Due to war, political persecution, and national instability, increasing numbers of refugees from the Near and Middle East, Africa, and the Balkans have sought for asylum in many European countries, especially Germany. In 2015, Germany experienced the largest asylum surge and arrival of refugees since World War II. The number of registered asylum seekers reached nearly half a million, most coming from Syria, but almost as many remain unregistered. Approximately 25% of asylum seekers are children.Reference Flüchtlinge 1 As a result of the withdrawal of American troops stationed in the Rhein-Neckar area in previous years, the city of Mannheim/Baden-Württemberg was able to provide one of the largest hosting facilities for refugees in Germany. Thus, increased visit and admission rates of refugees for various causes accrued.
Early and sufficient medical care for refugees is essential. Especially among children, who frequently suffer from infectious diseases, antibiotic therapy is frequently prescribed, but it may be less effective if the illness is caused by antibiotic-resistant organisms.Reference Logan 2 Treatment of infections caused by multidrug-resistant organisms (MDROs) is even more difficult in children because antibiotic treatment options are limited. Colonization with an MDRO has also been described among international travelers returning to Germany.Reference Hassing, Alsma, Arcilla, van Genderen, Stricker and Verbon 3 , Reference Lubbert, Straube and Stein 4 In Syrian war-injured patients, MDROs have also been isolated frequently.Reference Teicher, Ronat and Fakhri 5 Therefore, prevention strategies to avoid colonization and subsequent infection are warranted.
International uniform recommendations for MDRO screening in hospitalized patients from MDRO endemic countries do not exist. In Germany, the Commission for Hospital Hygiene and Infection Prevention (KRINKO) at the Robert Koch Institute (RKI), the German Society of Pediatric Infectious Diseases (DGPI), the Society of Tropical Pediatrics and International Child Health, and the Professional Association of Pediatrics recently published recommendations for refugee screening. 6 , Reference Pfeil, Kobbe, Trapp, Kitz and Hufnagel 7 Here, we report a high frequency of MDROs among pediatric refugees hospitalized at the University Children’s Hospital in Mannheim, Germany.
METHODS
Study Population
In this retrospective analysis, we reviewed laboratory screening results of all pediatric refugees seen at our hospital from October 2015 to March 2016 that were hospitalized in the Department of Pediatrics and the Department of Pediatric Surgery at the University Hospital Mannheim for various causes. Resident neonatal and pediatric patients with MDROs were excluded from the analysis. Routine screening procedures were conducted in accordance with recently published screening recommendations and already implemented institutional guidelines. 6 , Reference Pfeil, Kobbe, Trapp, Kitz and Hufnagel 7 Children were screened for multidrug-resistant Gram-negative (MRGN) bacteria (all patients) and VRE (oncologic patients only) via rectal swab and for MRSA (all patients) via nasopharyngeal swab. Most of the refugees were directly placed on contact precautions during the hospital stay. If the patient could not initially be isolated (due to lack of room capacity) and an MDRO was found after admission, contact precautions were initiated subsequently. The main demographic features as well as data related to the reason for admission were collected retrospectively by chart review.
Microbiological Investigation
According to the phenotypic definition of the KRINKO recommendation, Enterobacteriaceae detected by rectal swab were classified as 2MRGN/ESBL, 3MRGN, or 4MRGN. 8 , 9 Gram-negative bacteria resistant against 2, 3, or 4 antibiotic groups (penicillins with piperacillin as surrogate substance, cephalosporins with cefotaxime and/or ceftazidime as surrogate substance, and fluoroquinolones with ciprofloxacin as surrogate substance) were characterized as 3MRGN, while bacteria characterized as 4MRGN had additional resistance against carbapenems, with imipenem and/or meropenem as surrogate substance. In children, fluoroquinolones cannot be applied routinely, thus, the 2MRGN antibiotic group was implemented especially for neonates.
