Introduction
Viruses are numerically abundant and an integral part of life on Earth. However, there is very little known about viruses across many of Earth's environments, including the many extreme habitats that serve as astrobiology analogues, when compared with the understanding of viruses relevant to human health. Even with respect to human health, relatively little is known about viral responses to the space environment beyond Earth's atmosphere. Additionally, almost no research has been conducted to identify the impact of these viruses on the spacecraft systems used to sustain humans. Understanding viruses and virus–host interactions in space-relevant contexts will inform our grasp of their role(s) in both human spaceflight missions, including environmental control and life support systems (ECLSS), and in the search for life elsewhere. Here, we review viruses in diverse Earth environments, the very limited studies conducted on viruses in space and lay out a roadmap for future research. For a more extensive overview of environmental viruses and introduction to viruses, see Berliner et al. (Reference Berliner, Mochizuki and Stedman2018); for a specific review on the roles of viruses in spaceflight affecting human health, see Pavletic et al. (Reference Pavletić, Runzheimer, Siems, Koch, Cortesão, Ramos-Nascimento and Moeller2022); for a complementary review on the need to incorporate viruses more in astrobiology, see De La Higuera and Lázaro (Reference De La Higuera and Lázaro2022).
Viruses are sometimes defined as ‘very small obligate intracellular parasites’ (Acheson, Reference Acheson2011), and more broadly as ‘entities whose genomes are elements of nucleic acids that replicate inside living cells using the cellular synthetic machinery and causing the synthesis of specialized elements that can transfer the viral genome to other cells’ (Luria et al., Reference Luria, Darnell, Baltimore, Campbell and Hadar1978). These ‘specialized elements’ or virions are crucial to the definition of viruses. Viruses contain genetic information that can be transferred on massive scales due to the very large numbers of virions on Earth.
The sheer ubiquity of virions on Earth, with up to 1031 in the Earth's oceans alone (Suttle, Reference Suttle2005; Mushegian, Reference Mushegian2020), makes them an attractive target for the search for life on other worlds if one only knew what to search for! Viruses that infect bacteria and archaea typically range in size from tens to a few hundred nanometres, while viruses that infect eukaryotic organisms (e.g. amoebae or humans) can even range from tens to thousands of nanometres (Fig. 1). These so-called giant viruses can be larger than some bacteria or archaea and can even be infected by viruses themselves (Sommers et al., Reference Sommers, Chatterjee, Varsani and Trubl2021). For example, virophages have been observed to infect giant viruses that are themselves infecting cellular organisms (e.g. amoebae). In these cases, the virophage infection co-opts the giant virus and may improve the condition of the host organism (Roux et al., Reference Roux, Chan, Egan, Malmstrom, McMahon and Sullivan2017; Backstrom et al., Reference Backstrom, Yutin, Jorgensen, Dharamshi, Homa, Zaremba-Niedwiedzka, Spang, Wolf, Koonin and Ettema2019; Schulz et al., Reference Schulz, Roux, Paez-Espino, Jungbluth, Walsh, Denef, McMahon, Konstantinidis, Eloe-Fadrosh, Kyrpides and Woyke2020). Viruses can also alter the genetic architecture, phenotypic characteristics, reproduction strategy, infection dynamics and evolution of nearby viruses, which in turn influences how viruses interact with their hosts and ultimately, ecosystems. This spectrum of virus behaviours across all domains of life is also reflected in the productivity of their infected host cells (i.e. modulation of host-cell metabolism and tens to thousands of progeny virus particles per cell) and timing of single-cycle infection (e.g. tens to thousands of minutes). Notably, the timing for one cycle of virus growth correlates with the doubling time of healthy host cells (Jin and Yin Reference Jin and Yin2021).
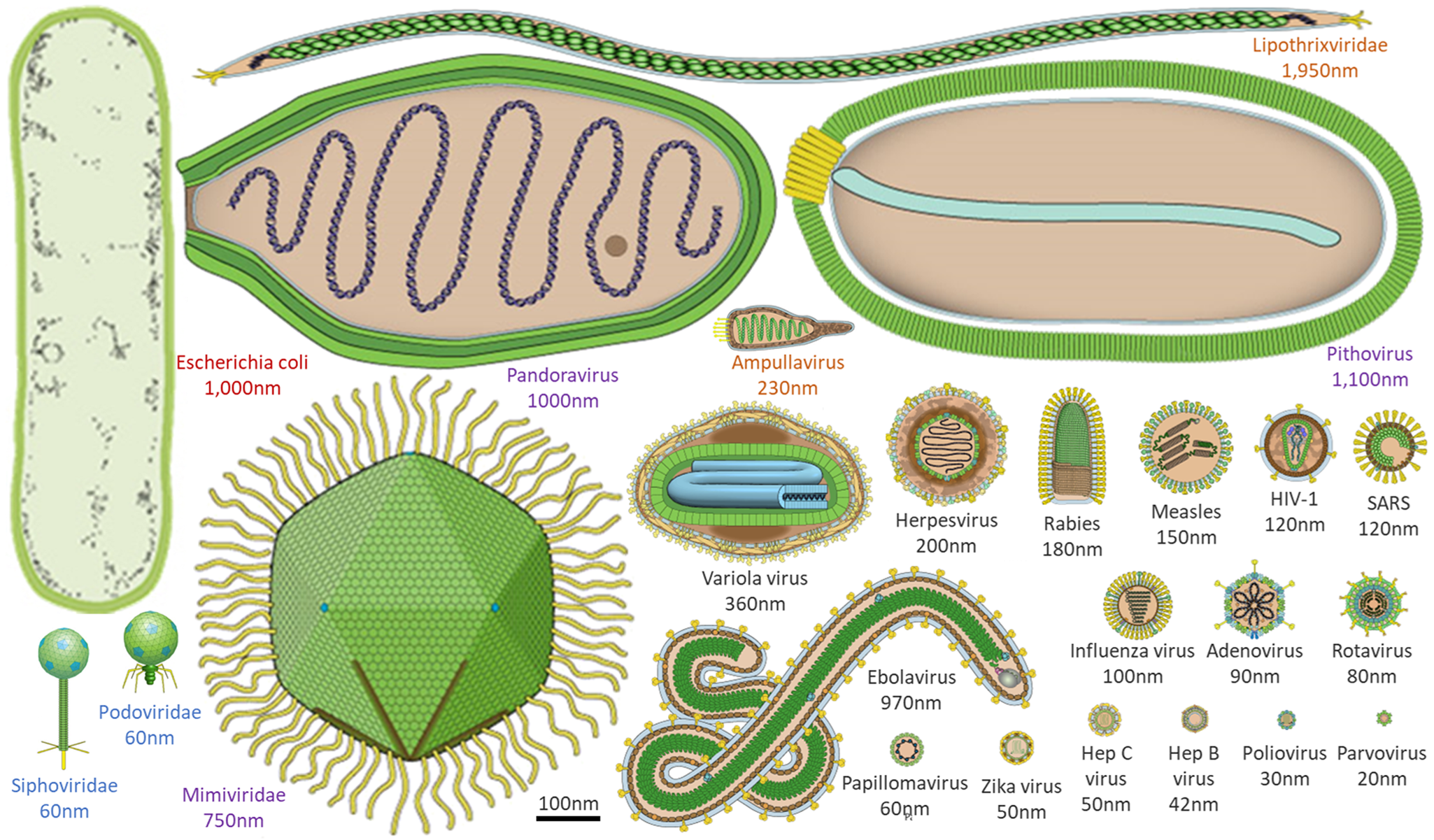
Figure 1. Morphological comparison of viruses, and a common bacterium at the same scale. Viral morphology and size are highly diverse, ranging from a few to thousands of nanometres. Here, an example bacterium (Escherichia coli, red text) is contrasted with example viruses that infect humans (black text), amoebae (purple text), archaea (orange text) and bacteria (blue text). Virus images adapted from ViralZone, Swiss Institute of Bioinformatics.
The diverse morphology of viruses which extends beyond that typically found in cellular life (Fig. 1), and the presence of unique genes for the virus coat or capsid, have already been used as a model for other life: ‘capsid-encoding organisms’ (Forterre and Prangishvili, Reference Forterre and Prangishvili2009). Virus genomes can be comprised of DNA or RNA, double-stranded or single-stranded, in multiple variations of each. Unlike cellular life, there are no universal viral genes to serve as a backbone upon which to build consistent evolutionary trees (Roux et al., Reference Roux, Adriaenssens, Dutilh, Koonin, Kropinski, Krupovic, Kuhn, Lavigne, Brister, Varsani and Amid2019; Tisza et al., Reference Tisza, Pastrana, Welch, Stewart, Peretti, Starrett, Pang, Krishnamurthy, Pesavento, McDermott, Murphy, Whited, Miller, Brenchley, Rosshart, Rehermann, Doorbar, Ta'ala, Pletnikova, Troncoso, Resnick, Bolduc, Sullivan, Varsani, Segall and Buck2020). The diversity of both virus morphology and viral nucleic acids makes them difficult to search for comprehensively, but also drives expanded toolkits that could be employed in the search for life on other worlds and therefore deserves further investigation.
Virus-like entities may even be detectable on other worlds where life-like processes are only just evolving. Although humans have become explicitly aware of viruses and their effects only in the past few centuries (Loeffler and Frosch, Reference Loeffler and Frosch1897; Beijerinck, Reference Beijerinck1898), they were likely present as soon as – or even before – the first cell arose on Earth (Moelling and Broecker, Reference Moelling and Broecker2019). Viral infections have played a key role in the evolution of life on Earth via the exchange of genes between viruses and their hosts, providing genetic diversity, killing dominant hosts thus maintaining balanced populations and possibly even inventing DNA itself (Forterre and Prangishvili, Reference Forterre and Prangishvili2009; Enard et al., Reference Enard, Cai, Gwennap and Petrov2016). Viral genome remnants are present in most, if not all, cellular genomes, including in humans, where viruses play key roles in human physiology (Bannert and Kurth, Reference Bannert and Kurth2004; Arneth, Reference Arneth2021). Further studies of the history of Earth's viruses may highlight opportunities for detecting emergent life on other worlds.
