Approximately 1.7 million cases of SARS-CoV-2 infection have been reported in the United States. 1 In Los Angeles County, >57,000 cases and 2,443 deaths have been reported, and more than half (n = 1,233) of these deaths have occurred in residential congregate settings. 2 Preliminary evidence shows that infection-control strategies focused solely on symptomatic individuals are insufficient to prevent transmission in congregate living facilities. Reference Mosites3-Reference Kimball6
The Veterans’ Affairs Greater Los Angeles Healthcare System (VAGLAHS) is a large healthcare facility that includes an inpatient tertiary-care center and multiple congregate living facilities on a single campus. As part of its coronavirus disease 2019 (COVID-19) response, VAGLAHS implemented a widespread laboratory surveillance program for SARS-CoV-2 in both hospital and residential facilities.
Herein, we describe the laboratory surveillance program; we discuss how data gathered influenced infection control measures; and we highlight key lessons learned during implementation.
Methods
Setting
VAGLAHS consists of a tertiary-care hospital with 160 acute-care beds (plus an inpatient psychiatry unit and geriatric dementia unit), a skilled nursing facility (SNF) with 3 units totaling 150 beds, and a 151-bed residential rehabilitation treatment center. Additionally, during the pandemic, VAGLAHS created temporary shelter units with capacity to shelter 218 homeless individuals.
Laboratory testing
Either a nasopharyngeal swab in universal/viral transport media or a cobas polymerase chain reaction (PCR) media dual swab kit was used with the cobas 6800/8800 diagnostic system (Roche, Basel, Switzerland) to collect nasopharyngeal or combined nasopharyngeal and oropharyngeal specimens, respectively. Reverse-transcription (RT)-PCR was performed by a reference laboratory, the VAGLAHS Pathology and Laboratory Medicine Service (PALMS) or the Veterans’ Affairs Long Beach Healthcare System (VALBHS).
Surveillance program planning and implementation
A daily meeting organized by facility leadership coordinated COVID-19 planning and implementation of testing. Participants included leadership from PALMS, infectious disease, nursing, inpatient medicine, outpatient medicine, SNF, the residential rehabilitation treatment center, and the temporary shelter units.
Results
Overview of testing availability, turnaround time, and capacity
From March 11 through 26, 2020 VAGLAHS sent all specimens for SARS-CoV-2 testing to a reference laboratory. During this time, results returned in a median of 5 days (interquartile range [IQR], 4–8). On March 27, PALMS completed their emergency use authorization for the US Food and Drug Administration and validation of in-house SARS-CoV-2 RT-PCR testing (30–60 tests per day capacity). From March 27 through 30, most RT-PCR testing was performed in house, decreasing turnaround time to a median of 2 days (IQR, 2–3). Finally, on March 31, the laboratory at VALBHS initiated SARS-CoV-2 testing using the cobas system and began accepting specimens from other VA facilities, substantially increasing testing capacity and further decreasing turnaround time to a median of 1 day (IQR, 1–1).
Summary of all testing
From March 11 to April 29, 2,230 tests for SARS-CoV-2 were performed on 1,781 individuals (Table 1). Of these individuals, 78 tested positive: 58 patients and 20 employees. Surveillance testing for patients was implemented over time as testing capacity increased, starting with the highest risk settings (Fig. 1). Employees were tested if they developed symptoms or had close contact with a known positive case without appropriate personal protective equipment. Upon entrance to all facilities, all individuals were asked about fever, respiratory symptoms, and/or close contact with persons known to have COVID-19; if any of these symptoms were present, the individual was appropriately triaged and was not allowed to enter the building.
Table 1. Characteristics of All Individuals Tested for SARS-CoV-2 Throughout the Surveillance Program by Location

Note. SD, standard deviation.
a Includes 8 patients and 20 employees.