Rectal swabs were cultivated for MRGN on selective media: CHROMAgar ESBL, (MAST Diagnostica, Merseyside, UK) and Cetrimid-Agar (bioMèrieux, Marcy-l'Étoile, France). For identification and susceptibility testing of resistant colonies, VITEK 2 (bioMèrieux) with Clinical and Laboratory Standards Institute (CLSI) interpretative standards were used. Decreased carbapenem susceptibility in Enterobacteriaceae was confirmed using molecular methods at the National Reference Center for Gram-negative bacteria. Nasopharyngeal swabs for MRSA detection were plated on ChromID MRSA Agar (bioMèrieux). Rectal swabs for VRE detections were plated on VRE Select Agar (BioRad, Berkeley, CA, USA).
RESULTS
In the course of 2015, an increasing number of refugees were accommodated in different refugee camps in Mannheim, Germany. Most of the refugees in the second half of 2015 came from Syria (40.2%), Afghanistan (16.1%), Iraq (13.5%), the Gambia (7.3%), Albania (4.3%), Iran (2.7%), Nigeria (2.7%) and from 19 other countries (13.2%). In the same time period, 28.1% of the refugees registered during an initial health investigation were children and adolescents (0–3 years, 5.6%; 4–6 years, 4.2%; 7–10 years, 5.2%; 11–15 years, 5% and 16–18 years, 8.1%).
We analyzed all pediatric refugees admitted to the Department of Pediatrics and the Department of Pediatric Surgery with respect to MDRO colonization. In total, 325 children were hospitalized; of these, 110 (33.8%) had a positive MDRO screening result. The numbers of hospitalized patients with a positive MDRO screening result were similar in both pediatric departments in 2015 and 2016. In the Department of Pediatrics, 66 of 179 hospitalized children (36.8%) were positive for an MDRO in 2015 and 27 of 77 hospitalized children (35.1%) were positive for an MDRO in 2016. In the Department of Pediatric Surgery, 11 of 40 (27.5%) were positive for an MDRO in 2015 and 6 of 29 (20.7%) patients were positive for an MDRO in 2016. However, the majority of patients were seen in pediatric emergency (881 patients) and on an outpatient basis (122 patients) without the necessity of admission. Among the 110 hospitalized children, 62 (56.4%) were male and 48 (43.6%) were female; the median age was 21 months (range, 1–202 months). The country distribution of hospitalized children reflected the general country distribution of all refugees in Mannheim; these children were refugees from Syria (51.8%), Afghanistan (22.9%), Irak (21.7%), Iran (1.2%), Bosnia (1.2%), and Nigeria (1.2%).
Between October 2015 and March 2016, the MDRO screening results of pediatric refugees in the Departments of Pediatrics and Pediatric Surgery revealed a peak of positive results in November and December 2015, which correlated with the high number of refugees in Mannheim during that time period (Figure. 1). The number of isolates decreased over the course of 2016, also corresponding to the decreasing hospitalized refugee numbers and total number of refugees in Mannheim. The rates of MDRO-colonized patients at our institution were similar between October 2015 and February 2016: October 2015, 0.29%; November 2015, 0.24%; December 2015, 0.26%; January 2016, 0.20%; February 2016, 0.24%. Only in March did we observe an unexplained decrease (0.08%) in the colonization rate.
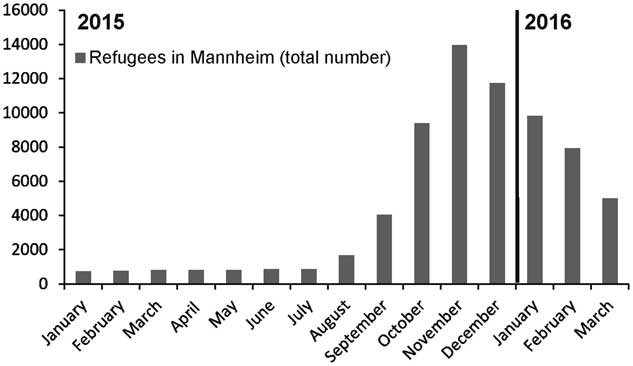
FIGURE 1 Number of refugees (adults and children) located in refugee camps in Mannheim, Germany, between January 2015 and March 2016.