On Earth, viruses have such a substantial impact that they drive major global biogeochemical cycles, and the same may be true on other worlds. The vast majority of marine viruses infect microbes (Suttle, Reference Suttle2005; Parikka et al., Reference Parikka, Le Romancer, Wauters and Jacquet2017), thus affecting many biological processes, including encoding photosynthesis genes that substantially contribute to atmospheric oxygen (O2), and help regulate global nutrient cycling (Greene and Reid, Reference Greene and Reid2013). Further understanding of the persistence of virus particles and viral impacts on microbial cellular chemistry may therefore help illuminate biosignatures on distant planets, or signatures of past life in the geologic records of other worlds.
Targeting the search for biosignatures should focus on locations that are currently or once were within the environmental ranges compatible with the persistence and replication of information molecules. The more that research is conducted on viral persistence and replication in extreme environments, the more that remarkable feats of persistence and replication may be discovered (e.g. viruses thawed from permafrost could infect a modern-day version of their host; Legendre et al., Reference Legendre, Bartoli, Shmakova, Jeudy, Labadie, Adrait, Lescot, Poirot, Bertaux, Bruley and Couté2014).
Viral persistence in and response to extreme conditions also has important implications for humans voyaging into space, as recently reviewed by Pavletić et al. (Reference Pavletić, Runzheimer, Siems, Koch, Cortesão, Ramos-Nascimento and Moeller2022). However, in addition to viral impacts on human physiology via direct infection and impacts on the microbiome, viral infection of plants or microbes could impact critical life support systems on deep space missions, including algal bioreactors (Matula and Nabity, Reference Matula and Nabity2019). Conditions such as microgravity affect fluid dynamics with implications for bacterial biology (as reviewed in Diaz et al., Reference Diaz, Li, Irwin, Calle and Callahan2019; Acres et al., Reference Acres, Youngapelian and Nadeau2021; Sharma and Curtis, Reference Sharma and Curtis2022), and presumably also virus–host interactions, but this is still an underdeveloped field.
The diversity of unique viral qualities and their integral role in life on Earth compels us to better understand the potential roles of viruses and virus-like entities in both astrobiology and space biology. There are multiple critical knowledge gaps in our understanding of viruses in the space environment and their potential role in other hypothetical biospheres in our Solar System and beyond.
In this review, we outline what new virus research here on Earth should investigate to address key scientific questions in planetary science, space biology and astrobiology. These include:
(1) What role(s) did viruses play in the origin and evolution of life on Earth?
(2) What are the environmental limits to the preservation and propagation of virions?
(3) What role(s) could viruses play in potential biospheres elsewhere, and how might those impacts be detectable as biosignatures?
(4) Can the detection of viruses beyond Earth be interpreted as a sign of life?
(5) How do artificial environments affect the human virome?
(6) Can virions be fossilized or otherwise preserved in a recognizable state?
Potential role(s) of viruses in the early stages of life
Increasing knowledge of the roles of viruses in the origins of life on Earth will benefit the search for life elsewhere. Viruses have and will continue to play a critical role in the evolution of life on Earth. Precursors of virus-like entities may even mark the beginning of life itself, making the virosphere potentially as old and significant as the cellular biosphere (Fig. 2; Janjic, Reference Janjic2018; Moelling and Broecker, Reference Moelling and Broecker2019). Viral signatures may be pivotal in the search for life elsewhere and in understanding evolutionary mechanics of other biospheres. Theoretical models predict that (depending on the information-transfer properties of the system) parasitic replicators will emerge anywhere in the Universe that life-like processes evolve (Eigen, Reference Eigen1971; Bresch et al., Reference Bresch, Niesert and Harnasch1980; Vignuzzi and López, Reference Vignuzzi and López2019; Vlok et al., Reference Vlok, Lang and Suttle2019). Indeed, even viruses themselves fall prey to parasitic replicators in the form of defective virus genomes and virophages that multiply at the expense of fully intact virus genomes (Perrault, Reference Perrault1981; Vignuzzi and López, Reference Vignuzzi and López2019; Roux et al., Reference Roux, Fischer, Hackl, Katz, Schulz and Yutin2023). The ‘RNA-world’ hypothesis characterizes the origin of life with self-replicating RNA, followed by ribonucleoproteins that later evolved into DNA and larger proteins (Müller et al., Reference Müller, Escobar, Xu, Węgrzyn, Nainytė, Amatov, Chan, Pichler and Carell2022). Viruses and virus-like replicators are the only known extant biological entities that contain all types of nucleic acid genomes (Table 1), including single-stranded and double-stranded RNA and DNA or mixtures thereof. RNA viruses serve as models for how RNA and ribonucleoproteins could have propagated via simple self-replicating RNA structures and ribozyme activity (Landweber et al., Reference Landweber, Simon and Wagner1998; Koonin et al., Reference Koonin, Senkevich and Dolja2006; Tyler, Reference Tyler2008; Durzyńska and Goździcka-Józefiak, Reference Durzyńska and Goździcka-Józefiak2015; Matsumura et al., Reference Matsumura, Kun, Ryckelynck, Coldren, Szilágyi, Jossinet, Rick, Nghe, Szathmáry and Griffiths2016; Weinberg et al., Reference Weinberg, Weinberg and Hammann2019). Viruses may have helped support the transition from the early RNA world to the current DNA world (Forterre, Reference Forterre2006; Diemer and Stedman, Reference Diemer and Stedman2012). Further, the recent acceptance of network-based clustering for virus taxonomy (Jang et al., Reference Jang, Bolduc, Zablocki, Kuhn, Roux, Adriaenssens, Brister, Kropinski, Krupovic, Lavigne and Turner2019) in addition to incorporation of three-dimensional (3D) structures (and other viral characteristics) have allowed deep evolutionary inferences that have identified newly proposed phyla of RNA viruses which may be a previously missing link in the transition from the RNA–peptide world to the DNA–RNA–peptide world (Zayed et al., Reference Zayed, Wainaina, Dominguez-Huerta, Pelletier, Guo, Mohssen, Tian, Pratama, Bolduc, Zablocki and Cronin2022). Further, beyond the origins of life, viruses played a role in the origins of the mitochondrion (Shutt and Gray, Reference Shutt and Gray2006) and of the placenta (Mi et al., Reference Mi, Lee, Li, Veldman, Finnerty, Racie, LaVallie, Tang, Edouard, Howes, Keith and McCoy2000). Viruses may even have been critical in the evolution of eukaryotes and multicellularity (Forterre and Gaïa, Reference Forterre and Gaïa2016; Lee et al., Reference Lee, Sherer, Kim, Rajic, Kaur, Urriola, Martini, Xue, Goldenfeld and Kuhlman2018; Guglielmini et al., Reference Guglielmini, Woo, Krupovic, Forterre and Gaia2019). Overall, viruses appear to have played many roles in the origin and evolution of early life.
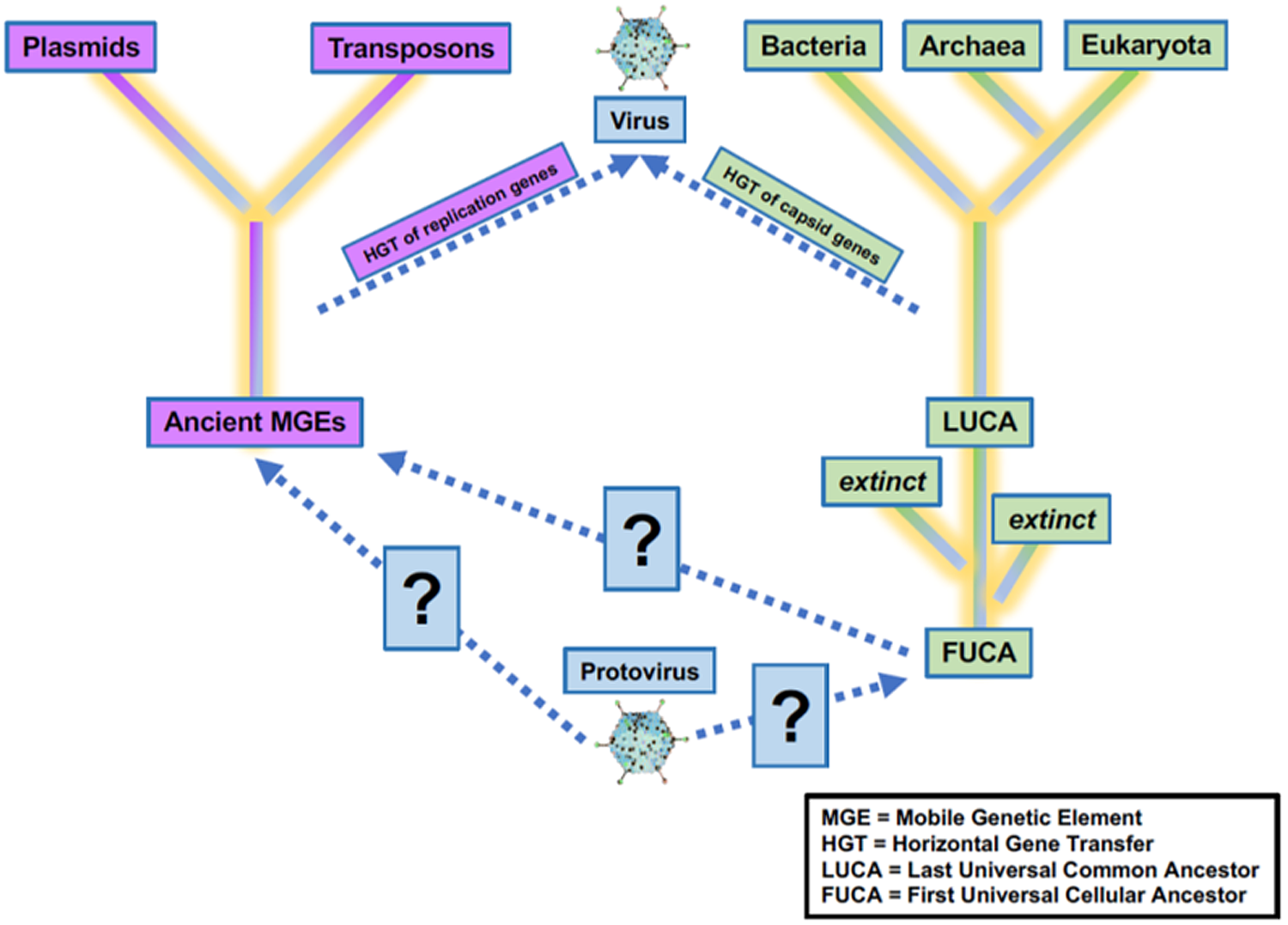
Figure 2. Uncertainty in the origin(s) and evolution of life on Earth. Viruses play a critical role in the evolution of life as we currently know them, and viruses or precursors of virus-like entities must be considered in origin experiments. Adapted from Harris and Hill (Reference Harris and Hill2021).
