disparities are complex and represent multiple domains and levels of influence. 22 Differential access to care or medications is a common driver of health disparities. Reference Essien, Dusetzina and Gellad14 Our study identified variations in treatment within the hospital visit, and differential access and utilization of antibiotics within hospitals may be a source of differential health outcomes. A variety of mechanisms plausibly lead to these observed differences, including structural racism and its effects on pre-hospital conditions and illness severity, implicit biases on the part of care teams, disparate access to subspecialty care, and differences in communication between caregivers and clinical teams.
To assess the intersectionality of race and socioeconomic status, we explored outcomes by COI. In unadjusted analyses, racial variations in antibiotic utilization between NH Black and NH White children remained across all COI levels. In our unadjusted analysis, the direction of antibiotic visit trends by COI was opposite in NH White and NH Black children, possibly suggesting that area opportunity and socioeconomic status affect care access and utilization in different ways for these populations. Similar findings were reported in an analysis of outpatient antibiotic prescribing. Reference Wattles, Feygin and Jawad5 In adjusted models, we did not find an independent association between COI and odds of receiving an antibiotic, suggesting that this area-based composite marker of socioeconomic status contributes less to inpatient antibiotic utilization than patients’ demographic characteristics and insurance status—a more direct marker of socioeconomic status.
In an exploratory analysis of select diagnoses, we found that racial and ethnic variations and directions of variations in antibiotic utilization are not consistent across diagnoses. The most common APR-DRG in our study, bronchiolitis and RSV pneumonia, demonstrates similar patterns of racial and ethnic differences in antibiotic utilization as another study using PHIS data that evaluated disparities in bronchiolitis management. Reference Honcoop, Poitevien, Kerns, Alverson and McCulloh23 Studies in outpatient settings consistently report higher variability in antibiotic utilization by race and ethnicity for indications in which antibiotics are not always indicated. Reference Fleming-Dutra, Hersh and Shapiro24–Reference El Feghaly, Herigon and Kronman26 Similarly, we report higher variability (though inconsistent directions of variability) in antibiotic visits for bronchiolitis and RSV pneumonia, infections of the upper respiratory tract, and other pneumonia. As in outpatient settings, these findings suggest that racial and ethnic differences were more pronounced for conditions in which antibiotic use was not always indicated. Stewardship programs seeking to ensure appropriate use or avoidance of antibiotics for specific diagnoses (e.g., RSV bronchiolitis) may directly influence pharmacoequity through standardization of antibiotic policies. Furthermore, stewardship programs that track and report differences in inpatient prescribing by race and ethnicity may counteract the potential for unconscious biases to lead to differential antibiotic treatment plans for hospitalized children. Notably, an examination of diagnosis-specific antibiotic utilization and appropriateness was beyond the scope of our analysis; such analyses for key diseases in which antibiotics are often over- or under-utilized could reveal specific areas for equity-oriented stewardship interventions.
Over the past decade, stewardship programs have successfully reduced antibiotic utilization, Reference Zay Ya, Win, Bielicki, Lambiris and Fink30 with the intent of decreasing unnecessary use and the subsequent development of antimicrobial resistance. However, without careful consideration of health equity principles, well-meaning efforts could increase disparities in treatment among vulnerable, racial and ethnic minority populations. In a recent review, Cichon and colleagues outline the following guidance, “Interventions to reimagine antimicrobial stewardship through a lens of equity”: (1) Improved access to antimicrobial stewardship; (2) Reduction in antimicrobial prescribing disparities; (3) Public health campaigns; and (4) Representation in decision-making. Reference Cichon, Green, Hilker and Inclusion31 Our findings confirm the opportunity for stewardship programs to stratify antibiotic use data by race and ethnicity as reported by the patient. In partnership with local health equity collaborators, hospital stewardship programs are well-positioned to address racial and ethnic inequities through their broad reach across service lines, collaborative efforts with quality improvement, and influential reporting to hospital administrators.
As a broad overview of patterns of inpatient antibiotic prescribing in pediatric settings, our analyses highlight questions that should be investigated in further studies. First, we did not aim to determine drivers of antibiotic utilization variations. It is possible that racial and ethnic differences in inpatient antibiotic utilization are pronounced for key diagnoses that may, but do not always, merit antibiotics (e.g., children admitted with asthma exacerbations in the setting of possible lower respiratory tract infections). Second, condition-specific studies are important to examine racial and ethnic differences in the appropriateness of antibiotic utilization. Alignment of specific antibiotics with specific diagnoses—and racial and ethnic differences in appropriate alignment—should be the focus of future diagnosis-specific investigation to identify differences amenable to antibiotic stewardship interventions. Third, future work could study differences among diagnoses for which there are national guidelines to determine differences in guideline adherence. Fourth, mixed-methods studies, with input from patients and families, could evaluate hypotheses about drivers and the impact of racial variations in antibiotic utilization.
Our study has several limitations. First, our data were obtained from an administrative and billing database and do not include detailed clinical information about patient encounters. We were unable to assess the appropriateness of antibiotic utilization, the accuracy of the assigned APR-DRGs or association of the antibiotic with the assigned APR-DRG. Second, as a large database, there may be incomplete data and errors in billing coding. Moreover, reporting of race and ethnicity within the dataset is not consistent across included hospitals and does not precisely capture multi-racial and ethnic backgrounds. Third, PHIS only includes data from freestanding US children’s hospitals and is not nationally representative. Therefore, findings may not be generalizable. Fourth, we elected to present relative likelihood of receiving an antibiotic and results of regression models as odds ratios to facilitate comparison with other equity literature. Notably, odds ratios may overestimate relative risk when outcomes are relatively common; thus, the magnitude of effect estimates from our logistic regression analysis should be considered illustrative. Fifth, although we identified significant associations between race and ethnicity and antibiotic utilization, as a retrospective cross-sectional analysis, we are unable to assess causality in these relationships. Recognizing that race and ethnicity are social constructs, future studies should explore social determinants of health, provider bias, and other potential aspects of structural racism and discrimination as potential drivers of variation in antibiotic utilization. Finally, it is important to be cautious in interpreting findings for groups representing a smaller proportion of our sample (NH Asian, NH Native Hawaiian/Other Pacific Islander, and NH American Indian).
Conclusion
Inpatient antibiotic utilization in US children’s hospitals differs by race and ethnicity and diagnosis. Inpatient antimicrobial stewardship programs could stratify local antibiotic use data by race and ethnicity. This type of stratification would shed further light on how differences in antibiotic utilization may contribute to inequities in hospitalization outcomes. Additional research is needed to examine differences by individual diagnoses, clinical outcomes, and drivers of variation.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/ice.2024.168.
Financial support
None.
Competing interests
BAW, JF, KJ, and MJS received grant support from Merck, unrelated to the present study. MJS receives funding support from Pfizer for clinical trials. All other authors report no conflicts of interest relevant to this article to disclose.