The majority of patients were colonized with MRGN bacteria (113 isolates), mostly 2MRGN/ESBL (87 isolates), and in a minority were colonized with MRSA (22 isolates), and 1 patient was colonized with VRE (Table 1). If a patient was readmitted to the hospital in 2015 or 2016, each isolate was counted only once. Escherichia coli 3MRGN was detected in 22 of 136 (16.2%) of the isolates; E. coli 4MRGN was detected in 2 of 136 (1.5%) of the isolates; and Klebsiella pneumoniae 3MRGN was detected in 2 of 136 (1.5%) of the isolates. Among 110 refugee patients, we detected single colonization with an MDRO in 84 patients (76.4%), co-colonization with 2 pathogens in 23 patients (20.9%), and triple colonization in 3 patients (2.7%). No MDRO isolates of Pseudomonas aeroginosa, Acinetobacter baumanii complex, Citrobacter freundii, Enterobacter cloacae, Serratia macescens, or Morganella morganii were detected among hospitalized pediatric refugees.
TABLE 1 MDRO Screening Results of Pediatric Refugees in the Departments of Pediatrics and Pediatric Surgery Between October 2015 and March 2016
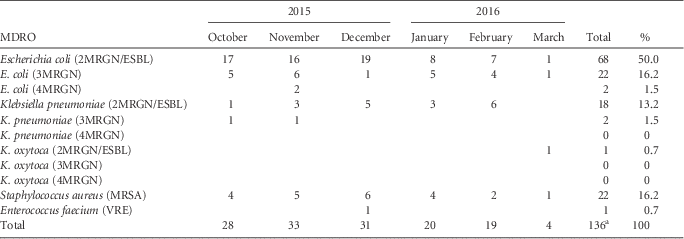
NOTE. MDRO, multiple-drug-resistant organism; ESBL, extended-spectrum β-lactamase; MRGN, multiple-drug-resistant Gram-negative, MRSA, methicillin-resitant Staphylococcus aureus; VRE, vancomycin-resistant enterococci.
a Among 110 refugee patients, we detected single colonization with an MDRO in 84 patients (76.4%), co-colonization with 2 pathogens in 23 patients (20.9%), and triple colonization in 3 patients (2.7%).
The majority of the hospitalized pediatric patients were admitted for infectious diseases such as gastroenteritis (33.9%), pneumonia (12.7%), bronchiolitis (8.1%), influenza (4.5%), and skin and soft-tissue infections (6.3%) (Table 2). In 3 patients colonized with MRSA, the pathogen was also the cause of skin and soft-tissue infection, and in 1 patient an E. coli 3MRGN was the cause of a pyelonephritis. Therefore, infections with MDRO in pediatric refugees were infrequently observed and occurred in only 3.6% of patients. All other patients were only colonized with an MDRO. Other children were hospitalized for neurological disorders (4.5%), hematologic/oncologic disorders (6.4%), surgery or due to trauma (9.1%), and other causes (1.8%).
TABLE 2 Causes of Hospitalization of Pediatric Refugees in the Department of Pediatrics and Pediatric Surgery Between October 2015 and March 2016

a In 3 patients colonized with MRSA, the pathogen was also the cause of a skin and soft-tissue infection, and in 1 patient an E. coli 3MRGN was cause of a pyelonephritis.
b Non-infectious causes for hospitalization included neurological disorders (5 cases; 4.5%), hematologic/oncologic disorders (7 cases; 6.4%), surgery/trauma patients (10 cases; 9.1%) and other causes (2 cases; 1.8%).
DISCUSSION
In this retrospective analysis of screened pediatric refugees, a large proportion of children (33.8%) were colonized with an MDRO. The most frequently detected pathogens were 2MRGN E. coli, followed by 3MRGN E. coli, MRSA, 2MRGN K. pneumonia, 3MRGN K. pneumonia, 4MRGN E. coli, and VRE. Only 4 infections caused by an MDRO were observed in screened pediatric refugees.