Table 1. Comparison of cellular and non-cellular entities—cells possess each of these four significant genetic and structural materials
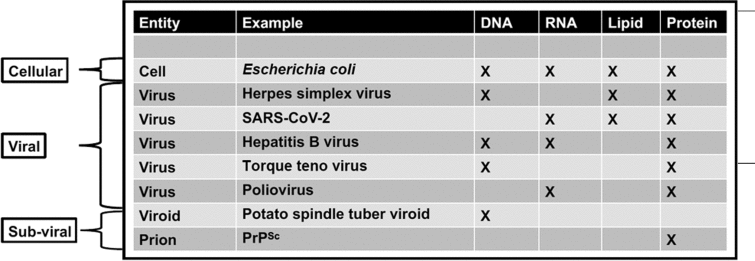
In the case of viruses and subviral elements, only reduced suites of these key molecules are present. Since non-cellular entities can possess either DNA or non-DNA genomes and may lack lipids, the search for extraterrestrial DNA, RNA or lipid alone would fail to detect some of these entities.
While uncovering the history of viruses is key to understanding the origin and evolution of life on Earth, an additional benefit is the development of new technologies that are potentially valuable for characterizing the diversity and history of non-terran life. For instance, comparative analysis of 3D atomic structures is a non-genomic method being used to interrogate virus origins that could also be applied to non-terran samples that may lack nucleic acids (Bamford et al., Reference Bamford, Grimes and Stuart2005). This method is employed to detect and classify viruses because there are no genes shared by all extant viruses (Roux et al., Reference Roux, Adriaenssens, Dutilh, Koonin, Kropinski, Krupovic, Kuhn, Lavigne, Brister, Varsani and Amid2019; Tisza et al., Reference Tisza, Pastrana, Welch, Stewart, Peretti, Starrett, Pang, Krishnamurthy, Pesavento, McDermott, Murphy, Whited, Miller, Brenchley, Rosshart, Rehermann, Doorbar, Ta'ala, Pletnikova, Troncoso, Resnick, Bolduc, Sullivan, Varsani, Segall and Buck2020). In comparison, all organismal cells conserve some genomic features such as ribosomal RNA (Table 1). Thus, the search for life elsewhere can tap into such techniques. In particular, we propose the following future research directions.
Future directions
(a) Investigate evolutionary relationships between RNA viruses and ribozymes (ribonucleotide enzymes) to evaluate the potential role of viruses in an RNA world, and the transition to a DNA world.
(b) Create tools and methods to enable inferences about early life through comparative analysis of extant viral life.
(c) Reconstruct ancient events to illuminate the origin(s) of viruses on Earth, and their possible roles in the emergence of all life.
(d) Develop phylogenies that consider all biological entities to attempt to connect and better constrain the shared and divergent evolutionary histories of viruses and cellular organisms.
Habitability, persistence and process limits of viruses and cellular life
Viruses have been known to remain active in a variety of harsh conditions, sometimes influencing the functioning and preservation of their hosts and sometimes reinfecting hosts after being preserved themselves yet still viable. Cellular life can also persist and operate under a variety of extreme conditions on Earth. However, the monitoring of virus–virus and virus–host activity together in extreme environments has been limited, despite these interactions being inherently different than those in milder environments. Therefore, fundamental concepts are not well understood about the types of environmental conditions and processes that are most conducive to the preservation and propagation of information molecules or molecular signatures of life. For example, understanding the upper and lower limits of viruses' persistence and activity under a range of conditions would inform where we might find viruses and other life on both Earth, in space and on other worlds. Additionally, long-term monitoring of viruses in extreme environments could inform their ability to persist under similar conditions on another planetary body. Multiple analytical techniques could be used to better determine viral activity in both dormant and active host environments and to better characterize viruses and low-level host activity as well. While viral process limitations are currently unknown for a range of conditions, viruses persist and interact with their hosts under a wide range of extreme conditions on Earth (Gil et al., Reference Gil, Mesa, Estrada-Ortiz, Lopez-Obando, Gómez and Plácido2021), including extremes in temperature, pH, pressure and salinity. These conditions manifest in both natural and built environments, and viruses have been observed in a variety of settings (Table 2). Understanding the extreme ranges of conditions in which viruses and their hosts can survive informs the search for life on other worlds, the operational considerations for planetary protection and viral monitoring during human spaceflight.
Table 2. Example locations where viruses have been observed through isolation or genomic studies
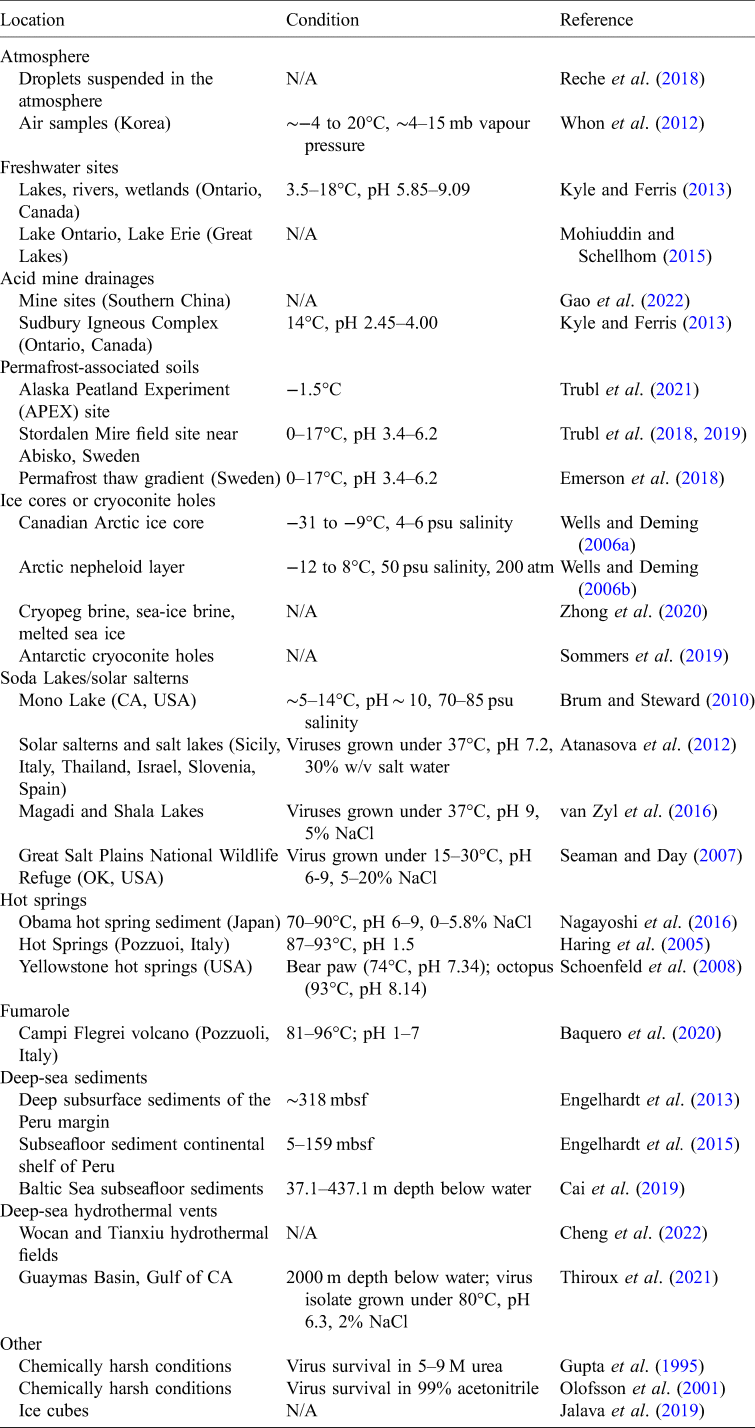
This table provides examples, and it is not a comprehensive review of all locations where viruses have been observed/isolated.
psu, practical salinity unit; mbsf, metres below sea floor.