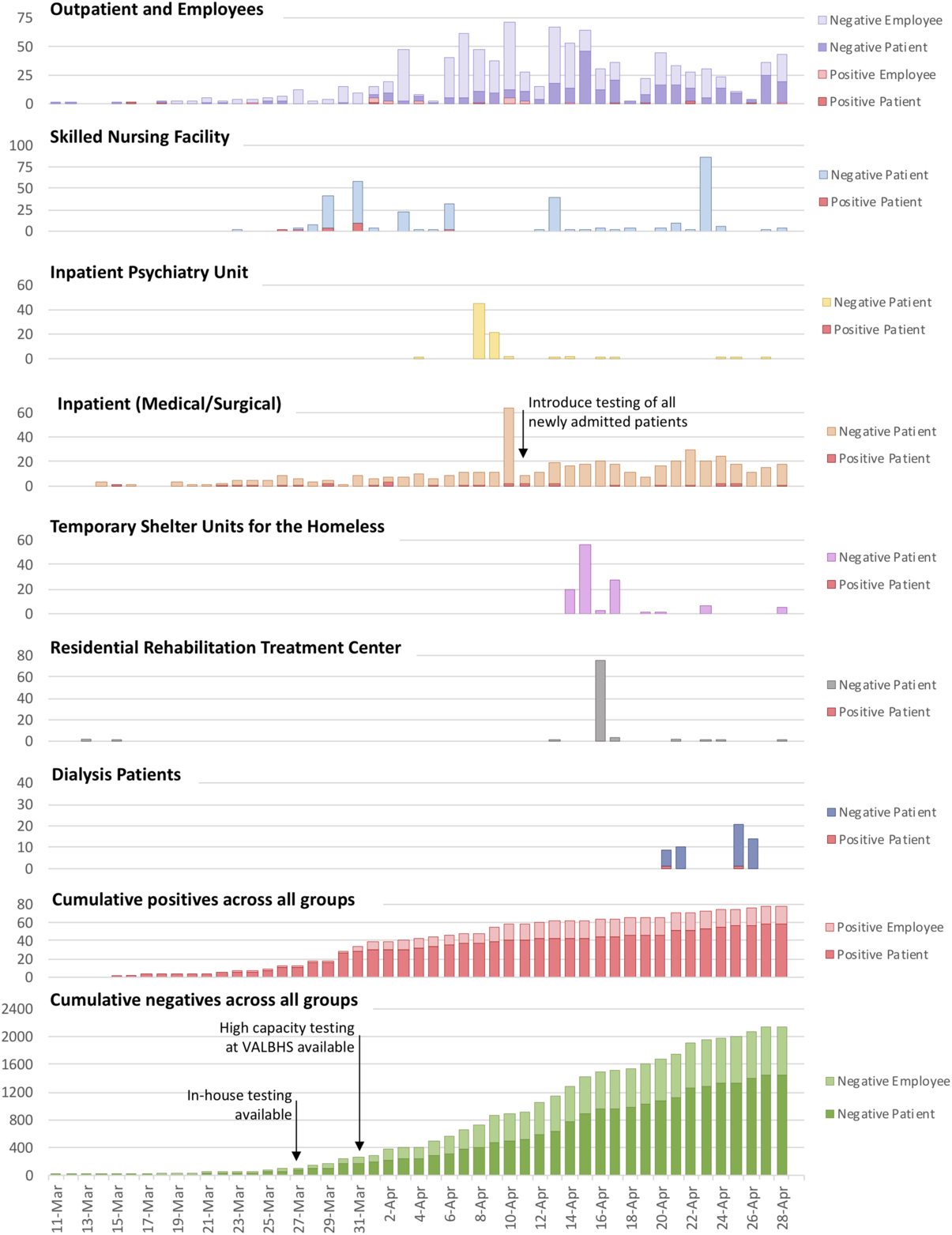
Fig. 1. Test results of widespread laboratory surveillance program for SARS-CoV-2 in hospital and congregate living settings, March 11–April 29, 2020.
Skilled nursing facility surveillance
On March 26 and 27, 2 symptomatic SNF residents were tested for SARS-CoV-2; on March 28, both tests returned positive. Given the high-risk implications of an outbreak and increased availability of in-house testing, all SNF residents (n = 100) were tested regardless of symptoms. This surveillance identified 14 asymptomatic SARS-CoV-2–positive residents from 2 SNF units who were moved to the tertiary-care facility and isolated under appropriate precautions. On April 3 and April 6, 57 residents who originally tested negative but lived in the same SNF unit as the SARS-CoV-2 positive individuals were retested, and 2 additional asymptomatic cases were identified. On April 13, a third round of testing was conducted among 39 residents who lived on the same unit as the SARS-CoV-2–positive residents were retested between April 3 and 6; none were positive. A fourth surveillance round (n = 87) on April 23 also yielded negative results.