Screening for MRGN in non-neonates is generally not established. In neonates, it has already been demonstrated that surveillance can contribute in predicting Gram-negative and MRGN sepsis.Reference Parm, Metsvaht and Sepp 10 , Reference Mammina, Di Carlo and Cipolla 11 In contrast, recent data in adults suggest that contact precautions even over 5 years may not lead to a lower MRGN incidence in a hospital setting.Reference Zahar, Poirel, Dupont, Fortineau, Nassif and Nordmann 12 MRSA screening for risk groups has been established for many years in Germany. 13 Still, data are conflicting regarding whether MRSA screening effectively can prevent or stop outbreaks or hinder further spread of this pathogen.Reference Gidengil, Gay, Huang, Platt, Yokoe and Lee 14 – Reference Patel, Thomas, Room, Wilson, Kearns and Gray 16
Two recent publications have described the prevalence of MDROs among refugees. In a study by Reinheimer et alReference Reinheimer, Kempf and Gottig 17 of 143 adult refugees and a study by Heudorf et alReference Heudorf, Krackhardt, Karathana, Kleinkauf and Zinn 18 of 119 unaccompanied refugee minors, colonization with MRGN bacteria was more frequent than in the general German population. An antibiotic-resistance surveillance study performed between 2009 and 2012 in Germany showed a MRSA prevalence of ~3.3% among outpatients and 3.4% among hospitalized patients, a MRGN E. coli prevalence of ~5.4% among outpatients and 6.9% among hospitalized patients, and a MRGN K. pneumonia prevalence of 2.1% among pediatric outpatients and 9.0% among hospitalized children.Reference Eckmanns, Richter and Feig 19 Most of the pediatric patients in the study by Heudorf et al. were from Afghanistan, whereas in our study most of the children came from Syria. Like our study, more individuals were colonized with 2MRGN than with 3MRGN. In our analysis, 4MRGN colonization was detected in 2 children.
The rise of Carbapenemase-producing Enterobacteriaceae in Europe is a serious concern in this context.Reference Albiger, Glasner, Struelens, Grundmann and Monnet 20 A Swiss study performed at the University Hospital Zurich detected a prevalence of 13.9% 2MRGN bacterial colonization and 7.9% 3MRGN bacterial colonization among travelers hospitalized abroad.Reference Nemeth, Ledergerber and Preiswerk 21 In an Italian sentinel surveillance study analyzing data from 48 Syrian asylum seekers with a median age of 20 years (23% children), 3 of 48 refugees (6.3%) carried MRSA and 11 of 48 (22.9%) carried MRGN.Reference Angeletti, Ceccarelli and Vita 22 Interestingly, in 4 of 48 refugees (8.3%), Pseudomonas strains (non-P. aeroginosa) were meropenem resistant. The relevance of this finding for high-risk groups with chronic lung diseases is currently under debate.
Herein, we have described the largest screened cohort of pediatric refugees to date with a high frequency of MDROs. The limitations of our study include the lack of an age-matched control group, but this was not possible due to the retrospective design of the study and the absence of universal MDRO screening. The sensitivity of detecting MRSA could also have been improved by using not only nasopharyngeal swabs but also swabs of additional sites (eg, rectum, axilla). Moreover, detailed refugee itineraries were not completely available due to lack of previous medical patient records and language barriers. Additionally, we can only speculate whether the children acquired their MDRO colonization during their travel or in their home country. Because most of the pediatric refugees were hospitalized in Mannheim for the first time and had not been in any other German hospital, it is unlikely that they acquired the MDRO at our hospital. Moreover, MDRO screening was performed directly after first contact in the emergency department, which makes an acquisition and consecutive colonization in the hospital before that time point also very unlikely. The sense and non-sense of screening for MDROs is under intense scrutiny.Reference Kaspar, Schweiger, Droz and Marschall 23 , Reference Seybold, Wagener, Jung and Sammet 24 Obviously, screening and pre-emptive isolation of risk groups can prevent nosocomial colonization and subsequent infection. Because the antibiotic armamentarium for MDROs is generally limited, the prevention of MDRO transmission is desirable. Increased costs of infection control measures and limited isolation capacities in hospitals have to be weighed against potential stigmatization and poorer patient care. Moreover, decolonization strategies for MRSA in refugees may not work well due to suboptimal conditions in camps, overcrowding, and language barriers to understanding medical indications. Therefore, we argue for a critical debate on MDROs, especially MRGN screening and isolation policies, not only for pediatric refugees but also for residential children.
ACKNOWLEDGMENTS
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.
Financial support: No financial support for this study was received.