In a variety of environmental settings, viruses can persist, maintain their integrity and even help preserve their hosts for extended periods of time. For example, viruses that adsorb to clays are protected from inactivation, thus enabling them to persist in soils for longer periods of time in the absence of a host (Bitton, Reference Bitton1975; Lance and Gerba, Reference Lance and Gerba1984; Lipson and Stotzky, Reference Lipson and Stotzky1985; Syngouna and Chrysikopoulos, Reference Syngouna and Chrysikopoulos2010). Virus particles can also be preserved via silicification and can become reactivated (i.e. infectious once again) when the silica is removed (Laidler and Stedman Reference Laidler and Stedman2010; Laidler et al., Reference Laidler, Shugart, Cady, Bahjat and Stedman2013). Further, upon thawing, viruses preserved in frozen permafrost for ~30 000 years were able to infect modern day versions of their hosts (Legendre et al., Reference Legendre, Bartoli, Shmakova, Jeudy, Labadie, Adrait, Lescot, Poirot, Bertaux, Bruley and Couté2014). Viruses can also influence the preservation of their biological hosts. For example, viruses that infect microbial mats can cause or expedite the microbial mats' fossilization into a stromatolite by (1) acting as a nucleation site for organomineralization, (2) altering microbial host metabolism to promote carbonate precipitation or (3) increasing the production of microbial extracellular substances (Pacton et al., Reference Pacton, Wacey, Corinaldesi, Tangherlini, Kilburn, Gorin, Danovaro and Vasconcelos2014; White et al., Reference White, Visscher and Burns2021). In summary, viruses can function as key structures for preservation and propagation of biological information, especially in extreme environments. In this context, a better understanding of which environmental and molecular factors drive this long-term persistence will enhance future searches for virus-like elements outside of Earth.
Throughout extreme environmental conditions, viruses can also extend protection to their hosts. For instance, viruses can confer host heat tolerance (Márquez et al., Reference Márquez, Redman, Rodriguez and Roossinck2007), and carry genes for sporulation that may drive their host to form an inactive spore, that is robust to unfavourable conditions (Trubl et al., Reference Trubl, Jang, Roux, Emerson, Solonenko, Vik, Solden, Ellenbogen, Runyon, Bolduc and Woodcroft2018; Van Goethem et al., Reference Van Goethem, Swenson, Trubl, Roux and Northen2019; Pelusi et al., Reference Pelusi, De Luca, Manfellotto, Thamatrakoln, Bidle and Montresor2021; Travers et al., Reference Travers Cook, Skirgaila, Martin and Buser2022). In another instance, viruses allow photosynthesis in cyanobacteria under desiccating conditions (Azua-Bustos et al., Reference Azua-Bustos, González-Silva, Arenas-Fajardo and Vicuña2012). Harsh environments, such as polar regions or hydrothermal vents, have a high incidence of temperate viruses, which can reside within their microbial hosts (lysogeny) until conditions are favourable for viral replication (Anderson et al., Reference Anderson, Sogin and Baross2014; Brum et al., Reference Brum, Hurwitz, Schofield, Ducklow and Sullivan2016). While in this lysogenic state, viruses may express genes that alter their microbial host's physiology and metabolism, thus increasing the host cell's ability to survive conditions in which resources are limited, including within sea ice, dry soil or acidic environments (Chen et al., Reference Chen, Golding, Sawai, Guo and Cox2005; Yu et al., Reference Yu, Chen, Shen, Zhao, Tang, Su, Wu, Qin, Xie, Zhang and Yu2015; Howard-Varona et al., Reference Howard-Varona, Hargreaves, Abedon and Sullivan2017; Lee et al., Reference Lee, Sieradzki, Nicolas, Walker, Firestone, Hazard and Nicol2021; Wu et al., Reference Wu, Davison, Gao, Nicora, Mcdermott, Burnum-Johnson, Hofmockel and Jansson2021).
By integrating into hosts that are dormancy capable, viruses may be able to persist through conditions that are incompatible with activity then reactivate when conditions improve. Extreme examples of organisms regaining function after dormancy include: (1) cyanobacteria reviving after a 672 day exposure to space outside of the International Space Station (ISS; Laranjeiro et al., Reference Laranjeiro, Harinath, Pollard, Gaffney, Deane, Vanapalli, Etheridge, Szewczyk and Driscoll2021), (2) cyanobacteria in Antarctica metabolizing within a week after rewetting following 20–30 years without stream flow (McKnight et al., Reference McKnight, Tate, Andrews, Niyogi, Cozzetto, Welch, Lyons and Capone2007), (3) nematodes reviving from 30 000-year-old permafrost in Siberia (Shatilovich et al., Reference Shatilovich, Tchesunov, Neretina, Grabarnik, Gubin, Vishnivetskaya, Onstott and Rivkina2018) and (4) rotifers in northeastern Siberia reactivating from 24 000-year-old permafrost (Shmakova et al., Reference Shmakova, Malavin, Iakovenko, Vishnivetskaya, Shain, Plewka and Rivkina2021). Microbes living under low-energy conditions in the South Pacific Gyre are claimed to have even retained their metabolic response after 101.5 million years (Morono et al., Reference Morono, Ito, Hoshino, Terada, Hori, Ikehara, D'Hondt and Inagaki2020). The diverse evidence of microbial and eukaryote dormancy noted above shows the potential for organisms to survive and even grow in space-like environments and planetary analogues. However, not much is known about any hitchhiking viruses, that have accompanied these long-persisting hosts, nor how they may have contributed to long host dormancies. Evaluating the persistence and reactivation of viruses in dormant hosts would hint at the plausibility of their presence in extreme non-terran environments.
The effects of viruses on extremophiles involve virus–host interactions that are fundamentally different relative to ideal environments (Dávila-Ramos et al., Reference Castelán-Sánchez, Lopéz-Rosas, García-Suastegui, Peralta, Dobson, Batista-García and Dávila-Ramos2019). The monitoring of viral persistence and activity in a wide variety of extreme environment types may indicate how viruses survived during early Earth, and what is applicable to alien biospheres. We need expanded experimentation to determine end member limits of viral persistence and activity under conditions like aridity, humidity, low/high O2 content, trapping in amber or soil/sediments, etc. Scoping out the broad viral environmental envelope would point to where virus-like entities might be found on both Earth and other worlds.
We posit that similar virus–host interactions are likely on any planetary body where life exists, thus it is critical to expand our understanding of these interactions on Earth to enable the detection and identification of such phenomena during space missions. For example, it is now feasible to computationally integrate how functions encoded in virus genomes interact with the material and energy resources of their hosts, thereby predicting the timing and levels of virus growth (Yin and Redovich, Reference Yin and Redovich2018). Successfully predicting viral activity in human-support space environments would help assure astronaut health.
Viral activity in low-energy Earth environments can be explored through development of high sensitivity tracer approaches. Bulk tracer methods, such as nucleic acid stable isotope probing (SIP; Radajewski et al., Reference Radajewski, Ineson, Parekh and Murrell2000; Manefield et al., Reference Manefield, Whiteley, Griffiths and Bailey2002), can provide detailed information about the level of stable isotope incorporation by microbes and have been applied to investigate virus activity in complex environments (Pasulka et al., Reference Pasulka, Thamatrakoln, Kopf, Guan, Poulos, Moradian, Sweredoski, Hess, Sullivan, Bidle and Orphan2018; Lee et al., Reference Lee, Sieradzki, Nicolas, Walker, Firestone, Hazard and Nicol2021, Reference Lee, Sieradzki, Hazard and Nicol2022; Starr et al., Reference Starr, Shi, Blazewicz, Koch, Probst, Hungate, Pett-Ridge, Firestone and Banfield2021; Trubl et al., Reference Trubl, Kimbrel, Liquet-Gonzalez, Nuccio, Weber, Pett-Ridge, Jansson, Waldrop and Blazewicz2021). Bulk methods such as SIP integrate over relatively large sample mass (Nuccio et al., Reference Nuccio, Blazewicz, Lafler, Campbell, Kakouridis, Kimbrel, Wollard, Vyshenska, Riley, Tomatsu and Hestrin2022), thus requiring more enriched substrate in complex systems. However, investigators have recently developed imaging mass spectrometry approaches that can detect viral replication at the single-particle level based on incorporation of rare stable carbon and nitrogen isotopes (Gates et al., Reference Gates, Condit, Moussatche, Stewart, Malkin and Weber2018; Pasulka et al., Reference Pasulka, Thamatrakoln, Kopf, Guan, Poulos, Moradian, Sweredoski, Hess, Sullivan, Bidle and Orphan2018; Mayali et al., Reference Mayali, Weber, Nuccio, Lietard, Somoza, Blazewicz, Pett-Ridge, Dumont and Hernández García2019). Because virion enrichment has been detected in individual particles using nanometre-scale secondary ion mass spectrometry (NanoSIMS), the approach is clearly equally viable for other very small samples. These studies have demonstrated sensitivity down to 100 nm-diameter virions. Further research is necessary to extend these methods to bacteriophage in the 50 nm-diameter range and experimentally apply them to extreme environmental and space-like conditions. Such powerful techniques could be used to evaluate the resilience of viruses to astrobiologically relevant conditions, thus we propose the following experimental directions.
Future directions
(a) Increase of environmental surveys and long-term monitoring of viral persistence and activity in extreme environments that can be used as analogues of planetary bodies and early life on Earth.
(b) Experimental work on the lower and upper limits to viral persistence and activity under a variety of environmental parameters.
(c) Evaluation of the persistence and activity of viruses in dormant and active hosts, combining techniques (e.g. SIP with metagenomics) to better characterize viruses and detect low-level host activity.