Inpatient psychiatry unit surveillance
On April 8 and 9, 45 patients from the inpatient psychiatry unit and 21 patients in the geriatric dementia unit all tested negative. Following this unit-wide testing, all new admissions were required to have a negative SARS-CoV-2 test.
Pre-existing and new inpatient admission surveillance
On April 10, universal hospital-wide SARS-CoV-2 testing was implemented on all inpatients (medical or surgical) who had not previously been tested or tested negative on admission. Overall, 32 inpatients were tested and 2 new positive cases were identified. Also, 22 patients with a previously negative test were retested; all remained negative. From April 10 onward, all new admissions were tested and all inpatients were retested every 7 days. Between April 10 and 29, 12 new inpatient admissions tested positive, 2 of whom lacked classic COVID-19 symptoms and would otherwise not have met symptom-based testing criteria.
Temporary shelter units for surveillance of homeless individuals
From April 14 to 17, 106 individuals participating in temporary shelter programs were tested for SARS-CoV-2; all tests were negative. Also, 2 individuals who had tested positive before arriving at the campus were housed in an area designated for SARS-CoV-2–positive patients for 14 days prior to shelter entry.
Surveillance in the residential rehabilitation treatment center
On April 16, 76 residents from the on-campus residential rehabilitation treatment center for substance use were tested; all were negative.
Surveillance among dialysis patients
From April 22 to 28, 55 patients receiving dialysis at VAGLAHS were tested; 2 tested positive. Although neither had symptoms or signs characteristic of COVID-19, both were admitted to acute care for isolation and monitoring.
Discussion
To our knowledge, this is the first description of a widespread laboratory surveillance program for SARS-CoV-2 on an integrated medical campus. In the early days of the pandemic, testing capacity was limited, so efforts focused predominantly on symptomatic individuals. As testing capacity increased in early April and the importance of asymptomatic transmission was recognized, we transitioned to a more comprehensive program in which we identified 22 asymptomatic individuals who would not otherwise have been diagnosed.
Two key components enabled the success of this widespread laboratory surveillance program: (1) close collaboration with PALMS to secure access to high-volume molecular testing and (2) strong coordination of staff from multiple disciplines to implement testing.
The early initiative taken by PALMS to develop an in-house test for SARS-CoV-2 was instrumental in increasing testing capacity and decreasing turnaround time. Without this in-house test, surveillance testing of the >100 residents in the SNF within a single week would not have been possible. Similarly, the roll-out of a high-volume, high-throughput testing system at VALBHS further increased capacity to >1,900 tests over 4 weeks. Finally, to avoid shortages in specimen swabs, VAGLAHS repurposed swab kits for Chlamydia trachomatis and Neisseria gonorrhoeae testing to test for SARS-CoV-2. Close coordination and frequent communication between nurses, physicians, and other staff, facilitated by daily leadership meetings, in both the hospital and congregate living facilities were critical in implementing the surveillance program.
Overall, the implementation of a widespread surveillance testing strategy likely prevented asymptomatic transmission of SARS-CoV-2, thereby preventing potential outbreaks of COVID-19 within an integrated medical campus.
Acknowledgments
We would like to thank Salem Toney and Dr Carmen Kletecka with Pathology and Laboratory Medicine Services for their tireless work to increase testing capacity and throughput during the pandemic. We would also like to thank Michael Simmons, RN, Dr Linda Sohn, and Dr Anjani Reddy for facilitating and coordinating the testing of hospitalized patients, SNF residents, and individuals in the temporary shelters and residential rehabilitation treatment center respectively.
Financial support
This work was supported in part by funds and facilities provided by the Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS).
Conflicts of interest
All authors report no conflicts of interest relevant to this article.