Biogeochemical cycling and biosignature detection
Viruses play critical roles in biogeochemical cycles on Earth. Our understanding of viruses as major players in Earth's biogeochemical cycles has been reshaped by the advent of metagenomic approaches that have enabled the study of uncultivated viruses (Kristensen et al., Reference Kristensen, Mushegian, Dolja and Koonin2010; Roux et al., Reference Roux, Brum, Dutilh, Sunagawa, Duhaime, Loy, Poulos, Solonenko, Lara, Poulain, Pesant, Kandels-Lewis, Dimier, Picheral, Searson, Cruaud, Alberti, Duarte, Gasol, Vaqué, Bork, Acinas, Wincker and Sullivan2016, Reference Roux, Adriaenssens, Dutilh, Koonin, Kropinski, Krupovic, Kuhn, Lavigne, Brister, Varsani and Amid2019). Marine virology has been particularly intensely studied, highlighting the potential for worlds with liquid oceans, such as Enceladus or Europa, to contain informational molecules such as virus genomes and proteins amongst their organic compounds (Postberg et al., Reference Postberg, Khawaja, Abel, Choblet, Glein, Gudipati, Henderson, Hsu, Kempf, Klenner, Moragas-Klostermeyer, Magee, Nölle, Perry, Reviol, Schmidt, Srama, Stolz, Tobie, Trieloff and Waite2018). On Earth, viruses have a huge impact on O2 concentrations by infecting the marine cyanobacteria that are responsible for ~25% of the O2 in Earth's atmosphere. At any moment, about half of marine cyanobacteria are infected, which can lead to either a decrease in O2 production (i.e. cells lysed by viruses) or an increase in the efficiency of their O2 production (Sieradzki et al., Reference Sieradzki, Ignacio-Espinoza, Needham, Fichot and Fuhrman2019). We need an improved understanding of what controls that balance, and how can that be used in biogeochemical modelling. Other work has demonstrated the activity of viruses in cold to sub-freezing temperature soils (Trubl et al., Reference Trubl, Kimbrel, Liquet-Gonzalez, Nuccio, Weber, Pett-Ridge, Jansson, Waldrop and Blazewicz2021; Wu et al., Reference Wu, Bottos, Danna, Stegen, Jansson and Davison2022) and illustrates the potential for viruses in biogeochemical processes of cold terrestrial worlds, such as Mars. Terrestrial worlds, or at least partly rocky bodies, such as comets, may host ice-lidded cryoconite holes in their polar ice caps that could result in the presence of liquid water to support active life (Zawierucha et al., Reference Zawierucha, Ostrowska and Kolicka2017). Could terrestrial worlds with thick mid-deck atmospheres, such as Venus, harbour life and virus-like entities? On Earth, we know that viruses exist within droplets in the atmosphere and can augment iron and sulphur metabolisms (Anantharaman et al., Reference Anantharaman, Duhaime, Breier, Wendt, Toner and Dick2014; Bonnain et al., Reference Bonnain, Breitbart and Buck2016; Roux et al., Reference Roux, Brum, Dutilh, Sunagawa, Duhaime, Loy, Poulos, Solonenko, Lara, Poulain, Pesant, Kandels-Lewis, Dimier, Picheral, Searson, Cruaud, Alberti, Duarte, Gasol, Vaqué, Bork, Acinas, Wincker and Sullivan2016; Dalcin Martins et al., Reference Dalcin Martins, Danczak, Roux, Frank, Borton, Wolfe, Burris and Wilkins2018). Since Venus's clouds contain sulphuric acid, viruses could be influencing biogeochemical processes in the planet's atmosphere as well. More intimately studying the role of viruses in biogeochemical processes in a variety of Earth analogue environments could inform any potential role viruses might play in biogeochemical processes on other worlds.
The widespread and frequent detection of genes used by viruses to hijack the metabolism of their host cell(s) and manipulate them to produce viral progeny strengthens the need for a conceptual shift towards calling virus-infected cells ‘virocells’, as this will help emphasize their differences from uninfected cells (Forterre, Reference Forterre2011, Reference Forterre2013). Viral infections can change a microbe's metabolic outputs, impacting the composition and quantity of their biosignatures in ways as profound as the organism's own genome. Combined experimental and in silico studies of virus growth on bacterial hosts have shown how the physiological state of the host cell can be reflected in the timing and level of virus production, whereas virus production is intimately linked to the availability of resources for protein synthesis (Mahmoudabadi et al., Reference Mahmoudabadi, Milo and Phillips2017). Since nutrient availability in the host's environment impacts its ability to produce viruses, the productivity of virus infection can provide a readout of the metabolic demands of the living host (You et al., Reference You, Suthers and Yin2002). Further, computational modelling suggests that viruses evolve to optimally use the finite metabolic energy resources of their host cells (Kim and Yin, Reference Kim and Yin2004). Virus growth may also be linked to host physiology in ways not previously appreciated; studies of viruses that infect bacteria, eukarya and archaea revealed delay times for virus production that correlate with the doubling times of their host cells (Jin and Yin, Reference Jin and Yin2021). Similar virus–host interactions could be at play in other systems, and we must be prepared to identify them.
Virus–host interactions and viral effects on microbial biosignatures can be evaluated through tracking stable isotopic signatures. Autotrophic microbial life preferentially incorporates the lighter isotope available for each biogenic element, a feature often used to identify ancient microbial life and sources of input materials in extant life. Yet the exact signatures of this process depend on which organisms are performing a given metabolic process, how many enzymatic reactions take place in relevant metabolic pathways and how the enzyme(s) work. For example, fractionation factors are different for denitrification via fungi versus bacteria (Ostrom and Ostrom, Reference Ostrom and Ostrom2017), or for methanogenesis depending on the initial substrate and the organisms involved (Whiticar, Reference Whiticar1999; Hornibrook et al., Reference Hornibrook, Longstaffe and Fyfe2000; Penning et al., Reference Penning, Claus, Casper and Conrad2006). When a virus infects a microbial host, it redirects its metabolism, thus possibly impacting these isotopic values. Such impact could be more dramatic if a virus were to encode an auxiliary metabolic gene that has a different fractionation factor than the host version of that gene. For example, kinetic measurements of virus and host versions of the same enzyme have revealed that the virus enzyme had a significantly lower k cat/K M value than the host enzyme (Thompson et al., Reference Thompson, Zeng, Kelly, Huang, Singer, Stubbe and Chisholm2011). Such viral influences can lead to large variations in isotopic signatures, leading to uncertainty when distinguishing between abiotic and biological processes and in the utility of a particular biosignature (Schwieterman et al., Reference Schwieterman, Kiang, Parenteau, Harman, DasSarma, Fisher, Arney, Hartnett, Reinhard, Olson, Meadows, Cockell, Walker, Grenfell, Hegde, Rugheimer, Hu and Lyons2018). While complex and potentially difficult for distinguishing biotic from abiotic processes, isotopic fractionation has the potential to reveal fundamental characteristics of biosphere metabolism, including the impact and contribution of viruses.
Isotopic fractionation measurements have long been applied to the geologic record of Earth to infer characteristics of the metabolisms of past biospheres and changes over time (Johnson et al., Reference Johnson, Johnson and Mardon2021). The isotope history of marine ecosystems throughout Earth's history is well-studied (Zerkle and Mikhail, Reference Zerkle and Mikhail2017; Krissansen-Totton et al., Reference Krissansen-Totton, Buick and Catling2015). However, the isotopic composition of icy environments through time on Earth is less well-characterized but may provide useful analogue environments applicable to other planetary bodies such as Mars (Havig and Hamilton, Reference Havig and Hamilton2019). Distinguishing viral influences on both present isotopic signatures and on their variation over multiple timescales may therefore provide a reference for interpreting isotopic biosignatures of past life on other worlds. To better understand the role of viruses in biogeochemical cycling on Earth in order to interpret and model geochemical cycles elsewhere, we propose the following experimental directions.
Future directions
(a) Improve understanding of how different viruses hijack their host's cellular machinery.
(b) Further explore the broad range of virus–host interactions and dynamics in various ecosystems.
(c) Quantitatively estimate the role and impact(s) of virocell metabolism on Earth's different biogeochemical cycles.
(d) Improve understanding of the extent and distribution of viral impacts on biosignatures for major Earth biogeochemical cycles, including the potential magnitude of such effects.
(e) Search for the existence of any generalizable biosignatures associated with viral metabolic reprogramming to reduce uncertainty associated with biosignatures for life detection.
Impact of viruses on human space exploration
Human spaceflight continues to evolve, and many new technologies and tests are being developed for the safety of crew members and planetary protection for future human-landed missions. However, many studies exclude viruses, despite their threats (and potential benefits) to many types of cellular life. Thus, questions remain as to how viruses interact with other viruses and their hosts in space environments. For example, how do natural versus artificial environments drive changes in the virome and how are viruses impacted and shaped by their environment? As human spaceflight expands to sustained missions beyond low Earth orbit (LEO), nearly every aspect of the mission (including ECLSS, human health and performance, lander operations and extravehicular activities (EVA)) must consider the impact of virus–host interactions. Otherwise, the impacts may prove to be analogous to the current concern about volatile contamination on the ISS where current instrument capabilities and measurements are less sensitive or erroneous due to environmental conditions untested prior to flight (Regberg et al., Reference Regberg, Bell, Davis, Roeschel, Rucker, Tschirschwitz and Wallace2022).
Viral response to spaceflight environments
Viral studies in microgravity have been very limited, with most microbiology investigations focused on bacteria. Those have shown a variety of species-specific and even strain-specific morphological and physiological responses to the low fluid shear force and lack of liquid media convection in microgravity (as reviewed in Diaz et al., Reference Diaz, Li, Irwin, Calle and Callahan2019; Acres et al., Reference Acres, Youngapelian and Nadeau2021; Sharma and Curtis, Reference Sharma and Curtis2022). Without externally applied forces, nearly everything in a microgravity environment can remain suspended or quiescent. Brownian motion dominates, thus reducing the ability for host cells to gather nutrients (Zea et al., Reference Zea, Prasad, Levy, Stodieck, Jones and Shrestha2016), and thereby influencing the size, structure and organization of these host cells. Additionally, the physical limitations of microgravity could potentially modify the infiltration capabilities of viruses. Further, the lack of buoyancy effects may also influence viral dispersal and host encounter rates in both the spacecraft cabin atmosphere and quiescent fluid systems. Another response to microgravity is aggregation (Zea et al., Reference Zea, Nisar, Rubin, Cortesão, Luo, McBride, Moeller, Klaus, Müller, Varanasi, Muecklich and Stodieck2018; Domnin et al., Reference Domnin, Parfenov, Kononikhin, Petrov, Shevlyagina, Arkhipova, Koudan, Nezhurina, Brzhozovskiy, Bugrova, Moysenovich, Domnin, Parfenov, Kononikhin, Petrov, Shevlyagina, Arkhipova, Koudan, Nezhurina, Brzhozovskiy, Bugrova and Moysenovich2022), which might limit the ability of viruses outside an aggregate to encounter a host surface or increase host encounter rate once one host in an aggregate is lysed. Even with external forces (fans, pumps, etc.) moving liquid and gas loops, it is still unknown how well viruses can adhere to encountered surfaces. Simulated microgravity has been associated with a thicker cell membrane envelope in Escherichia coli (Zea et al., Reference Zea, Nisar, Rubin, Cortesão, Luo, McBride, Moeller, Klaus, Müller, Varanasi, Muecklich and Stodieck2018), which could inhibit the ability of viruses to infect hosts. In contrast, lower membrane integrity was observed in Vibrio fischeri (now known as Aliivibrio fischeri; Vroom et al., Reference Vroom, Rodriguez-Ocasio, Lynch, Ruby and Foster2021), which could make hosts more susceptible to membrane-disrupting events.
The effects of microgravity on virus dynamics can be measured through environmental sequencing of surfaces aboard space stations and crewmember microbiomes, yet there is a dismaying lack of virus-centric metagenomic analyses (according to Mora et al., Reference Mora, Wink, Kögler, Mahnert, Rettberg, Schwendner, Demets, Cockell, Alekhova, Klingl, Krause, Zolotariof, Alexandrova and Moissl-Eichinger2019, there has been a single study). Although there may be limitations in sampling size, frequency and depth of coverage due to the technical and logistical challenges of obtaining spacecraft samples, several data sets from the ISS are already available for reanalysis (Be et al., Reference Be, Avila-Herrera, Allen, Singh, Checinska Sielaff, Jaing and Venkateswaran2017; Singh et al., Reference Singh, Wood, Karouia and Venkateswaran2018; Urbaniak et al., Reference Urbaniak, Sielaff, Frey, Allen, Singh, Jaing, Wheeler and Venkateswaran2018, Reference Urbaniak, Morrison, Thissen, Karouia, Smith, Mehta, Jaing and Venkateswaran2022; Checinska Sielaff et al., Reference Checinska Sielaff, Urbaniak, Mohan, Stepanov, Tran, Wood, Minich, McDonald, Mayer, Knight and Karouia2019; Avila-Herrera et al., Reference Avila-Herrera, Thissen, Urbaniak, Be, Smith, Karouia, Mehta, Venkateswaran and Jaing2020). In future studies, the virus-to-host ratio for known virus–host pairs is one statistic that can be estimated, and its distribution explored spatially, temporally and in response to natural or experimental perturbations. Targeted and untargeted metagenomic sequencing of microgravity samples would have complementary strengths allowing for comprehensive analyses that include data from broad surveys at the species and genus taxonomic levels and to narrowly focused studies concerning specific strain and variant population dynamics. Viral dispersal dynamics, host adsorption, infection and progeny productivity in spaceflight environments are extremely understudied and deserve significant further work.
Environmental control and life support systems
ECLSS are imperative for supporting human spaceflight. These flight-proven technologies on the ISS control air composition and temperature, food and water and waste remediation. However, long-duration missions beyond LEO will require robust alternatives that do not rely on frequent resupply missions (Anderson et al., Reference Anderson, Sargusingh, Gatens, Perry, Schneider, Macatangay, Toomarian, McKinley and Shaw2019). Closing the carbon loop through bioregenerative technologies is one approach for providing ECLSS. Algal photobioreactors can remove carbon dioxide (CO2), liberate O2, remove or alter waste and produce edible biomass (Matula and Nabity, Reference Matula and Nabity2019; Fahrion et al., Reference Fahrion, Mastroleo, Dussap and Leys2021). These photobioreactors can withstand the dynamic temperature environment experienced within the ISS thermal control loops (Matula and Nabity, Reference Matula and Nabity2021; Matula et al., Reference Matula, Nabity and McKnight2021). Preliminary spaceflight studies using algae for ECLSS observed thriving cultures (Helisch et al., Reference Helisch, Keppler, Detrell, Belz, Ewald, Fasoulas and Heyer2020; Poughon et al., Reference Poughon, Laroche, Creuly, Dussap, Paille, Lasseur, Monsieurs, Heylen, Coninx, Mastroleo and Leys2020). Likewise, extremophilic algae, lichen, cyanobacteria and fungi included in experiments mounted on the outside of the ISS and Space Shuttle survived weeks to months-long missions (de Vera et al., Reference De Vera, Alawi, Backhaus, Baqué, Billi, Böttger, Berger, Bohmeier, Cockell, Demets and De la Torre Noetzel2019; Malavasi et al., Reference Malavasi, Soru and Cao2020). However, these studies did not characterize the full microbiome within the non-axenic cultures. This current lack is extremely important. For example, virophages can have profound implications for microbial nutrient cycling, often referred to as the microbial loop. Predator–prey simulation models indicate that the presence of virophages regulates helper virus and algal host dynamics altering carbon flux through the microbial loop in aquatic ecosystems (Yau et al., Reference Yau, Lauro, DeMaere, Brown, Thomas, Raftery, Andrews-Pfannkoch, Lewis, Hoffman, Gibson and Cavicchioli2011). To survive unfavourable conditions (e.g. microgravity, viral infection and antimicrobials), some microbes can form protective barriers such as biofilms. Bacteria exude a variety of organic matter dubbed extracellular polymeric substances, which forms the biofilm and acts like glue securing and protecting the bacteria allowing them to remain hydrated, control local pH and other services. Biofilms can benefit certain systems such as the rhizosphere (plants root zones) aiding in food production. However, biofilms can also be detrimental by corroding or clogging machinery that supports ECLSS, or preventing wound healing in skin abrasions, to name just a couple of examples (Landry et al., Reference Landry, Morey, Bharat, Haney and Panesar2020). Recently, highlighted in Justiniano et al. (Reference Justiniano, Goeres, Sandvik, Kjellerup, Sysoeva, Harris, Warnat, McGlennen, Foreman, Yang and Li2023) are the knowledge gaps in our understanding of microbiomes (including viruses) and biofilm formation in space, although we do know that viruses can adapt new strategies to prevent bacterial growth or kill organisms within biofilms. Understanding potential biome or virome shifts within these systems over long durations may elucidate the need for specific algal species selection, causes of viral or bacterial-based system failures and operational considerations (Fig. 3; Matula and Nabity, Reference Matula and Nabity2019).
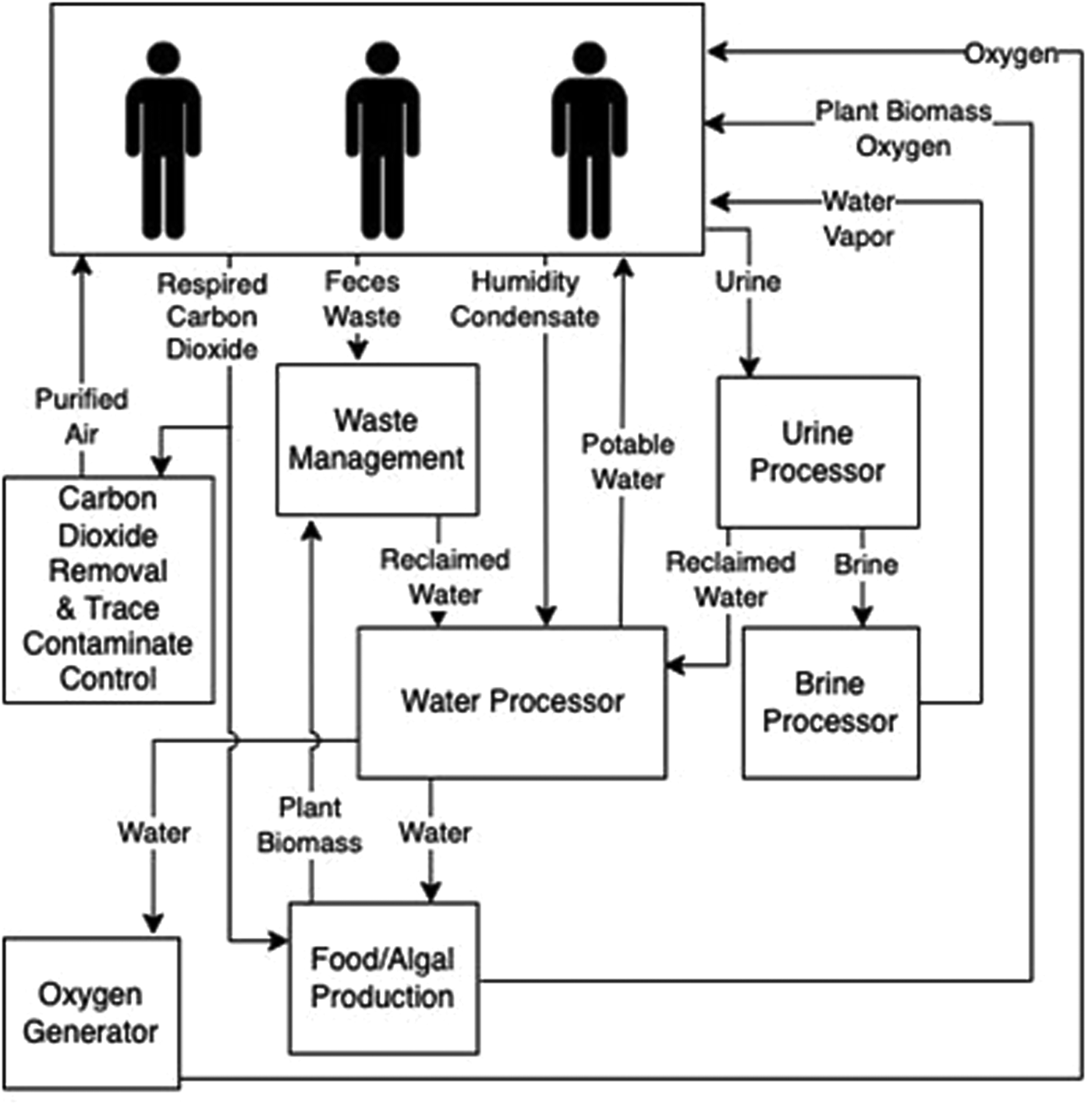
Figure 3. Review of spaceflight ECLSS potentially impacted by viruses. A simplified overview of environmentally controlled life-support systems for spacecraft and surface habitats that could be positively or negatively impacted by viruses.
Bioregenerative ECLSS
Efforts on the ISS have focused on plants for CO2 removal and O2 production, as a source of multivitamins, for urine reuse and to promote psychological well-being (Dzhos et al., Reference Dzhos, Golubkina, Antoshkina, Kondratyeva, Koshevarov, Shkaplerov, Zavarykina, Nechitailo and Caruso2021). On Earth, plant viruses can be a threat to agriculture, and disease management relies on rapid and accurate viral identification (Rubio et al., Reference Rubio, Galipienso and Ferriol2020). Many plants have been grown under microgravity, including flower-producing species, herbs and vegetables. However, due to the stress of spaceflight, viral infection of these plants can be hugely influential on functionality. A recent study found that spaceflight factors significantly affect tomato plants (Dzhos et al., Reference Dzhos, Golubkina, Antoshkina, Kondratyeva, Koshevarov, Shkaplerov, Zavarykina, Nechitailo and Caruso2021), increasing the productivity of the plants and the concentration of some vitamins such as carotenoids, which are important for crew members on long-term space missions. Importantly, plants grown from seeds that were in space for half a year prior to germination were resistant to viral infection. Moreover, seed resilience of many plant species (amongst other organisms) was tested by keeping them outside the ISS for 558 or 682 days, thus exposing them to high levels of radiation (Tepfer and Leach, Reference Tepfer and Leach2017). All seeds were able to germinate after the shorter flight, but only seeds with a stronger coat could survive the longer radiation exposure. While these studies show promise for cultivating plants on long-term voyages, our understanding of plant–virus interactions on short flights is extremely limited and we have no information for long-term flights.
Human physiology and the viral ecology of crewed spacecraft
In space-bound crewmembers, latent viruses are shown to reactivate more than in matched controls. Sometimes this reactivation occurs before leaving Earth and is presumably stress related. However, there are several plausible reactivation triggers associated with spaceflight itself, such as ultraviolet or ionizing radiation (Pavletíc et al., Reference Pavletić, Runzheimer, Siems, Koch, Cortesão, Ramos-Nascimento and Moeller2022), but precisely which mechanisms might be responsible for increased virus reactivation in crewmembers have not been determined. Reactivation has included herpes simplex virus, Epstein–Barr virus and varicella zoster virus (Stowe et al., Reference Stowe, Pierson, Feeback and Barrett2000; Mehta et al., Reference Mehta, Cohrs, Forghani, Zerbe, Gilden and Pierson2004; Pierson et al., Reference Pierson, Stowe, Phillips, Lugg and Mehta2005; Rooney et al., Reference Rooney, Crucian, Pierson, Laudenslager and Mehta2019). After 6–12 months in space, crewmembers experienced significant changes in their microbiome (both internal and external) that led to rashes and hypersensitivity episodes (Voorhies et al., Reference Voorhies, Mark Ott, Mehta, Pierson, Crucian, Feiveson, Oubre, Torralba, Moncera, Zhang and Zurek2019). These symptoms warrant further investigation into the effects of long-term space environment exposure on humans combined with their accompanying microbes and viruses.
The community composition of viruses and other organisms on the ISS is mostly comprised of viruses that only infect non-human cells, such as bacteriophages. This composition differs from that of terrestrial space analogues (e.g. MDRS and HI-SEAS), thus pointing towards the space environment as the likely trigger (Mora et al., Reference Mora, Wink, Kögler, Mahnert, Rettberg, Schwendner, Demets, Cockell, Alekhova, Klingl, Krause, Zolotariof, Alexandrova and Moissl-Eichinger2019). Although these viruses do not infect humans, they can be transported back to Earth and released into Earth environments via plants, humans and other entities that can harbour them. Continued efforts are needed for quarantining people within space stations for long-term space travel and increased screening for crewmembers returning to Earth. A viable way to test viral transmission and educate humans, especially those preparing for space travel is through simulations that consider epidemiology parameters (Patel et al., Reference Patel, Sharma, Reddy, Princy, Tsaramirsis, Pavlopoulou, Koçer and Piromalis2021). Although it might be tempting to contemplate elimination of viruses from spacecraft wherever possible, we should be sensitive to the unanticipated positive roles viruses may play and the unreasonable resources that viral elimination would entail. As well, a virus's presence can be beneficial by sometimes warding off more pathogenic relatives by cross-protection or superinfection exclusion (Folimonova et al., Reference Folimonova, Achor and Bar-Joseph2020). Immunologic ‘eustress’ can promote a healthy organism, as is seen by comparison to the physiological deficits of germ-free animals (Round and Mazmanian, Reference Round and Mazmanian2009). Indeed, in some cases, viruses can be symbiotic with their host organisms (Roosinck, Reference Roosinck2011). As such, viruses are key players in crewmember health and safety and in the ecology of crewed environments.
Spaceflight operational impacts
Given the differences in viral dispersal patterns observed in low-gravity environments and the extreme conditions under which viruses persist and interact with hosts (see Section ‘Viral response to spaceflight environments’), even surfaces often considered sterile, or at least clean, must be investigated. Some bacterial species – for example, Bacillus pumilis strain SAFR-032 (Tirumalai et al., Reference Tirumalai, Rastogi, Zamani, O’Bryant Williams, Allen, Diouf, Kwende, Weinstock, Venkateswaran, Fox and Setlow2013) – are refractory to pre-flight sterilization, and some could be tolerant as well given the extreme diversity of viral morphotypes (including lipid-free viruses, see Fig. 1). Even with perfect sterilization of equipment (which is essentially unobtainable), it is not currently possible to achieve a virus-free environment on a manned space mission because of post-launch viral shedding by crewmembers (vide supra). The frequency of sampling, maintaining and cleaning life support systems will impact day-to-day spaceflight operations, during both cruise phase and orbital or surface sustained missions. EVA and associated planetary protection protocols must also be accommodated: for example, protecting areas with high-scientific value from contamination (e.g. Rummel et al., Reference Rummel, Beaty, Jones, Bakermans, Barlow, Boston, Chevrier, Clark, de Vera, Gough, Hallsworth, Head, Hipkin, Kieft, McEwen, Mellon, Mikucki, Nicholson, Omelon, Peterson, Roden, Sherwood Lollar, Tanaka, Viola and Wray2014). Modifications of operations may entail landing further from a planetary surface sample site, designing new vehicles, requiring more stringent cleaning protocols or considering entirely new sample sites (e.g. Meyer et al., Reference Meyer, Bakermans, Beaty, Bernard, Boston, Chevrier, Conley, Feustel, Gough, Glotch, Hays, Junge, Lindberg, Mellon, Mischna, Neal, Pugel, Quinn, Raulin, Rennó, Rummel, Schulte, Spry, Stabekis, Wang and Yee2019). Additionally, contamination restrictions during EVA will narrow the design of space suit systems (Willson et al., Reference Willson, Rask, George, deLeon, Bonaccors, Blank, Slocombe, Silburn, Steele, Gargarno and McKay2014). Attempts to dictate planetary protection protocols without fully understanding viral dispersal and survivability will potentially result in overly restrictive constraints that ultimately hinder scientific discovery or alternatively inadequate protocols that do not achieve proper containment.
To understand the impact of viruses on human space exploration, we suggest that the following experiments be prioritized.
Future directions
(a) Characterize differences in dispersal, aggregation and adsorption processes of viruses in both fluid and air microgravity environments.
(b) Compare the ability of viruses to adsorb to and infect hosts during and after microgravity-induced cellular changes.
(c) Investigate virus–host interactions, especially in response to perturbation effects on virus-to-host ratios.
(d) Develop enhanced capabilities for onboard biosurveillance.
(e) Investigate viromes in built environments relevant to space, including the ISS.
(f) Explore virus–host dynamics for both human- and plant-associated microbiomes in space.
(g) Assess space-associated triggers that cause latent viral infections to become virulent.
(h) Quantitatively estimate the role and impact(s) of virocell metabolism in spaceflight environments.
(i) Identify viral loading impacts significant to both planetary protection requirements, and preservation of human health. Explore desirable modifications to spaceflight operations (travel, landing and EVA) that could ameliorate such loading impacts.
(j) Characterize viral influence on microbial biofilm composition and growth dynamics in human, algal and plant systems under various space-related conditions.
Detection of viruses on Earth and elsewhere
A variety of instrumentation exists that can detect both microbial biomarkers and viruses. These types of techniques have often been used on Earth to study viruses (Sommers et al., Reference Sommers, Chatterjee, Varsani and Trubl2021; Vincent and Vardi, Reference Vincent and Vardi2023). However, these same techniques can be difficult to conduct with automated systems in space and have yet to be designed specifically for space missions. Therefore, it is currently unclear if and how informational molecules can be reliably detected in environmental samples beyond Earth. Engineering investigations must be paired with science goals to further develop innovative, flight-ready technologies to detect and characterize viruses, virus-like particles and virus genomes efficiently and accurately. Once developed, such technologies could then be applied in Earth analogue systems to test performance and limitations for detecting viruses and virus-like particles. Additionally, these instruments and protocols can be incorporated into standard operating procedures for planetary sample missions.
While autonomous virus-detection technology is not yet ready, the future is promising. For example, solid-state nanopore-based biosensors are currently being designed for spaceflight and have proven capability for evaluating different biomarkers (i.e. DNA/RNA, proteins and whole viral particles – spanning from a few nanometres to over 100 nanometres in diameter). Solid-state nanopore sensors would be particularly useful in space missions because they also can measure particle-size distributions within virus populations and discriminate between viral particles by monitoring the change in electrical current as particles pass through an electrically biased pore or by measuring the mass density of viruses (Zhou et al., Reference Zhou, Li, Tan, Zlotnick and Jacobson2011; Arjmandi et al., Reference Arjmandi, Van Roy and Lagae2014; McMullen et al., Reference McMullen, De Haan, Tang and Stein2014; Akpinar and Yin, Reference Akpinar and Yin2015; Wu et al., Reference Wu, Chen, Zhou, Wang, Xia, Ma, Luo and Liu2016). Researchers have adapted flow cytometry to the scale of virus particles; specifically, flow virometry employs more powerful lasers, reduced diameter fluid flows, wider-angle sampling of scattered light and fluorescent labelling of particles (Bhat et al., Reference Bhat, Cao and Yin2022). Additionally, microscopy has been used to evaluate microbial and viral populations on Earth. Microscopy technologies paired with spectroscopic techniques, can inform the physical shapes and boundaries belonging to certain chemical or mineralogic compositions (Zhang et al., Reference Zhang, Hung, Song and He2013). While an atomic force microscope was used during the Phoenix Mars Lander to investigate Martian soils (Pike et al., Reference Pike, Staufer, Hecht, Goetz, Parrat, Sykulska-Lawrence, Vijendran and Madsen2011) and recently, a scanning electron microscope was added to the ISS (Own et al., Reference Own, Martinez, Cushing, DeRego, Own, Weppelman, Thomas-Keprta, Rahman and Pettit2018) higher performance complex space-qualified microscopes have yet to be developed that can reliably detect virus-like particles. Another technique that could be applied to space virology is NanoSIMS, which performs in situ quantitative trace sample analysis with exceptional sensitivity and spatial resolution (Mayali, Reference Mayali2020; Pett-Ridge and Weber, Reference Pett-Ridge, Weber and Navid2022). NanoSIMS has been used to detect viruses (Gates et al., Reference Gates, Condit, Moussatche, Stewart, Malkin and Weber2018) and map their elemental and isotopic distributions in complex communities and in mineralized samples (Pacton et al., Reference Pacton, Wacey, Corinaldesi, Tangherlini, Kilburn, Gorin, Danovaro and Vasconcelos2014). Also, the Network for Life Detection is currently using NanoSIMS to discern between abiotic and biological signatures based on the differences in elemental and isotopic patterns of cellular organisms versus minerals and other precipitates. These technologies complement spectroscopic methods by providing orthogonal evidence for viral and non-viral life (Zhang et al., Reference Zhang, Hung, Song and He2013). Overall, there are multiple instrument designs either waiting to be developed or currently being developed for detecting viruses and life beyond Earth.
Environmental virology techniques could also complement life detection instrumentation. In conjunction with other previously described instruments, high fidelity sequencing methods and high-resolution structure analysis could also confirm the presence of viruses on other worlds (Bamford et al., Reference Bamford, Grimes and Stuart2005). Moreover, to reduce signal-to-noise problems, relic or environmental DNA can be removed from a system using propidium monoazide to enhance detectability of intact viruses and microbes (Wagner et al., Reference Wagner, Malin, Knapp and Illmer2008; Weinmaier et al., Reference Weinmaier, Probst, La Duc, Ciobanu, Cheng, Ivanova, Rattei and Vaishampayan2015). Thus, methods of environmental virology could also be necessary for life detection and returned samples (Janjic, Reference Janjic2018; Ricciardi et al., Reference Ricciardi, Cassey, Leuko and Woolnough2022; Table 3). Much work remains to integrate both engineering and science perspectives. While some instrumentation, such as light, electron and atomic force microscopy, has been used on Mars and in space on the ISS (Pike et al., Reference Pike, Staufer, Hecht, Goetz, Parrat, Sykulska-Lawrence, Vijendran and Madsen2011; Own et al., Reference Own, Martinez, Cushing, DeRego, Own, Weppelman, Thomas-Keprta, Rahman and Pettit2018), instrumentation in development must also be tested in extreme analogue environments to explore and overcome limitations. Moreover, for planetary protection purposes, utilizing instrumentation for detecting viruses should be incorporated into standard operating procedures for flight and sample return missions. To fully understand the presence of viruses in space environments, new technologies need to be developed and include the following.
Table 3. Possible virus detection methods: how can we detect extraterrestrial viruses?
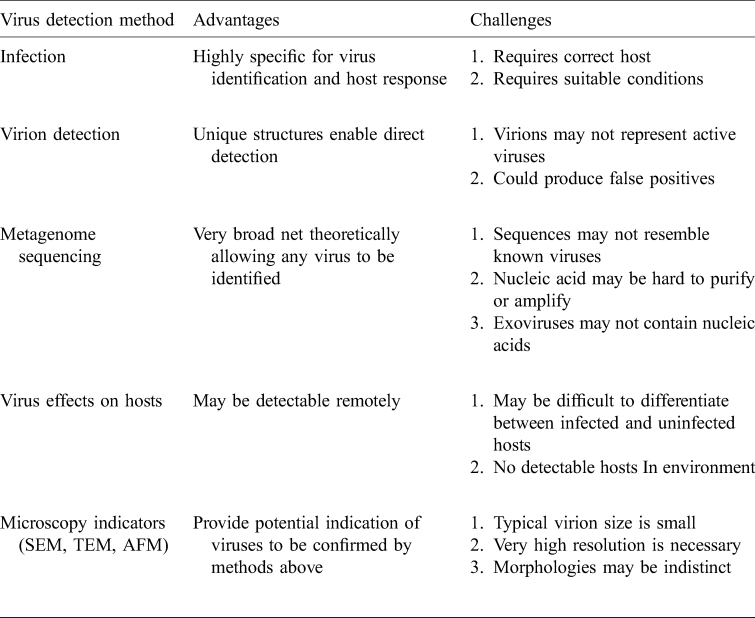
Of the currently known methods for virus detection, those suitable for astrobiology applications are challenging to imagine but we can leverage off the suite of methods already in use on Earth.
Future directions
(a) Develop innovative autonomous flight-ready technologies for efficient and accurate detection and characterization of virus genomes.
(b) Apply such technologies to Earth analogue systems to explore prospects and limitations for detecting viruses and virus-like particles.
(c) Incorporate measurements of viruses in standard operating procedures for sample return missions and subsequent examinations.
Conclusion
For effective pursuit of astrobiology questions, it is critical to understand how life on Earth functions, as it is currently the only place where life is confirmed to exist. Viruses are key contributors to Earth's ecosystems; however, much remains unknown regarding their influence on cellular life, role in evolutionary history and their fundamental physical interactions with the Earth system. Basic ecological factors, such as persistence and decay under various scenarios, also remain underexplored. Likewise, safe and effective crewed deep space travel will require a thorough understanding of the human microbiome in space, including viruses. Here, we have outlined ways in which focused astrovirological investigations can broadly advance the goals of space science. Across the diverse disciplines that make up astrobiology and space biology, we highlight several classes of investigations which cannot afford to neglect viruses. Most of the diversity of life on Earth is comprised of viruses, whether diversity is defined by number of species, type of genetic information, number of individuals (Breitbart et al., Reference Breitbart, Salamon, Andresen, Mahaffy, Segall, Mead, Azam and Rohwer2002; Roossinck, Reference Roossinck2012; Paez-Espino et al., Reference Paez-Espino, Eloe-Fadrosh, Pavlopoulos, Thomas, Huntemann, Mikhailova, Rubin, Ivanova and Kyrpides2016; Parikka et al., Reference Parikka, Le Romancer, Wauters and Jacquet2017; Mushegian, Reference Mushegian2020), absence of any universally present gene, mode of replication or number of unique (i.e. non-homologous) genes (Koonin and Wolf, Reference Koonin and Wolf2012). Viruses should be explicitly considered in organism-level astrobiological investigations. The ability to detect virus-like entities must be a point of assessment for instruments and missions to directly detect extraterrestrial organisms for planetary protection, human spaceflight safety and sample return (Janjic, Reference Janjic2018; Ricciardi et al., Reference Ricciardi, Cassey, Leuko and Woolnough2022). When organisms are used as model systems, viruses should be adequately represented to reduce biases and increase utility of diverse astrobiological studies.
‘Whether viruses are alive or not may be a moot question, but if a virion (or virus-like particle) were to be unequivocally detected in an extraterrestrial sample, very few people would claim that this would not be evidence for life – wherever that sample was from’.
– Berliner et al. (Reference Berliner, Mochizuki and Stedman2018)
Financial support
The work of G.T., J.P-R., N.M. and P.K.W. was supported by the US Department of Energy (DOE) Office of Science, Office of Biological and Environmental Research Genomic Science programme award SCW1632, and by Lawrence Livermore National Laboratory LDRD award 21-LW-060, under the auspices of the DOE under contract DE-AC52-07NA27344. The work conducted by S.R. was supported by the U.S. Department of Energy Joint Genome Institute (https://ror.org/04xm1d337), DOE Office of Science User Facility and the Office of Science of the U.S. Department of Energy operated under contract no. DE-AC02-05CH11231. Work by K.M.S. was supported by the U.S. National Science Foundation, MCB-1929273 and MCB-2025305. Work by J.Y. was supported by the U.S. National Science Foundation (MCB-2029281 and CBET-2030750). Work in the Kaelber lab was supported in part by the U.S. National Institutes of Health, R21GM140345.
Conflict of interest
The authors report no conflict of interest.









